Abstract
Modulation of the low affinity adenosine receptor subtype, the A2b adenosine receptor (A2bAR), has gained interest as a therapeutic target in various pathologic areas associated with cardiovascular disease. The actions of the A2bAR are diverse and at times conflicting depending on cell and tissue type and the timing of activation or inhibition of the receptor. The A2bAR is a promising and exciting pharmacologic target, however, a thorough understanding of A2bAR action is necessary to reach the therapeutic potential of this receptor. This review will focus on the role of the A2bAR in various cardiovascular and metabolic pathologies in which the receptor is currently being studied. We will illustrate the complexities of A2bAR signaling and highlight areas of research with potential for therapeutic development.
Adenosine levels increase markedly during hypoxia, inflammation, and cell injury and have been implicated as cellular signaling mediators in adaptation to cellular stress (Bodin and Burnstock, 1998; Bodin and Burnstock, 2001; Linden, 2005; Fredholm, 2007; Eltzschig and Carmeliet, 2011). Adenosine acts on four G-protein coupled receptors, the A1, A2a, A2b, and A3 adenosine receptors (ARs) (Fredholm et al., 2011). The actions of adenosine on these receptors are wide and varied. In this review we will focus on the A2bAR, which has recently garnered interest in being used therapeutically in various avenues, including asthma, inflammatory bowel disease, diabetes, and sickle cell disease among others (Elzein et al., 2008; Hasko et al., 2008; Kolachala et al., 2008; El-Tayeb et al., 2011; Field et al., 2014). Yet, there have been controversial reports as to whether the receptor is protective or injurious in such pathologies. The A2bAR is a low affinity adenosine receptor subtype, requiring micromolar range adenosine levels for activity, while the other receptors function at the nanomolar range (Fredholm et al., 2001; Fredholm et al., 2011). As adenosine is released from cells during injury, hypoxia, or cellular stress, (Bodin and Burnstock, 1998; Bodin and Burnstock, 2001; Linden, 2005; Fredholm, 2007; Eltzschig and Carmeliet, 2011) and as A2bAR expression is elevated by similar stresses (Eltzschig et al., 2003; Nguyen et al., 2003; Kolachala et al., 2005; St Hilaire et al., 2008), the A2bAR has been described as the adenosine receptor which is most active in pathologic states and hence a promising therapeutic target. In light of this, we will review the literature on the role of the A2bAR in various cardiovascular and metabolic systems in which the receptor is currently being investigated as a potential pharmacologic target. As will be highlighted in this review, the actions of the A2bAR are varied and dependent on timing, expression, and model system studied. The A2bAR is an exciting therapeutic target, however, appropriate understanding of the actions of this receptor will be necessary to fully realize its therapeutic potential.
Inflammatory processes underlying cardiovascular and metabolic diseases
Inflammation is an important mediator of cardiovascular (Lusis, 2000; Glass and Witztum, 2001; Woollard and Geissmann, 2010; Zernecke and Weber, 2010; Libby et al., 2011) and metabolic pathology (Wellen and Hotamisligil, 2005; Hotamisligil, 2006). Furthermore, clinical markers of inflammation, like C-reactive protein (CRP), are associated with an increased risk of cardiovascular disease (Kaptoge et al., 2010). Much research has focused on the regulation of the inflammatory response by the A2bAR. We will review those findings related to cardiovascular and metabolic disease and those that illustrate the complexity of A2bAR signaling.
In 2006, Yang et al. generated an A2bAR knockout (KO) mouse model (Yang et al., 2006) and showed that the A2bAR is highly expressed on macrophages and that A2bAR KO mice have a mild increase in plasma levels of the pro-inflammatory cytokines, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), and decreased plasma levels of the anti-inflammatory cytokine, IL-10, following lipopolysaccharide (LPS) stimulation as compared to wild type (WT) mice (Yang et al., 2006). These results suggest that the A2bAR dampens the inflammatory response. Further studies have shown an anti-inflammatory role for the A2bAR. For example, a nonspecific adenosine agonist, 5′-N-ethylcarboxamidoadenosine (NECA) increases the production of IL-10 from LPS-activated RAW 264.7 macrophages and an A2bAR antagonist attenuates this effect (Nemeth et al., 2005). Additionally, activation of the A2bAR with BAY 60-6583, a specific A2bAR agonist, inhibits superoxide generation by neutrophils (van der Hoeven et al., 2011).
Importantly, in vivo studies have elucidated mechanisms in addition to alteration of cytokine levels that explain how the A2bAR affects the pathogenesis of inflammation. A2bAR KO mice have increased expression of adhesion molecules intracellular adhesion molecule (ICAM-1) and E-selectin, which increases leukocyte rolling and adhesion (Yang et al., 2006). Furthermore, A2bAR KO mice exhibit enhanced neutrophil infiltration into tissues under hypoxic conditions (Eckle et al., 2008a). These studies suggest that the A2bAR prevents inflammatory cell transmigration into the tissue of interest, one of the first steps in the inflammatory response. In addition the A2bAR affects alternative macrophage activation. Adenosine and NECA enhance the expression of markers of alternative macrophages (arginase 1, tissue inhibitor of matrix metalloproteinase-1, and macrophage galactose-type C-type lectin-1) (Csoka et al., 2012). Pharmacologic inhibition of the A2bAR or genetic lack of the A2bAR prevents NECA-induced expression of these alternative macrophage markers (Csoka et al., 2012), suggesting that A2bAR promotes alternative macrophage development. Finally, a unique role for the A2bAR in regulating the nuclear factor kappa B (NFκB) pathway was demonstrated. The C-terminus of the A2bAR binds to the p105 subunit of NFκB, which prevents polyubiquitination and degradation of p105 and subsequently inhibits NFκB activation (Sun et al., 2012). Intriguingly, this effect occurs independent of ligand binding to the receptor (Sun et al., 2012), suggesting a role for the A2bAR in regulating inflammation even in the absence of endogenous ligand.
