Abstract
A single center, open-label, dose-finding adaptive study was conducted in twelve healthy overweight female subjects. The study was to evaluate the safety and tolerability of the capsaicinoids (CAPs) from Capsicum extract in a beadlet form compared to placebo in a healthy overweight population. The investigational product capsaicinoids (CAPs) from Capsicum extract in a beadlet form (Capsimax®) a proprietary encapsulated form of Capsicum extract in beadlet form supplemented at 2 mg, 4 mg, 6 mg, 8 mg and 10 mg of CAPs. An ascending dose protocol evaluated a total dose of 10 mg daily given in five divided doses (2 mg, 4 mg, 6 mg, 8 mg and 10 mg of CAPs). Each dose was given for a week. Safety and tolerability were assessed. Primary outcomes were tolerability assessments and reports of adverse events. Tolerability assessments were observed on skin color and any changes in skin, bowel movement, digestion, mouth or throat, hair color or changes in hair color, urination includes frequency and burning sensations, breathing, any changes in their health. Secondary outcomes were body weight, body mass index (BMI), blood pressure (SBP/DBP), vital signs, electrocardiograms, clinical chemistry parameters including liver function tests, lung function tests and kidney function tests and complete blood count (CBC). No dose effective changes were observed. The escalating dose levels of CAPs in a beadlet form product found was tolerable and safe for weight management studies. Tolerability assessments and safety blood markers showed no significant changes from baseline. No significant serious adverse events were reported throughout the duration of the study. Further longer term studies are required to explore the tolerability of the product. This trial is registered with ISRCTN: # ISRCTN10975080.
1. Introduction
Obesity rate increased alarmingly in U.S. by 27.7 percent in 2014 [1]. Increased morbidity from diabetes, hypertension, cardiovascular disease, and inflammatory disorders is common in developing and developed countries [2, 3]. More than one-quarter of health care costs related to obesity and associated disorders. Promoting weight loss, decreasing body fat, maintenance of negative energy balance, reducing energy intake, and increasing energy expenditure through physical activity reduce body weight [1]. The interest in natural herb supplements such as Gymnema sylvestre, green tea, Sphaeranthus indicus, Garcinia mangostana, Dolichos biflorus, and Piper betle leaves for treatment of obesity was rapidly growing because of their minimal side effects. The spicy varieties of Capsicum were investigated for their effects on weight loss and thermogenesis. Commonly called chili peppers, or simply “chilies,” Capsicum is used as a food additive in many regions due to its pungency, aroma, and color [4]. It was safely consumed in large amounts in many countries, especially in Mexico and Korea, where per capita consumption reaches 15 grams per day. More moderate consumption up to 5 grams daily per capita of Capsicum was reported in Thailand and India. In U.S., consumption of all peppers increased, from an average of 6.94 kg per person in 2005 to 8.66 kg per person in 2012 and consumption of bell peppers grew from 4.17 kg to 5.03 kg, while chili pepper consumption grew from 2.77 kg to 3.36 kg [5]. The daily consumption of all peppers was approximately 24 g/person and chili pepper was 9 g/person. Capsicum (Capsicum annuum L. or Capsicum frutescens L.) and paprika (Capsicum annuum L.) were generally recognized as safe (GRAS) for their intended use in food (21 CFR 182.10; 582.10; 21 CFR 182.20; 582.20; 21 CFR 73.340) [6].
The most abundant forms of capsaicinoids found in hot red peppers are capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide), dihydrocapsaicin, and nordihydrocapsaicin. About 70% of the burning sensation experienced from consumption of chili red peppers is attributed to capsaicin [7]. Capsaicinoids (CAPs) are detected rapidly using gas chromatography or supercritical fluid extraction and supercritical fluid chromatography (SFE/SFC). Approximately 3 mg of capsaicinoids is present within 1 g of dried red pepper. Capsaicin is used extensively as treatment for pain. Evidences showed that capsaicin has effects on digestive tract, regulating homeostasis and other deleterious effects. The TRPV1 channels are expressed in many neurons in the GI tract [6]. Capsicum and capsaicinoids improve metabolism and hormone function [8], stabilize blood glucose [9, 10], reduce insulin and leptin resistance [11] and endothelial function [12], inhibiting LDL-cholesterol oxidation [13], and prevent cancer due to antioxidant activity [14–16]. Spicy food consumption showed highly consistent inverse associations with total mortality among both men and women after adjustment with potential risk factors. The adjusted hazard ratios for death were 0.90 (95% confidence interval 0.84 to 0.96), 0.86 (0.80 to 0.92), and 0.86 (0.82 to 0.90) for those who ate spicy food 1 or 2, 3 to 5, and 6 or 7 days a week, respectively. People consuming spicy foods 6 or 7 days a week showed a 14% relative risk reduction in total mortality. Inverse associations were also observed for deaths due to cancer, ischemic heart diseases, and respiratory diseases [15].
