Abstract
We report 2 patients with drug-resistant epilepsy caused by KCNT1 mutations who were treated with quinidine. Both mutations manifested gain of function in vitro, showing increased current that was reduced by quinidine. One, who had epilepsy of infancy with migrating focal seizures, had 80% reduction in seizure frequency as recorded in seizure diaries, and partially validated by objective seizure evaluation on EEG. The other, who had a novel phenotype, with severe nocturnal focal and secondary generalized seizures starting in early childhood with developmental regression, did not improve. Although quinidine represents an encouraging opportunity for therapeutic benefits, our experience suggests caution in its application and supports the need to identify more targeted drugs for KCNT1 epilepsies.
KCNT1 mutations have recently been implicated in a range of epilepsy syndromes including severe autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE)1,2 and epilepsy of infancy with migrating focal seizures (EIMFS).3,4 Mutations result in KCNT1 channel gain of function. This gain of function, the magnitude of which correlates with the clinical severity, can be reduced by quinidine in vitro.5 A recent case report described improvement in seizure control with quinidine in a patient with KCNT1-EIMFS.6 Here, we report 2 patients with different epilepsy phenotypes caused by KCNT1 mutations with different responses to quinidine therapy. We also analyze the cases for additional factors that might have resulted in variable therapeutic response and discuss how this could help development of future tailored therapies for such disorders. This work was approved by our institutional review board.
Case Reports
Patient 1
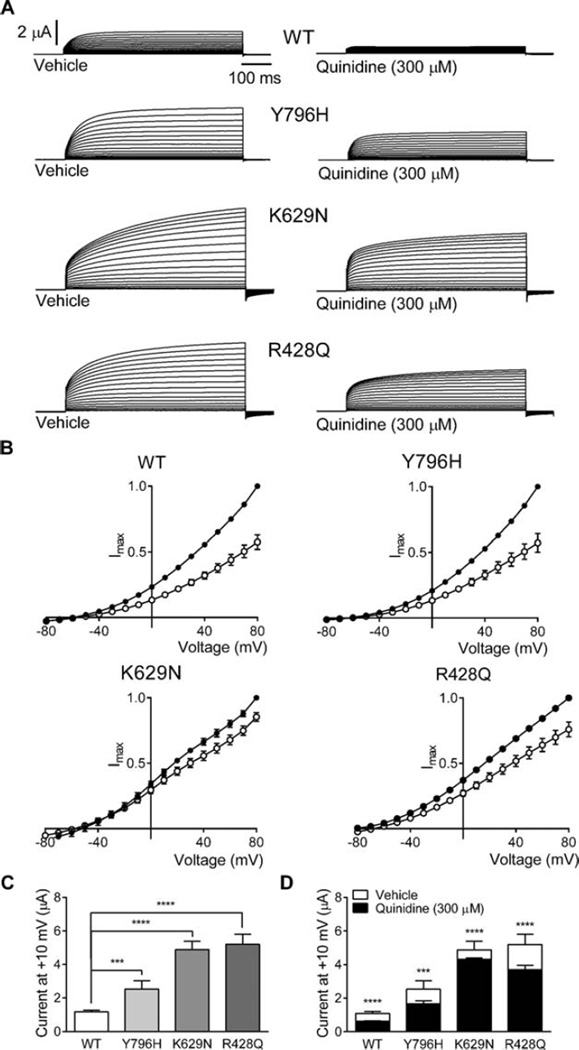
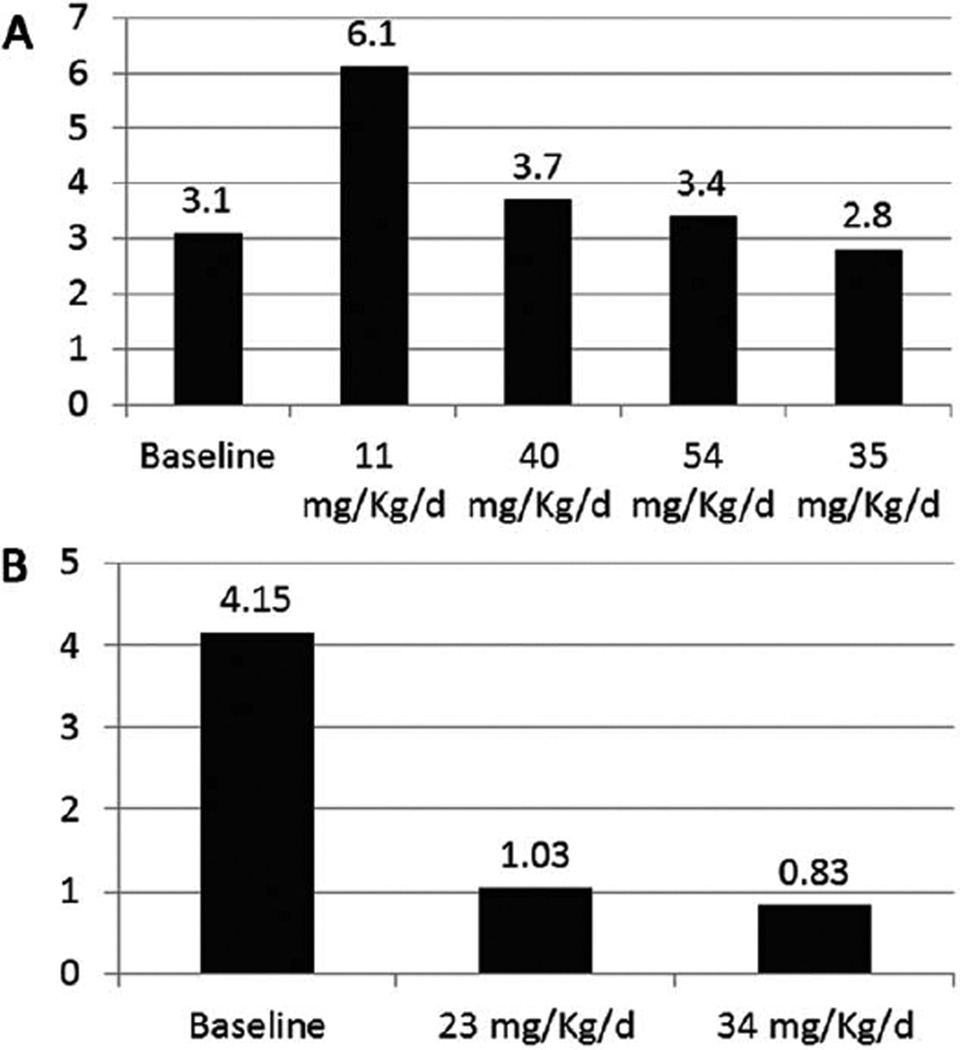
Patient 1 is an 11-year-old female who had normal growth and development until the age of 18 months, when she developed nighttime “gagging” spells initially attributed to “allergies,” followed by nocturnal generalized tonic–clonic seizures as of the age of 2.5 years. Initial electroencephalogram (EEG) was normal, but long-term monitoring demonstrated that the gagging spells were nocturnal focal seizures arising from the right hemisphere. Her nocturnal tonic–clonic seizures rapidly progressed in frequency to 10 to 15 per night. Over the following year, she