On the other hand, other studies have suggested that the A2bAR promotes inflammation. Early work performed in intestinal epithelial cells (Sitaraman et al., 2001), cultured bronchial smooth muscle cells (Zhong et al., 2004), lung fibroblasts (Zhong et al., 2005), and primary murine alveolar macrophages (Pedroza et al., 2011) suggested a proinflammatory role for the A2bAR through increased release of the proinflammatory cytokine, IL-6. NECA treatment of the intestinal epithelial cell line, T84, which only expresses the A2bAR and not the other adenosine receptor subtypes, increases IL-6 secretion (Sitaraman et al., 2001). The studies in cultured bronchial smooth muscle cells, lung fibroblasts, and alveolar macrophages showed that the nonspecific adenosine receptor agonist, NECA, induces IL-6 secretion and that this response is attenuated by an A2bAR antagonist (Zhong et al., 2004; Zhong et al., 2005; Pedroza et al., 2011). Furthermore, Ryzhov et al. generated an A2bAR KO mouse using a different targeting construct and determined the ability of the A2bAR to mediate two actions of NECA: (1) stimulation of IL-6 release and (2) inhibition of LPS-induced TNF-α release (Ryzhov et al., 2008). In this study, NECA increases plasma IL-6 levels in WT but not A2bAR KO mice, suggesting that the A2bAR promotes inflammation by increasing IL-6 levels (Ryzhov et al., 2008). They also found that NECA decreases LPS-induced TNF-α levels in both WT and A2bAR KO mice, suggesting that the A2bAR does not dampen inflammation in response to LPS (Ryzhov et al., 2008). Despite these results that suggest that A2bAR activation by NECA promotes inflammation, this study also found that macrophages derived from A2bAR KO mice secrete more TNF-α than WT mice (Ryzhov et al., 2008), which is consistent with the results from Yang et al. (2006).
These studies underlie important considerations in studying the A2bAR as well as other receptors. First, genetic lack of a receptor does not always predict the results of pharmaceutical modulation of the receptor. Knockout mouse models are helpful in determining the role of a receptor in pathologic processes; however, developmental effects of genetic alteration can impact the interpretation of the results. Lack of the A2bAR throughout development may upregulate other proteins to compensate for lack of the A2bAR. Pharmacologic modulation of a receptor, unlike knockout mouse models, determines how alteration of cellular signaling affects a particular point in time, but may not mimic lifelong lack of a receptor. Second, use of pharmacologic modulators requires thorough understanding of the action of the agents on potentially other targets. The presence and activity of other receptors may influence the effect of A2bAR on particular process. For example, the A2bAR receptor antagonist, MRS 1754, prevents adenosine from suppressing TNF-alpha expression only in the absence of the A2aAR (Kreckler et al., 2006). Furthermore, NECA is a nonspecific adenosine receptor agonists that can activate all four of the adenosine receptors (Fredholm et al., 2011), which makes it more difficult to conclude which receptor is responsible for certain actions of the compound.
Different cell types, each expressing the A2bAR may have opposing downstream outcomes in response to modulation of the receptor. The initial in vivo studies that supported an anti-inflammatory role for the A2bAR showed that TNF-α levels following LPS injection are greater in A2bAR KO mice, than control counterparts (Yang et al., 2006). Bone marrow-chimeric mice studies illustrated that A2bAR signaling on hematopoietic cells is more important than A2bAR signaling in nonhematopoietic cells in affecting cytokine levels following LPS injection (Yang et al., 2006). In a different disease model, that of cecal ligation and puncture-induced sepsis, A2bAR signaling on nonhematopoietic cells was the main contributor to reducing cytokine and chemokine production (Csoka et al., 2010). In contrast, in a pulmonary ischemia-reperfusion model, the lungs of the A2bAR KO mice had reduced levels of TNF-alpha and IL-6 as compared to WT mice (Anvari et al., 2010). These effects were largely due to A2bAR expression on non-bone marrow-derived cells (Anvari et al., 2010).
A2bAR signaling in acute and chronic inflammation may also yield differing results. For example, in a model of acute antigen-induced anaphylaxis, A2bAR KO mice have greater mortality, increased mast cell degranulation, and cytokine production (Hua et al., 2007). However, in a model of chronic exposure to pulmonary allergen, A2bAR KO mice have reduced eosinophilic infiltrate, reduced levels of IL-4 (a cytokine induced by allergens), and reduced airway smooth muscle remodeling inflammation as compared to WT mice, suggesting that lack of the A2bAR was protective against chronic pulmonary inflammation and airway remodeling (Zaynagetdinov et al., 2010). Pharmacologic studies also support this conclusion as the A2bAR antagonist, CVT-6883, inhibits allergen-induced chronic pulmonary inflammation (Mustafa et al., 2007). Furthermore, the A2bAR is protective in acute inflammation as evidenced by the findings that A2bAR KO mice have increased pulmonary inflammation following acute injury from hypoxia (Eckle et al., 2008a) or ventilator-induced lung injury (Eckle et al., 2008b). On the other hand, mice deficient in adenosine deaminase (ADA), an enzyme responsible for adenosine breakdown, have elevated levels of adenosine, and develop features of chronic pulmonary inflammation, like fibrosis (Chunn et al., 2005). This effect is thought to be mediated by adenosine signaling through the A2bAR, as pharmacologic inhibition of the A2bAR in ADA KO mice ameliorates the signs of chronic pulmonary inflammation (Sun et al., 2006). However, ADA/A2bAR double KO mice have increased pulmonary inflammation and airway damage leading to early death, a finding that supports the protective role of A2bAR signaling in acute lung injury (Zhou et al., 2009).
Overall, when interpreting studies on the role of the A2bAR in inflammation-induced processes, it is important to consider timing of A2bAR activation or inhibition (pharmacologic or genetic), specificity of pharmacologic agents, model systems, and cell types as well as acute versus chronic responses studied. In addition, when studying inflammation in vivo, the influence of housing facilities with potentially somewhat different pathogen screens, could impact experimental outcomes.