Capsaicinoids (CAPs), potential ingredient to support body weight, reduce ad libitum energy intake [17–19], increase thermogenesis and energy expenditure [7, 9, 20–22], and increase lipolysis [7, 9, 20, 21, 23–26]. Since studies have used a variety of treatments (chilies and dietary supplements) within the research design, the results for a given outcome measure the ingested treatment, dose, and product quality. However, assuming the overall effects as noted above, epidemiological data seem to support an association between consumption of CAPs containing foods and a lower incidence of obesity and associated disorders [15, 27].
CAPs are major naturally occurring pungent principles in red-hot pepper (Figure 1) [28]. The current study was a pilot study designed to study the safety and tolerability of capsaicinoids from Capsicum extract fruit in a beadlet form, administered to a group of 12 healthy overweight female subjects to escalating dosages of CAPs from Capsicum extract fruit (i.e., 2–10 mg capsaicinoids). Each dose is administered for a week (7 days). Daily diary data was maintained by subjects to show general tolerability and record adverse events.
Figure 1.
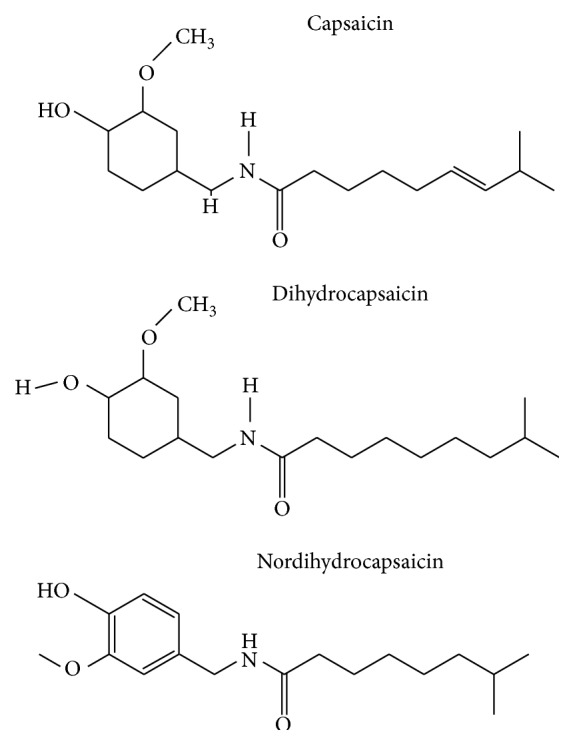
The structures of capsaicinoids.
2. Materials and Methods
2.1. Subjects
The study is designed to test different doses of CAPs product (2 mg CAPs, 4 mg CAPs, 6 mg CAPs, 8 mg CAPs, and 10 mg CAPs) on tolerability, safety, and anthropometric and metabolic measures in overweight healthy subjects. The study was a single center, dose-finding, open-label, and adaptive study design with twelve (12) overweight women taking escalating doses of the study product, each dose for six (6) weeks. An ascending dose protocol evaluated a total dose of up to 10 mg daily given in different doses (2 mg, 4 mg, 6 mg, 8 mg, and 10 mg of CAPs). Each dose is administered to subjects for a week (7 days). Safety and tolerability are assessed by recording vital signs, electrocardiograms, clinical chemistry parameters, urinalysis, and adverse events. The study was conducted at Medicus-Staywell Clinical Research, Northridge, CA. Institutional review board (IRB) approval was received (Copernicus Group, IRB, Cary, NC). All experimental procedures were performed under the Helsinki Declaration. All participants completed a detailed medical history, prior and concomitant medications, and physical activity questionnaire.