started to regress and eventually lost all of her expressive speech and fine motor skills; at age 5 years, she was still ambulatory, but very ataxic. In the following year, she became nonambulatory and noncommunicative and had to be fed by Gtube. EEG showed multifocal discharges emanating from the right and left posterior head regions and right temporal region. Frequent prolonged bursts of generalized poly-spike and wave discharges were also noted in sleep. Extensive metabolic and genetic investigations were negative. Whole exome sequencing (WES) revealed a heterozygous de novo KCNT1 mutation (NM_020822.1:c.2386T>C; p.[Tyr796His], Y796H). This exact variant has been previously reported in a family with ADNFLE.2 Functional testing in vitro showed that the Y796H mutation resulted in channels with a significantly greater magnitude of peak current as compared to the wild-type (WT) channels (Fig 1A, C). However, this effect was on the milder side of the functional spectrum when compared to K629N, the mutation identified in Patient 2, and R428Q, the mutation reported in the recently published case.6 Quinidine (300 µM) produced significant inhibition (36.1±6.7%) of the Y796H channel, bringing current levels down toward WT control values (see Fig 1B, D). When seen for evaluation for quinidine therapy, the patient was having multiple nocturnal mostly tonic, rarely tonic–clonic, seizures/ day. She had diffuse decrease in tone. Reflexes and plantar responses were normal. Twelve antiepileptic medications and the ketogenic diet had failed. EEG showed a diffusely slow background with bilateral frontal spikes interictally, and during her recorded generalized tonic seizures an electrodecremental response. Magnetic resonance imaging (MRI) showed global atrophy. She was admitted and started on quinidine 11mg/kg/day in 3 divided doses achieved gradually over 3 days. Over the following month, her mean quinidine serum level was 0.6 µg/ml (0.4 and 0.8 µg/ml; therapeutic for cardiac effects: 2–5 µg/ml). She was readmitted, and the dose was increased over 3 days to 40mg/kg/day in 3 divided doses. Her mean level rose during the following 2 months to 2.4 µg/ml (2.4, 2.1, 3.3, and 1.7 µg/ml). She was then readmitted again, and the was dose increased over 3 days to 54.2 µg/kg/day. The level however did not rise over the next month; mean was 1.7 µg/ml (2, 1.3 µg/ml). The