Vascular stenosis
The current management of symptomatic coronary artery disease includes coronary reperfusion with percutaneous coronary intervention (PCI), which involves balloon angioplasty and stent placement at the site of the previous arterial blockage. Drug eluting stents are preferred over bare metal stents in most patients because they reduce the rate of restenosis (Roiron et al., 2006), a gradual narrowing of the vessel diameter that occurs following PCI. The rate of restenosis of drug eluting stents ranges from 3 to 20% depending on the type of stent used (with lower rates of restenosis for second generation drug eluting stents) and the length of follow up of the trial and the size of the plaque studied (Dangas et al., 2010). The pathogenesis of restenosis is believed to involve arterial injury and neointimal proliferation with macrophage accumulation (Hoffmann et al., 1996; Kearney et al., 1997; Komatsu et al., 1998; Kornowski et al., 1998). Research into medical therapy that can reduce the rate of restenosis after PCI has gathered interest. One such agent that is being investigated is cilostazol, a phosphodiesterase inhibitor that results in an increase in intracellular cyclic AMP (cAMP). It is currently approved for the treatment of intermittent claudication and has reduced the rate of restenosis following PCI with bare metal stents (Douglas et al., 2005) and with drug eluting stents (Lee et al., 2011). The A2bAR, which when activated also increases cAMP levels, has been studied as a therapeutic target in restenosis.
The A2bAR has been shown to be protective against restenosis following vascular injury. In one study, a guidewire-induced femoral artery injury model in mice was used to mimic angioplasty (Yang et al., 2008). A2bAR KO mice have increased neointimal formation following femoral artery injury as compared to WT mice (Yang et al., 2008). In addition, the chemokine receptor CXCR4, which promotes cell migration and neointimal hyperplasia, is upregulated in platelets, macrophages, and leukocytes derived from A2bAR KO mice (Yang et al., 2008). Furthermore, the A2bAR plays a role in vascular smooth muscle cell (VSMC) proliferation, one of the pathologic steps of restenosis, as VSMCs from A2bAR KO mice proliferate faster than those derived from WT mice (Yang et al., 2008). This is consistent with earlier studies that showed an inhibitory effect of cAMP and A2bAR on vascular smooth muscle proliferation (Dubey et al., 1998; Dubey et al., 1999; Dubey et al., 2000; Burnstock, 2002; Jackson et al., 2011). These studies suggest that A2bAR activation may be beneficial in preventing restenosis following PCI, a conclusion that was recently tested. In accordance, Bot et al. showed that activation of the A2bAR with BAY 60-6583, the A2bAR specific agonist, for 18 days following wire-induced vascular injury of the left common carotid artery in Apolipoprotein E (ApoE) deficient mice reduces lumen stenosis and media size (Bot et al., 2012). As a mechanism to explain this result, the authors noted that in vivo and in vitro treatment with BAY 60-6585 decreases VSMC proliferation (Bot et al., 2012). Furthermore, the authors also demonstrated that BAY 60-6583 increases collagen content, which suggests that A2bAR activation may also promote plaque stability (Bot et al., 2012). Overall, these studies show promising effects of A2bAR agonism on reducing restenosis.
Vascular tension
Adenosine has long ago been described as a vasodilator (Drury and Szent-Gyorgyi, 1929). Despite its vasodilatory effects, this nucleoside has never been used as an antihypertensive due to its short half-life and ability to stimulate four different receptors (Gessi et al., 2011). Vascular smooth muscle cells (VSMCs) are important in the regulation of vascular tension and have high expression of the A2bAR (Yang et al., 2006). VSMCs contract or dilate in response to changes in calcium (Ca2+) levels that result from ligand stimulation of surface receptors (Wynne et al., 2009). It is known that activation of the A2bAR by coupling to Gs and Gq results in downstream increases in cAMP and IP3, respectively (Wynne et al., 2009). IP3 results in elevated cytosolic Ca2+ and contraction of VSMCs whereas increases in cAMP results in decreased Ca2+ and relaxation of VSMCs (Wynne et al., 2009). These changes in Ca2+ levels cause perturbation of the cytoskeleton leading to VSMC contraction or relaxation (Wynne et al., 2009). Ca2+ bound to calmodulin in VSMCs activates myosin light chain kinase (MLCK), which phosphorylates the regulatory light chains of myosin by adding a phosphate group to serine-19 (Wynne et al., 2009). The phosphorylation of myosin light chains causes cycling of myosin heads on actin, which culminates in VSMC contraction (Wynne et al., 2009). Thus, the specific nature of downstream signaling of the A2bAR (via IP3 or cAMP) in VSMCs alters the final impact of A2bAR stimulation on modulation of VSMC contractility and vascular tension.
Several in vitro studies have examined the role of the A2bAR in relaxation of VSMCs, using A2-type adenosine receptor agonist-treated, precontracted isolated aortas using a force transducer to measure tension. One study showed that NECA, the nonspecific adenosine receptor agonist as well as CGS21680, the A2aAR specific agonist, elicit vasodilation in Prostaglandin F2α (PGF2α)-precontracted porcine arterial rings (Balwierczak et al., 1991) When potassium chloride (KCl) is used to precontract the arterial rings, neither NECA nor CGS21680 treatment causes vasodilation (Balwierczak et al., 1991). This study suggests that the A2aAR (and perhaps the A2bAR) are important for vasodilation of porcine arterial rings. In a second study found that when rat renal arteries are precontracted with phenylephrine, NECA, but not CGS21680, treatment induces relaxation (Martin and Potts, 1994). Furthermore, blocking nitric oxide (NO) release from endothelial cells, attenuates the vasodilatory response of the VSMCs to NECA, thereby illustrating that the A2bAR is likely responsible for vasodilation in the renal artery, and that this effect is due to the A2bAR regulation of NO release from endothelial cells (Martin and Potts, 1994). Thus, A2bAR activation has differing affects on vessel tension across species and the effect depends on the initial precontracting agents used on the tissues.