Inclusion and Exclusion Criteria. Healthy female subjects between 25 and 55 years of age and with body mass index (BMI) between 25 and 34.9 kg/m2 were included. All participants signed informed consent forms. Subjects agreed to all study visits and procedures.
Pregnant and lactating women were not included. Subjects were excluded based on regular ingestion of chili peppers (raw or powdered form), black pepper, or ginger or foods known to contain chili peppers, black pepper, or ginger more than 3 times per week. Subjects with known allergies to foods were excluded. Subjects were excluded for any prescribed medications for chronic diseases such as inflammatory disorders, metabolic disorders, diabetes, cardiovascular disease, cancer, HIV/AIDS, and obesity. Subjects with recent history of alcoholism (within 12 months) or drug abuse were excluded.
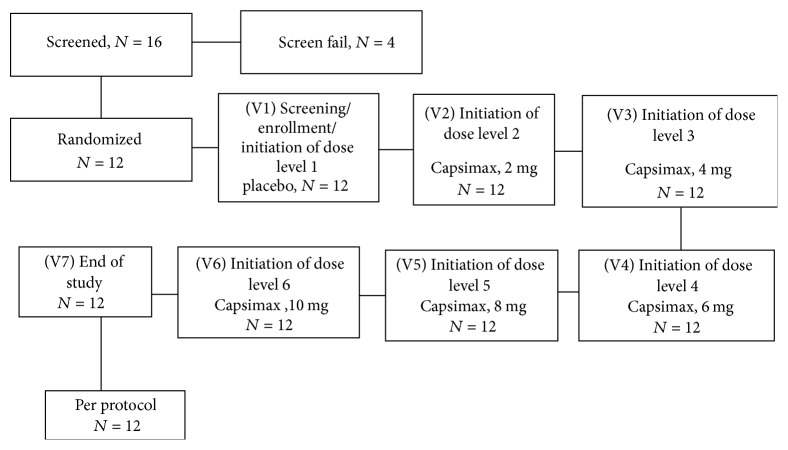
Study design and number of visits of participants are reported in Figure 2. Medical history, review of concomitant medications, vital signs, demographic assessments, anthropometric measurements, physical examination, and laboratory assessments including urine collection for pregnancy and blood draws for CBC and CMP were assessed. Subjects were given study product and instructions (Visit 1).
Figure 2.
Study design.
Subjects returned to the site every week and tolerability reviews were conducted using open-ended questions. Subjects fasting samples were collected at baseline and after 6 weeks of product administration. Subject's diaries were reviewed to record AEs. Subjects were given 2 mg CAPs (100 mg Capsimax), 4 mg CAPs (200 mg Capsimax), 6 mg CAPs (300 mg Capsimax), 8 mg CAPs (400 mg Capsimax), and 10 mg CAPs (500 mg Capsimax) during each visit. Each dose was administered for a week (7 days). Vital signs, medical history, and tolerability survey were recorded for subjects at each visit. Blood samples were analyzed for safety markers such as complete blood count (CBC) and comprehensive metabolic panel (white blood cells, red blood cells, hemoglobin (Hb), hematocrit, mean platelet volume (MPV), mean corpuscular volume (MCV), red cell distribution width (RDW), platelet count, mean corpuscular hemoglobin, neutrophil, lymphocyte, monocytes, eosinophil, basophil, total protein, albumin, globulin, ratio of albumin and globulin, calcium, chloride, sodium potassium, and anion gap) and liver, lung, and kidney function tests (serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), alkaline phosphatase, bilirubin, carbon dioxide, blood urea nitrogen (BUN), and creatinine blood urea nitrogen). Frequency and intensity of adverse event (AE) and serious AEs were recorded based on the diaries and interviews during each visit.