dose was then decreased and maintained during the following month to 34mg/kg/ day. Seizure frequency showed no improvement from baseline (Fig 2A). During the first hospitalization the patient had, before quinidine was started during the first 24 hours of admission, 10 seizures which were detected by video EEG monitoring (10 seizures/24 hours). She then had 52 seizures over the next 5 days while quinidine was being started and increased (13 seizures/24 hours). During the second hospitalization she had 23 seizures over 4 days (5.45 seizures/24 hours), and during the third hospitalization she had 39 seizures over 5 days (7.8 seizures/24 hours). EEGs done during each admission did not show any significant changes. Developmentally she was noted to be minimally more alert and interactive. At the 54.2 µg/ kg/day dose, some prolongation of the QT interval was observed, which precluded further increases.
FIGURE 1.
Quinidine inhibition of hKCNT1 currents expressed in Xenopus oocytes.17,18 (A) Representative current traces obtained from oocytes expressing wild-type (WT), Y796H, K629N, and R428Q channels in vehicle control and in the presence of 300 µM quinidine. Scale bars apply to all traces. Oocytes were held at −90mV and stepped from −80mV to 80mV for 600 milliseconds every 5 seconds. (B) Normalized average current–voltage relationships for WT, Y796H, K629N, and R428Q channels in the presence of vehicle (solid circles) and 300 µM quinidine (open circles; WT, n = 20; Y796H, n = 9; K629N, n = 16; R428Q, n = 23). Currents were normalized to the value in the absence of quinidine at a test potential of +80 mV (Imax). (C) Average peak currents at +10mV for WT (n = 127), Y796H (n = 63), K629N (n = 118), and R428Q (n = 38) channels. The peak currents for each mutant channel at +10mV were compared to the peak currents for the WT channel at +10mV. (D) Comparison of average current amplitude for WT and mutant channels illustrating the degree of block by 300 µM quinidine (WT, n = 13; Y796H, n = 9; K629N, n = 43; R428Q, n = 24) derived from measurements made at +10mV (see C for vehicle control n values; ***p< 0.001; ****p< 0.0001). Data for Y796H and R428Q are reformatted from our other study,5 but are the same underlying data. Data are presented as mean-± standard error of the mean, and Student t test was used to test statistical significance.
FIGURE 2.