Vascular resistance has also been measured in isolated tissues by using perfusion pressure as a surrogate measure. One study used hypoxic gas to precontract isolated rat lungs and found that NECA, but not CGS21680, reduces vascular resistance independent of NO (Haynes et al., 1995). Similar results were found using methoxamine to precontract rat mesenteric arterial beds (Rubino et al., 1995). These studies suggest that activation of one of the other adenosine receptors, possibly the A2bAR, plays a role in regulating vascular resistance in the lung and mesenteric vasculature. On the other hand, NECA induces the release of endothelin-1 (ET-1), a vasoconstrictor, and vascular remodeling agent, from cultured human pulmonary artery endothelial cells (PAECs) and pulmonary artery smooth muscle cells (PASMCs) (Karmouty-Quintana et al., 2012). Furthermore, NECA-induced increase in ET-1 is inhibited by an A2bAR antagonist, suggesting an effect of A2bAR on vascular remodeling via ET-1 (Karmouty-Quintana et al., 2012)
In vivo studies have also been undertaken to interrogate the role of the A2bAR in controlling vascular tension and blood pressure. In one study, blood pressure was measured by tail cuff and by arterial catheterization in young (8–12 weeks old) WT and A2bAR KO mice at baseline as well as following adenosine infusion and showed no difference between genotypes, suggesting that the A2bAR plays no role in blood pressure regulation in young mice (Yang et al., 2006). In a mouse model of bleomycin-induced pulmonary hypertension secondary to pulmonary fibrosis, A2bAR signaling was found to play a role in vascular remodeling and proliferation. Interestingly, pharmacologic inhibition or genetic ablation of the A2bAR attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension (Karmouty-Quintana et al., 2012). While bleomycin treatment in WT mice leads to vascular remodeling, including increased vascular smooth muscle mass and collagen content, this effect does not occur in A2bAR KO mice or WT mice treated with an A2bAR antagonist (Karmouty-Quintana et al., 2012). In correlate to the in vitro findings described above, the authors also found that bleomycin treatment increases plasma ET-1 levels, and that this effect is attenuated in mice treated with the A2bAR antagonist. Another study found A2bAR KO mice showed decreased systolic and mean arterial blood pressure with angiotensin II infusion as compared to WT mice (Zhang et al., 2013). Furthermore, angiotensin II-induced ET-1 production in the kidneys of WT mice but not in A2bAR KO mice or mice treated with the A2bAR antagonist (Zhang et al., 2013). Furthermore, the authors found increased levels of A2bAR mRNA in kidney biopsies of hypertensive chronic kidney disease patients (Zhang et al., 2013). Together, these in vivo models show that the A2bAR may promote vascular remodeling via factors such as ET-1.
As noted, there are seemingly conflicting reports on the role of the A2bAR in regulating vascular dilation and resistance, possibly due to differences in downstream signaling (Gs and/or Gq), cell type, and model system tested. In addition, results are likely influenced by the examined species, as well as the methods employed to measure vascular tension and resistance.
Atherosclerosis
Cardiovascular disease (CVD) encompasses coronary artery disease (myocardial infarction, angina pectoris, heart failure), cerebrovascular disease (stroke, transient ischemic attack), peripheral artery disease, and aortic atherosclerosis and aneurysm. CVD is the leading cause of death in the United States (Murphy et al., 2013). Atherosclerosis is an underlying etiologic factor in many types of cardiovascular disease. Atherosclerosis begins in adolescence with fatty streaks, which progress to plaques in adulthood and finally results in thrombotic events that cause occlusion of vessels leading to clinically significant morbidity and mortality (reviewed in Weber and Noels [2011]). The pathogenesis of atherosclerosis (reviewed in [Weber and Noels, 2011]) involves endothelial dysfunction, subendothelial accumulation of low density lipoprotein (LDL) and subsequent oxidation. Oxidized LDL promotes endothelial cell expression of adhesion molecules (like ICAM-1 and E-selectin) and secretion of chemokines (like TNF-α), leading to the intimal accumulation of lipid-laden macrophages, called foam cells. Further accumulation of cholesterol, inflammatory cells, and apoptotic cells forms the necrotic core of the atheroma, which is encapsulated by collagen and proliferating smooth muscle cells (Hansson and Hermansson, 2011; Moore and Tabas, 2011; Weber and Noels, 2011). Intervention at one or several of these steps in the development of atherosclerosis may prevent subsequent thrombotic events.
The function of the A2bAR in the pathogenesis of atherosclerosis has been studied. In one approach, Gessi et al. investigated the effect of adenosine on foam cell formation (Gessi et al., 2010) in hypoxic conditions (1% O2) using an in vitro model of foam cell formation, which involved differentiating the human myelomonocytic cell line U937 or human peripheral blood mononuclear cells into macrophages and then incubating the cells with oxidized LDL to form foam cells. The authors found that hypoxia selectively induces A2bAR expression in U937 cells, differentiated macrophages, and foam cells (Gessi et al., 2010). Moreover, adenosine promotes hypoxia-inducible factor-1α (HIF-1α) protein (Gessi et al., 2010) and HIF-1α induces expression of vascular endothelial growth factor (VEGF) and promotes foam cell formation via the A2bAR and A3AR specifically (Jiang et al., 2007; Sluimer et al., 2008; Gessi et al., 2010). These results suggest that antagonists to the A2bAR and A3AR may be beneficial in preventing atherosclerotic plaque formation (Gessi et al., 2010).
In contrast, a later study using an in vivo mouse model of atherosclerosis found that the A2bAR is protective against plaque formation (Koupenova et al., 2012). To promote atherosclerosis development, A2bAR KO mice were bred onto an ApoE background (A2bAR, ApoE double KO) and the mice were fed Western diet. A2bAR, ApoE double KO mice have increased plasma and liver cholesterol and triglycerides and larger atherosclerotic plaque lesions as compared to ApoE mice (Koupenova et al., 2012). Importantly, injection of the A2bAR specific agonist, BAY 60-6583, into ApoE mice reduces atherosclerotic plaque formation and plasma lipid levels (Koupenova et al., 2012). As a mechanism to explain the effect of the A2bAR on lipid metabolism, hepatocytes isolated from A2bAR, ApoE double KO mice express higher levels of sterol regulatory element binding protein-1 (SREBP-1), a key transcription factor in lipogenesis and cholesterol synthesis, while BAY 60-6583, reduces hepatocyte expression of SREBP-1 (Koupenova et al., 2012). As such, this study identified the A2bAR as atheroprotective secondary to modulation of lipid metabolism and plaque formation.
Ischemic preconditioning
Adenosine signaling has been implicated in mediating cardioprotective cellular responses to myocardial ischemia (Xi et al., 2009). Ischemic preconditioning, which involves repeated short episodes of ischemia and reperfusion prior to myocardial infarction (MI), has been shown to reduce infarct size after MI (Murry et al., 1986). Interestingly, A2bAR gene expression is elevated in the hearts of individuals with ischemic heart disease as compared to healthy controls (Eckle et al., 2012) and A2bAR expression increases after ischemic preconditioning (Eckle et al., 2008c).
Using a model of murine in situ preconditioning, Eckle et al. showed that A2bAR signaling is necessary for the cardioprotective effect of ischemic preconditioning (Eckle et al., 2007). Ischemic preconditioning reduces infarct size in WT, A1AR KO, A2aAR KO, and A3AR KO mice, but not in A2bAR KO mice after 2 hours of reperfusion (Eckle et al., 2007). Furthermore, activation of the A2bAR with BAY 60-6583 reduces infarct size after ischemia (Eckle et al., 2007). Further studies by the same group showed that A2bAR signaling stabilizes the circadian rhythm protein, period 2 (Per2) within 2 hours to promote a protective metabolic switch in carbohydrate utilization (Eckle et al., 2012). The group also showed that A2bAR signaling in bone marrow-derived cells is primarily responsible for the cardioprotective effect in ischemia-reperfusion injury, suggesting an additional role for A2bARregulation of inflammation in mediating cardioprotective effect (Koeppen et al., 2012). In contrast, amore recent study did not find a role for the A2bAR in acute ischemic preconditioning (Maas et al., 2010). The authors report no difference in infarct size following ischemic preconditioning in A2bAR KO mice as compared to WT mice (Maas et al., 2010). However, consistent with the original report, they find that treatment with BAY 60-6583 reduces infarct size and conclude that the A2bAR may play a role in a later phase of ischemic preconditioning (Maas et al., 2010).
Metabolic disease
The A2bAR is expressed in organs that regulate metabolic processes, namely the pancreas (Yang et al., 2006; Nemeth et al., 2007), liver (Koupenova et al., 2012), fat, and muscle (Dixon et al., 1996; Johansson et al., 2007; Johnston-Cox et al., 2012). Given the role of the A2bAR in modulating inflammation and cardiovascular processes, several groups have investigated A2bAR signaling in metabolic disease. The following studies underscore how signaling through a single receptor can have varying effects depending on timing and model system used.
In a model of type I diabetes mellitus, the nonspecific adenosine receptor agonist, NECA, reduces diabetes development as measured by plasma glucose levels and pancreatic insulin content (Nemeth et al., 2007). This effect is attenuated by A2bAR antagonists and is not reproduced by A2aAR, A1AR or A3AR agonists (Nemeth et al., 2007). These results suggest that A2bAR activity inhibits the development of type I diabetes. The authors suggest that the mechanism for this effect involves reduction in the production of proinflammatory cytokines (Nemeth et al., 2007).
Figler et al. used diabetic KKAY mice to model type 2 diabetes mellitus and study the role of A2bAR agonism and antagonism on glucose disposal (Figler et al., 2011). The authors found that the A2bAR antagonist, ATL-801, reduces hepatic glucose production, and increases glucose uptake in skeletal muscle and brown adipose tissue in KKAY mice as measured by hyperinsulinemic-euglycemic clamps (Figler et al., 2011). Notably, these treatments were given to the mice for 2 days only (Figler et al., 2011). The authors also found that acute challenge with NECA (35 min prior to oral glucose tolerance test) results in delayed glucose disposal and increased fasting glucose levels in WT but not in A2bAR KO mice (Figler et al., 2011), suggesting that activation of the A2bAR, at least acutely, impairs glucose uptake. As a correlate and potential mechanistic explanation, the authors find that 4 hours following treatment with NECA plasma IL-6 levels are elevated (Figler et al., 2011). Moreover, the authors showed that administration of ATL-801 to WT mice concurrently with high fat diet (HFD) feeding for 10 weeks reduces fasting blood glucose levels (Figler et al., 2011). This study suggests that inhibition of the A2bAR acutely impacts glucose disposal.
In contrast, studies using a HFD-induced diabetes model to study the role of the A2bAR on glucose metabolism find the A2bAR to be protective against the development of type 2 diabetes (Johnston-Cox et al., 2012). Following 16 weeks of HFD, A2bAR KO mice have increased fasting glucose and insulin levels as well as impaired glucose tolerance and insulin clearance as compared to WT mice (Johnston-Cox et al., 2012). Moreover, administration BAY 60-6583 for 2 weeks concurrent with HFD feeding lowers fasting glucose levels and improves glucose and insulin tolerance (Johnston-Cox et al., 2012). The A2bAR may modulate whole body glucose metabolism by regulating insulin receptor substrate-2 (IRS-2) levels. A2bAR KO mice having decreased levels of IRS-2, which is associated with impaired insulin signaling in tissues (Johnston-Cox et al., 2012). Interestingly, there is a positive association between levels of A2bAR and IRS-2 mRNA in human adipose tissue (Johnston-Cox et al., 2012). In addition, macrophage expression of A2bAR is a major contributor to control of insulin sensitivity and glucose tolerance through the regulation of inflammatory cytokines, which impair insulin signaling (Johnston-Cox et al., 2014). Furthermore, A2bARKO mice have increased classical (proinflammatory) macrophage activation and impaired alternative (anti-inflammatory) macrophage activation (Csoka et al., 2014). Given our understanding of the ability of the A2bAR to affect acute and chronic inflammation differently (as illustrated above), it is not surprising that results using different models of diabetes and using different time courses of A2bAR activation or inhibition (acute vs. chronic) may have opposing results.
Metabolic disease is complex and involves the regulation and coordination of many organs. While the A2bAR affects glucose disposal, it also affects other aspects of whole body energy homeostasis. For one, the A2bAR plays a role in regulating adipogenesis (Eisenstein et al., 2014). Activation of the receptor with BAY 60-6583 inhibits adipogenesis (Eisenstein et al., 2014). Furthermore, the A2bAR regulates insulin secretion from pancreatic β-cells (Rusing et al., 2006; Johnston-Cox et al., 2012). In vitro studies have also suggested that the A2bAR stimulates gluconeogenesis and glycogenolysis in rat hepatocytes (Harada et al., 2001; Yasuda et al., 2003). Given the many actions of the A2bAR on metabolic organs, it is necessary to consider all the effects of agonists or antagonists when designing therapeutic agents.