Changes in seizure frequency during quinidine therapy in Patients 1 (A) and 2 (B). Seizure frequency was documented by review of the patient’s daily seizure calendar, which we had established that the families were keeping reliably before the start of the baseline period. Baseline was the 1 month before starting quinidine therapy, and average baseline daily seizure frequency was calculated over that month. During quinidine therapy, seizure frequency was calculated over the period of the stable dose of quinidine, which was always for a minimum of 1 month or longer if the patient was kept on the specific stable quinidine dose for a longer period of time. We monitored both of our patients with inpatient video-electoencephalographic recordings before initiation of the quinidine therapy to confirm the nature of the seizures and that the families were reliably recognizing seizures, and did not identify any striking discrepancies. For an individual patient, over the course of the follow-up, there was no difference in their seizure type, severity, or duration based on the families’ reports and based on the inpatient monitoring that also occurred throughout the inpatient hospitalizations during dose increases.
Patient 2
Patient 2 is a 3-year-old male who was hospitalized as a neonate for 4 months for episodes of multiple daily focal seizures and status epilepticus that required pentobarbital coma. The electroclinical picture was consistent with EIMFS. Seizures usually lasted 10 seconds to 3 minutes and consisted of: (1) unilateral upper and lower extremity clonic activity with adversive head turning and nystagmus, (2) eye deviation, (3) generalized tonic stiffening, and (4) lip smacking with asymmetric jerking of the extremities. EEG showed diffuse delta slowing with very frequent multifocal spikes and electrographic seizures occurring as frequently as every 2 to 10 minutes, lasting an average of about 30 seconds and emanating independently from C3, C4, T3, T4, O1, or O2. Extensive metabolic workup was negative; ammonia, lactate, pyruvate, amino acids, acylcarnitine profile, creatine, guanidinoacetate, biotinidase, pipecolic acid, alpha aminoadipic acid semialdehyde (serum), organic acids, sulfocysteine, succinyl purines (urine), glucose, protein, amino acids, lactate, neurotransmitter metabolites, neopterin, biopterin, 5-methyl tetrahydrofolate, folate, homocysteine, and pyridoxal-5-phospahate (cerebral spinal fluid [CSF]) were all normal. He also had negative workup including muscle biopsy for mitochondrial diseases. DNA testing for known epilepsy syndromes was unremarkable. He continued to have multiple seizures per day, which evolved to tonic seizures over the following months. At 3 years, WES revealed a de novo KCNT1 mutation (NM_020822.2:c.1887G>C; p.[Lys629Asn], K629N). Functional testing in vitro showed that K629N also resulted in a gain-of-function phenotype, the magnitude of which was quite striking when compared to both WT and Y796H, but not R428Q (see Fig 1A, C). Quinidine (300 µM) was less effective in inhibiting K629N channel current (11.5±1.7% inhibition) as compared to WT (42.7±2.7% inhibition), Y796H (36.1±6.7% inhibition), or R428Q (25.8±3.2% inhibition; see Fig 1B, D). When evaluated for quinidine therapy, he was having multiple daily seizures. Eight antiepileptic medications and the ketogenic diet had failed. His examination showed microcephaly, central hypotonia, extremity hypertonia, and hyperreflexia with bilateral Babinski reflex. EEG showed interictal multifocal spikes and ictal electrodecremental fast beta rhythms and multifocal subclinical electrographic seizures. MRI showed diffuse atrophy. He was admitted and started on 12mg/kg/day of quinidine in 3 divided doses, which was gradually increased over 4 days to 22.6mg/kg/ day in 3 divided doses. Comedications were kept at the same doses and the levels remained within the ranges established before quinidine therapy. Over the next 2 months, quinidine serum level was 0.3 µg/ml. QT interval did not show any significant changes, so the patient was readmitted and the dose was increased gradually over 4 days to 34.4mg/kg/day divided into 3 doses. During the first hospitalization before quinidine was started, the patient had during the first 12 hours of admission 8 seizures detected by video EEG monitoring (16 seizures/24 hours). He then had 4 seizures over the next 2 days (2 seizures/24 hour) while quinidine was being started and increased. During the second hospitalization he had 6 seizures over 48 hours (3 seizures/24 hours), and during the third admission he had 33 seizures over 4 days (8.25 seizures/ 24 hours). EEGs done during each admission did not show any significant changes. Over the next month, the mean level was 0.77 µg/ml (1.1, 0.6, and 0.6 µg/ml). Seizure frequency decreased by 80% as shown in Figure 2B. Developmentally he was more alert and more interactive.