Although A2bAR signaling in metabolic disease is complex, it is likely relevant to human disease as several studies have shown associations between A2bAR expression, inflammation and metabolic disease in human tissue. SNPs within the human A2bAR gene, ADORA2B, are associated with HOMA-insulin resistance and plasma levels of IL-6 and CRP (Figler et al., 2011). Furthermore, A2bAR expression is elevated in adipose tissue of obese individuals as compared to lean individuals and is associated with downstream targets of insulin signaling (Johnston-Cox et al., 2012).
Conclusion
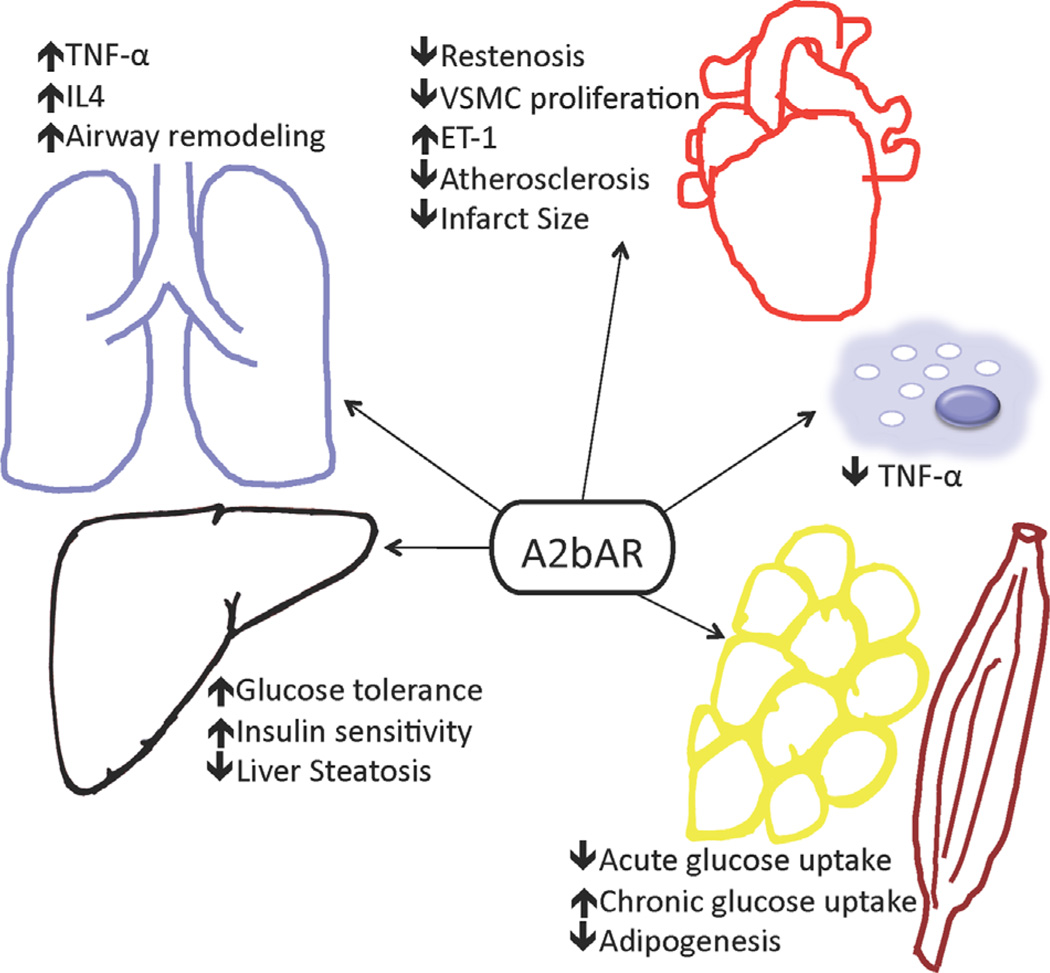
As illustrated in this review, A2bARs function in many pathologic processes and may lead to different downstream effects depending on the cell type, chronicity, and timing of the activation or inhibition of signaling (Fig. 1). As a result of these pleiotropic actions, a thorough understanding of the effect of A2bAR modulation in different organs and at different time points is necessary in targeting the A2bAR for novel therapeutics in the treatment of cardiovascular and metabolic disease. In most of the above-described comparative studies, unless specified, the generic background is C57BL/6 mice. Considering the complexity of this receptor response, means other than systemic pharmacological intervention should be considered, such as nanoscience technology-medicated tissue receptor targeting.
Figure 1.
The A2bAR plays many roles in different organs and pathologies.
Acknowledgments
Funding: This work was supported by the National Heart, Lung and Blood Institute Grant (HL93149) to KR, and by the Boston Nutrition Obesity Research Center (DK046200) to K.R., an established Investigator with the American Heart Association. A.E was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases NRSA F30 award (DK098834). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest: None.
Compliance with Ethical Standards
Literature Cited
- Anvari F, Sharma AK, Fernandez LG, Hranjec T, Ravid K, Kron IL, Laubach VE. Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;140:871–877. doi: 10.1016/j.jtcvs.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balwierczak JL, Sharif R, Krulan CM, Field FP, Weiss GB, Miller MJ. Comparative effects of a selective adenosine A2 receptor agonist, CGS 21680, I and nitroprusside in vascular smooth muscle. Eur J Pharmacol. 1991;196:117–123. doi: 10.1016/0014-2999(91)90416-n. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm Res. 1998;47:351–354. doi: 10.1007/s000110050341. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Bot I, de Vries H, Korporaal SJ, Foks AC, Bot M, van Veldhoven J, Ter Borg MN, van Santbrink PJ, van Berkel TJ, Kuiper J, Ijzerman AP. Adenosine A(2)B receptor agonism inhibits neointimal lesion development after arterial injury in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2197–2205. doi: 10.1161/ATVBAHA.112.252924. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth ZH, Pacher P, Bai P, Hasko G. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63:850–866. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscso B, Himer L, Vizi ES, Blackburn MR, Deitch EA, Hasko G. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JS, Jr, Holmes DR, Jr, Kereiakes DJ, Grines CL, Block E, Ghazzal ZM, Morris DC, Liberman H, Parker K, Jurkovitz C, Murrah N, Foster J, Hyde P, Mancini GB, Weintraub WS. Coronary stent restenosis in patients treated with cilostazol. Circulation. 2005;112:2826–2832. doi: 10.1161/CIRCULATIONAHA.104.530097. [DOI] [PubMed] [Google Scholar]
- Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and total protein synthesis in vascular smooth muscle cells. Hypertension. 1999;33:190–194. doi: 10.1161/01.hyp.33.1.190. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. Adenosine inhibits growth of human aortic smooth muscle cells via A2B receptors. Hypertension. 1998;31:516–521. doi: 10.1161/01.hyp.31.1.516. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Shue H, Jackson EK. A(2B) receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension. 2000;35:267–272. doi: 10.1161/01.hyp.35.1.267. [DOI] [PubMed] [Google Scholar]
- Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008a;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008b;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5’-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- Eisenstein A, Carroll SH, Johnston-Cox H, Farb M, Gokce N, Ravid K. An adenosine receptor-Kruppel-like factor 4 protein axis inhibits adipogenesis. J Biol Chem. 2014;289:21071–21081. doi: 10.1074/jbc.M114.566406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tayeb A, Michael S, Abdelrahman A, Behrenswerth A, Gollos S, Nieber K, Muller CE. Development of polar adenosine A2A receptor agonists for inflammatory bowel disease: Synergism with A2B antagonists. ACS Med Chem Lett. 2011;2:890–895. doi: 10.1021/ml200189u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzein E, Kalla RV, Li X, Perry T, Gimbel A, Zeng D, Lustig D, Leung K, Zablocki J. Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J Med Chem. 2008;51:2267–2278. doi: 10.1021/jm7014815. [DOI] [PubMed] [Google Scholar]
- Field JJ, Nathan DG, Linden J. The role of adenosine signaling in sickle cell therapeutics. Hematol Oncol Clin North Am. 2014;28:287–299. doi: 10.1016/j.hoc.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, Kim JK, LaNoue KF, Linden J. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60:669–679. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BBAPIJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Gessi S, Fogli E, Sacchetto V, Merighi S, Varani K, Preti D, Leung E, Maclennan S, Borea PA. Adenosine modulates HIF-1{alpha}, VEGF, IL-8, and foam cell formation in a human model of hypoxic foam cells. Arterioscler Thromb Vasc Biol. 2010;30:90–97. doi: 10.1161/ATVBAHA.109.194902. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Varani K, Borea PA. Adenosine receptors in health and disease. Adv Pharmacol. 2011;61:41–75. doi: 10.1016/B978-0-12-385526-8.00002-3. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- Harada H, Asano O, Hoshino Y, Yoshikawa S, Matsukura M, Kabasawa Y, Niijima J, Kotake Y, Watanabe N, Kawata T, Inoue T, Horizoe T, Yasuda N, Minami H, Nagata K, Murakami M, Nagaoka J, Kobayashi S, Tanaka I, Abe S. 2-Alkynyl-8-aryl-9-methyladenines as novel adenosine receptor antagonists: Their synthesis and structure-activity relationships toward hepatic glucose production induced via agonism of the A(2B) receptor. J Med Chem. 2001;44:170–179. doi: 10.1021/jm990499b. [DOI] [PubMed] [Google Scholar]
- Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J, Jr, Obiako B, Thompson WJ, Downey J. Adenosine-induced vasodilation: Receptor characterization in pulmonary circulation. Am J Physiol. 1995;268:H1862–H1868. doi: 10.1152/ajpheart.1995.268.5.H1862. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94:1247–1254. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, Tilley SL. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med. 2007;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Gillespie DG. 2′,3′-cAMP, 3′-AMP, and 2′-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. Am J Physiol Heart Circ Physiol. 2011;301:H391–H401. doi: 10.1152/ajpheart.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Li T, Qiu Y, Rui Y, Chen W, Lou Y. RNA interference for HIF-1 alpha inhibits foam cells formation in vitro. Eur J Pharmacol. 2007;562:183–190. doi: 10.1016/j.ejphar.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Johansson SM, Salehi A, Sandstrom ME, Westerblad H, Lundquist I, Carlsson PO, Fredholm BB, Katz A. A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochem Pharmacol. 2007;74:1628–1635. doi: 10.1016/j.bcp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Johnston-Cox H, Eisenstein AS, Koupenova M, Carroll S, Ravid K. The macrophage A2B adenosine receptor regulates tissue insulin sensitivity. PLoS One. 2014;9:e88775. doi: 10.1371/journal.pone.0098775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston-Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG, LeBrasseur N, Ravid K. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One. 2012;7:e40584. doi: 10.1371/journal.pone.0040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty-Quintana H, Zhong H, Acero L, Weng T, Melicoff E, West JD, Hemnes A, Grenz A, Eltzschig HK, Blackwell TS, Xia Y, Johnston RA, Zeng D, Belardinelli L, Blackburn MR. The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J. 2012;26:2546–2557. doi: 10.1096/fj.11-200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M, Pieczek A, Haley L, Losordo DW, Andres V, Schainfeld R, Rosenfield K, Isner JM. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation. 1997;95:1998–2002. doi: 10.1161/01.cir.95.8.1998. [DOI] [PubMed] [Google Scholar]