Discussion
Using the approach of a translational paradigm for in vitro studies to inform novel therapies for epilepsy, we treated 2 patients with quinidine.7 Case 1, who had a novel phenotype resembling severe nocturnal frontal lobe epilepsy, albeit with additional posterior and generalized EEG changes, which could be considered intermediate between EIMFS and typical nocturnal frontal lobe epilepsy, demonstrated no response, whereas Case 2, who had neonatal onset EIMFS, had a clinically meaningful improvement.
Earlier work suggested possible poor accumulation of quinidine in the CSF of human subjects (4–37%, average±16%).8 However, work in rodents showed it can achieve concentrations higher than those in the serum.9 CSF access is also supported by the antiepileptic effects of quinidine in a number of animal models.10,11 These have included focal penicillin, electrically induced seizures, and bupivacaine-induced generalized seizures. These findings, and the effects of quinidine on KCNT1 channel dysfunction, supported using it in our 2 patients. Quinidine metabolism is enhanced by enzyme inducers, which is probably the reason why our second patient had low levels, as he was on phenobarbital.12 Although quinidine has been reported to interact with a number of medications,13–16 it has not been reported to specifically affect the metabolism of clobazam, levetiracetam, or phenobarbital. The levels of these medications remained within the ranges documented before quinidine was initiated. This argues in favor of a true response to quinidine in Patient 2. Quinidine can cause prolongation of the QT interval; thus, both patients underwent continuous electrocardiographic (ECG) monitoring throughout their inpatient admissions during which the quinidine dosages were being increased, and also underwent routine ECGs and Holter monitoring on a monthly basis during the outpatient follow-up visits.
The available data are too limited to reach firm conclusions about any possible correlation between specific mutations and the response to quinidine. Our first patient had a severe presentation and did not respond to quinidine although she carries the Y796H mutation that was previously found in a family with ADNFLE, and that is on the more mild side of the in vitro functional spectrum. Our second patient had a favorable response albeit less so than the patient reported by Bearden et al,6 although the mutation he carries is the least sensitive mutation to quinidine in vitro. The patient of Bearden et al6 showed the best response, and carried the mutation that was the most sensitive to quinidine (see Fig 1D). This raises the possibility that the in vitro effect of quinidine may predict, in some but not in all patients, clinical response. The current limited and variable data, however, both in terms of in vitro effects and clinical responses makes it impossible to draw any clear conclusions about in vitro responses and clinical responses. Thus, our data justifies a need for further studies of quinidine in KCNT1-related epilepsies, and illustrates that in vitro work can guide development and investigation of clinical therapies. Our cases also demonstrate that clinical response can vary. This cautions against wide and indiscriminate use of quinidine in patients outside carefully designed protocols and provides information that can help in the design of future controlled studies. Specifically, our data, combined with the previous work, make the important practical point that there is some phenotype– genotype correlation and that anyone considering quinidine therapy should first assess the quinidine response of the specific mutation found in the patient. We feel that this work will directly contribute to the more careful application of quinidine going forward, which is becoming a pressing concern as we learn of an increasing number of patients with mutations of unknown significance in KCNT1 being considered for quinidine therapy. We also note that making the case for this functional assessment before targeted treatment is initiated would have the additional benefit of ensuring that quinidine is not initiated when there is a benign variant, or a mutation that results in loss rather than gain of function, which is also becoming an increasing concern.
Differences in response to quinidine may relate to: (1) interindividual variability in crossing the blood–brain barrier; (2) differences in the efflux of quinidine from the brain;9 (3) the developmental window in which quinidine is given; (4) injury resulting from previous severe seizures; (5) limitations on dosage due to quinidine prolongation of QT interval; or (6) additional unrecognized pathophysiological factors that lead to different phenotypes, as may be the case in our Patient 1.
In conclusion, the therapeutic effects of quinidine in KCNT1 positive epilepsy remain largely unknown. The question of how much quinidine may help, if it all, and in which specific types of KCNT1 positive epilepsy and for which mutations can only be resolved by more detailed clinical evaluation, ideally using objective measurements of seizures since we may expect responses to be inflated by expectation in the absence of objective measures. Ideally, evaluation of quinidine in KCNT1 animal models would guide such clinical evaluations. Although the current limited data do not appear to support the idea of substantial clinical benefit of quinidine, quinidine does clearly illustrate a new potential paradigm for the development and clinical evaluation of genetically targeted therapies in epilepsy.
Acknowledgments
This study was supported by grants from the National Health and Medical Research Council (S.B., I.S., S.M.), NIH National Institute of Neurological Disorders and Stroke (S.B.), and NIH Centers without Walls “Epi4K” (I.S.), and an early career fellowship from the National Health and Medical Research Council (S.M.).
We thank faculty and staff who took care of these patients, including Drs W. Gallentine, S. Kansagra, C. Pizoli, and E. Smith; Dr A. Helseth for her technical help; and the nursing staff of the Duke Pediatric Epilepsy Program for the excellent care they provided for these patients.
Footnotes
M.A.M. did the major write-up of the protocol, started the therapy, followed the patients, and did the major write-up of the manuscript. Y.J. contributed to all the above steps with M.A.M. M.C. contributed to the cardiac aspects of all of the above. V.S., S. Petrov., R.S., A.G., S.B., I.S., S.M., and M.B. contributed to the development of the concepts, to clinical data analysis, and to the manuscript write-up. A.M. contributed to the implementation of the protocol. C.J.M., M.L., and S.Petrou performed the in vitro studies, analyzed those data, and contributed to manuscript write-up. M.A.M., D.G., Y.J., and S.Petrou all contributed to the overall design of the study. D.G. developed the overall concept and contributed to the design of the study, data analysis, and writing of the manuscript. M.A.M. and Y.J. contributed equally.
Potential Conflicts of Interest
S.B.: grants, UCB Pharma, Novartis Pharmaceuticals, Sanofi-Aventis, Jansen Cilag; patent, SCN1A testing (held by Bionomics; no financial return); consultancy, Bionomics, Athena Diagnostics. I.S.: editorial board, Annals of Neurology, Epileptic Disorders, Neurology; paid educational presentations, UCB, Athena Diagnostics; speaking fees, UCB, Athena Diagnostics, Transgenomics, GlaxoSmithKline, Biocodex; travel expenses, UCB, GlaxoSmithKline, Biocodex; patent, Diagnostic and Therapeutic Methods for EFMR (WO/2009/086591). D.G.: consultancy, Jazz Pharmaceuticals, Vertex Pharmaceutical; grants, UCB Pharma, European Commission; paid scientific presentation, Janssen Pharmaceutica.