- Koeppen M, Harter PN, Bonney S, Bonney M, Reithel S, Zachskorn C, Mittelbronn M, Eckle T. Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury. Anesthesiology. 2012;116:1245–1257. doi: 10.1097/ALN.0b013e318255793c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: A basis for A2bR overexpression in colitis. Cell Mol Life Sci. 2005;62:2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachala V, Ruble B, Vijay-Kumar M, Wang L, Mwangi S, Figler H, Figler R, Srinivasan S, Gewirtz A, Linden J, Merlin D, Sitaraman S. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155:127–137. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu R, Ueda M, Naruko T, Kojima A, Becker AE. Neointimal tissue response at sites of coronary stenting in humans: macroscopic, histological, and immunohistochemical analyses. Circulation. 1998;98:224–233. doi: 10.1161/01.cir.98.3.224. [DOI] [PubMed] [Google Scholar]
- Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: Contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31:224–230. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- Koupenova M, Johnston-Cox H, Vezeridis A, Gavras H, Yang D, Zannis V, Ravid K. A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation. 2012;125:354–363. doi: 10.1161/CIRCULATIONAHA.111.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, Kang SJ, Park SJ, Lee JH, Choi SW, Seong IW, Lee NH, Cho YH, Shin WY, Lee SJ, Hyon MS, Bang DW, Choi YJ, Kim HS, Lee BK, Lee K, Park HK, Park CB, Lee SG, Kim MK, Park KH, Park WJ. A randomized, double-blind, multicenter comparison study of triple antiplatelet therapy with dual antiplatelet therapy to reduce restenosis after drug-eluting stent implantation in long coronary lesions: Results from the DECLARE-LONG II (Drug-Eluting Stenting Followed by Cilostazol Treatment Reduces Late Restenosis in Patients with Long Coronary Lesions) trial. J Am Coll Cardiol. 2011;57:1264–1270. doi: 10.1016/j.jacc.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas JE, Wan TC, Figler RA, Gross GJ, Auchampach JA. Evidence that the acute phase of ischemic preconditioning does not require signaling by the A 2B adenosine receptor. J Mol Cell Cardiol. 2010;49:886–893. doi: 10.1016/j.yjmcc.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PL, Potts AA. The endothelium of the rat renal artery plays an obligatory role in A2 adenosine receptor-mediated relaxation induced by 5′-N-ethylcarboxamidoadenosine and N6-cyclopentyladenosine. J Pharmacol Exp Ther. 1994;270:893–899. [PubMed] [Google Scholar]
- Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2010. Natl Vital Stat Rep. 2013;61:1–117. [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D. Effect of a specific and selective A(2B) adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther. 2007;320:1246–1251. doi: 10.1124/jpet.106.112250. [DOI] [PubMed] [Google Scholar]
- Nemeth ZH, Bleich D, Csoka B, Pacher P, Mabley JG, Himer L, Vizi ES, Deitch EA, Szabo C, Cronstein BN, Hasko G. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- Pedroza M, Schneider DJ, Karmouty-Quintana H, Coote J, Shaw S, Corrigan R, Molina JG, Alcorn JL, Galas D, Gelinas R, Blackburn MR. Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS One. 2011;6:e22667. doi: 10.1371/journal.pone.0022667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiron C, Sanchez P, Bouzamondo A, Lechat P, Montalescot G. Drug eluting stents: An updated meta-analysis of randomised controlled trials. Heart. 2006;92:641–649. doi: 10.1136/hrt.2005.061622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino A, Ralevic V, Burnstock G. Contribution of P1-(A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1995;115:648–652. doi: 10.1111/j.1476-5381.1995.tb14981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusing D, Muller CE, Verspohl EJ. The impact of adenosine and A(2B) receptors on glucose homoeostasis. J Pharm Pharmacol. 2006;58:1639–1645. doi: 10.1211/jpp.58.12.0011. [DOI] [PubMed] [Google Scholar]
- Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861–869. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- St Hilaire C, Koupenova M, Carroll SH, Smith BD, Ravid K. TNF-alpha upregulates the A2B adenosine receptor gene: The role of NAD(P)H oxidase 4. Biochem Biophys Res Commun. 2008;375:292–296. doi: 10.1016/j.bbrc.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Duan Y, Eisenstein AS, Hu W, Quintana A, Lam WK, Wang Y, Wu Z, Ravid K, Huang P. A novel mechanism of control of NFkappaB activation and inflammation involving A2B adenosine receptors. J Cell Sci. 2012;125:4507–4517. doi: 10.1242/jcs.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven D, Wan TC, Gizewski ET, Kreckler LM, Maas JE, Van Orman J, Ravid K, Auchampach JA. A role for the low-affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils. J Pharmacol Exp Ther. 2011;338:1004–1012. doi: 10.1124/jpet.111.181792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard KJ, Geissmann F. Monocytes in atherosclerosis: Subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne BM, Chiao CW, Webb RC. Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin II and endothelin-1. J Am Soc Hypertens. 2009;3:84–95. doi: 10.1016/j.jash.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, Zvara DA, Xu Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009;47:684–690. doi: 10.1016/j.yjmcc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci U S A. 2008;105:792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda N, Inoue T, Horizoe T, Nagata K, Minami H, Kawata T, Hoshino Y, Harada H, Yoshikawa S, Asano O, Nagaoka J, Murakami M, Abe S, Kobayashi S, Tanaka I. Functional characterization of the adenosine receptor contributing to glycogenolysis and gluconeogenesis in rat hepatocytes. Eur J Pharmacol. 2003;459:159–166. doi: 10.1016/s0014-2999(02)02832-7. [DOI] [PubMed] [Google Scholar]
- Zaynagetdinov R, Ryzhov S, Goldstein AE, Yin H, Novitskiy SV, Goleniewska K, Polosukhin VV, Newcomb DC, Mitchell D, Morschl E, Zhou Y, Blackburn MR, Peebles RS, Jr, Biaggioni I, Feoktistov I. Attenuation of chronic pulmonary inflammation in A2B adenosine receptor knockout mice. Am J Respir Cell Mol Biol. 2010;42:564–571. doi: 10.1165/rcmb.2008-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernecke A, Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc Res. 2010;86:192–201. doi: 10.1093/cvr/cvp391. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y. Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res. 2013;112:1466–1478. doi: 10.1161/CIRCRESAHA.111.300166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Belardinelli L, Maa T, Feoktistov I, Biaggioni I, Zeng D. A(2B) adenosine receptors increase cytokine release by bronchial smooth muscle cells. Am J Respir Cell Mol Biol. 2004;30:118–125. doi: 10.1165/rcmb.2003-0118OC. [DOI] [PubMed] [Google Scholar]
- Zhong H, Belardinelli L, Maa T, Zeng D. Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am J Respir Cell Mol Biol. 2005;32:2–8. doi: 10.1165/rcmb.2004-0103OC. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Mohsenin A, Morschl E, Young HW, Molina JG, Ma W, Sun CX, Martinez-Valdez H, Blackburn MR. Enhanced airway inflammation and remodeling in adenosine deaminase-deficient mice lacking the A2B adenosine receptor. J Immunol. 2009;182:8037–8046. doi: 10.4049/jimmunol.0900515. [DOI] [PMC free article] [PubMed] [Google Scholar]