References
- 1.Derry CP, Heron SE, Phillips F, et al. Severe autosomal dominant nocturnal frontal lobe epilepsy associated with psychiatric disorders and intellectual disability. Epilepsia. 2008;49:2125–2129. doi: 10.1111/j.1528-1167.2008.01652.x. [DOI] [PubMed] [Google Scholar]
- 2.Hern SE, Smith KR, Bahlo M, et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 2012;44:1188–1190. doi: 10.1038/ng.2440. [DOI] [PubMed] [Google Scholar]
- 3.Coppola PP, Chiron C, Robain O, Dulac O. Migrating partial seizures in infancy: a malignant disorder with developmental arrest. Epilepsia. 1995;36:1017–1024. doi: 10.1111/j.1528-1157.1995.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 4.Barcia G, Fleming MR, Deligniere A, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44:1255–1260. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milligan CJ, Li M, Gazina EV, et al. KCNT1 gain of function in 2 epilepsy phenotypes is reversed by quinidine. Ann Neurol. 2014;75:581–590. doi: 10.1002/ana.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bearden D, Strong A, Ehnot J, et al. Targeted treatment of migrating partial seizures of infancy with quinidine. Ann Neurol. 2014;76:457–461. doi: 10.1002/ana.24229. [DOI] [PubMed] [Google Scholar]
- 7.Marquez MF, Bonny A, Hernandez-Castillo E, et al. Long-term efficacy of low doses of quinidine on malignant arrhythmias in Brugada syndrome with an implantable cardioverter-defibrillator: a case series and literature review. Heart Rhythm. 2012;9:1995–2000. doi: 10.1016/j.hrthm.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Ochs HR, Greenblatt DJ, Lloyd BL, et al. Entry of quinidine into cerebrospinal fluid. Am Heart J. 1980;100:341–346. doi: 10.1016/0002-8703(80)90148-9. [DOI] [PubMed] [Google Scholar]
- 9.Westerhout J, Smeets J, Danhof M, de Lange EC. The impact of P-gp functionality on non-steady state relationships between CSF and brain extracellular fluid. J Pharmacokinet Pharmacodyn. 2013;40:327–342. doi: 10.1007/s10928-013-9314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gigout S, Louvel J, Kawasaki H, et al. Effects of gap junction blockers on human neocortical synchronization. Neurobiol Dis. 2006;22:496–508. doi: 10.1016/j.nbd.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Voss LJ, Jacobson G, Sleigh JW, et al. Excitatory effects of gap junction blockers on cerebral cortex seizure-like activity in rats and mice. Epilepsia. 2009;50:1971–1978. doi: 10.1111/j.1528-1167.2009.02087.x. [DOI] [PubMed] [Google Scholar]
- 12.Data JL, Wilkinson GR, Nies AS. Interaction of quinidine with anticonvulsant drugs. N Engl J Med. 1976;294:699–702. doi: 10.1056/NEJM197603252941305. [DOI] [PubMed] [Google Scholar]
- 13.Ngui JS, Chen Q, Shou M, et al. In-vitro stimulation of warfarin metabolism by quinidine: increases in the formation of 4′3- and 10-hydroxywarfarin. Drug Metab Dispos. 2001;29:877–886. [PubMed] [Google Scholar]
- 14.Ngui JS, Tang W, Stearns RA, et al. Cytochrome P450 3A4-mediated interaction of diclofenac and quinidine. Drug Metab Dispos. 2000;28:1043–1050. [PubMed] [Google Scholar]
- 15.Damkier P, Brøsen K. Quinidine as a probe for CYP3A4 activity: intrasubject variability and lack of correlation with probe-based assays for CYP1A2, CYP2C9, CYP2C19, and CYP2D6. Clin Pharmacol Ther. 2000;68:199–209. doi: 10.1067/mcp.2000.108532. [DOI] [PubMed] [Google Scholar]
- 16.von Moltke LL, Greenblatt DJ, Duan SX, et al. Inhibition of desipramine hydroxylation (cytochrome P450-2D6) in-vitro by quinidine and by viral protease inhibitors: relation to drug interactions in vivo. J Pharm Sci. 1998;87:1184–1189. doi: 10.1021/js980197h. [DOI] [PubMed] [Google Scholar]
- 17.Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 18.Petrou S, Ugur M, Drummond RM, et al. P2X7 purinoceptor expression in Xenopus oocytes is not sufficient to produce a pore-forming P2Z-like phenotype. FEBS Lett. 1997;411:339–345. doi: 10.1016/s0014-5793(97)00700-x. [DOI] [PubMed] [Google Scholar]