2.2. Product Information
Capsimax, a proprietary product, consists of capsaicinoids (CAPs) obtained from dried red fruits of Capsicum annuum L. The Capsicum extract was standardized into a beadlet form with food grade carbohydrates useful for food applications. Capsimax is a faint, pinkish-white colored, free-flowing, uniform spheroidal beadlet with a spicy odor characteristic of dried ripe fruits of Capsicum. The novel delivery technology masks the irritating effects of CAPs in GI. The product was standardized to 2% capsaicinoids, of which 1.2–1.35% is capsaicin, 0.6–0.8% is dihydrocapsaicin, and 0.1–0.2% is nordihydrocapsaicin. The product has 15–25% extract from Capsicum, 45–55% sucrose, and 30–35% cellulose gum coatings. Placebo and Capsimax capsules (providing 2 mg, 4 mg, 6 mg, 8 mg, and 10 mg CAPs) from Capsicum extract in beadlet form are provided by OmniActive Health Technologies Ltd., India. The placebo capsules were same in appearance and consisted of cellulose. The capsules were manufactured under GMP conditions (Mumbai, India).
2.3. Statistical Analysis
A modified per-protocol analysis was performed including all subjects who had at least one poststudy product exposure visit. Mean baseline values and change from baseline were determined. Data are expressed as mean ± standard deviation unless otherwise specified. Primary endpoints were tolerability assessments and AEs and secondary end points were blood safety markers (complete blood count (CBC) and comprehensive metabolic panel (CMP)). Change in assessments for tolerability and safety measures is assessed by ANOVA in a repeated measures design. This allowed for an assessment of the main effect of time, dose, and time by dose interaction. A paired sample t test was used to assess changes over time for each group. Mean, standard deviation and significance and categorical variables are summarized as counts and percentages. Data derived from diary entries, clinical chemistry assessments, questionnaires, and other relevant assessments at baseline and after supplementation. Statistical Software for Social Sciences (SPSS version 19) was used to run all analysis. Results are considered statistically significant at p < 0.05.
3. Results
3.1. Descriptive Characteristics
Table 1 provides descriptive baseline characteristics of the participants. In the current study, 58% subjects were Hispanics and 42% were non-Hispanic subjects. Overweight healthy women participated in the study (mean BMI: 28 kg/m2). Blood pressure was normal at baseline and at postsupplementation.
Table 1.
Baseline characteristics of the subjects.
| Baseline characteristics | Subjects details |
|---|---|
| N | 12 females |
| Age (Mean ± SD) | 43.0 ± 8.18 |
| BMI (Mean ± SD) | 28.7 ± 2.95 |
| Marital status | |
| Single, N | 6 |
| Married, N | 5 |
| Divorced, N | 1 |
| Ethnicity | |
| Hispanic, N | 7 |
| Non-Hispanic, N | 5 |
3.2. Tolerability Study
Twelve female subjects participated in the study. All participants completed the study. The primary endpoints were daily diary tolerability assessments and reports of adverse events. Subjects tolerability survey reports suggest that escalating doses of CAPs (2–10 mg CAPs) had no significant changes in the skin, bowel movements, hair, digestion, urination, mouth or throat, and breathing. At week 2, only 92% of the subjects reported no changes in their urination. Subjects reported no changes in their overall health. Four incidences of adverse events such as sprained ankle, elevated blood pressure, stomach ache, and increased urination are reported. However, in investigator evaluation, these incidences unrelated to product consumption. All subjects completed the study and no dropouts were reported. No serious adverse events were reported.
3.3. Anthropometric Measurements and Vital Signs
No significant changes were observed for vital signs, body weight, body mass index, and blood pressure over baseline (SBP/DBP, Table 2). CAPs dose escalation did not affect blood pressure.
Table 2.
Body mass index and blood pressure.
| Details | Baseline | Placebo | Capsaicinoids from Capsimax | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | |||||||
| Mean ± SD | Mean ± SD | 2 mg | 4 mg | 6 mg | 8 mg | 10 mg | |
| BMI | 28.68 ± 3 | 28.65 ± 3 | 28.76 ± 3 | 28.69 ± 3 | 28.77 ± 3.15 | 28.67 ± 3 | 28.71 ± 3 |
| SBP | 122 ± 17 | 115 ± 15 | 118 ± 20 | 115 ± 15 | 119 ± 16 | 117 ± 15 | 122 ± 21 |
| DBP | 74.83 ± 10 | 71.17 ± 8 | 72.33 ± 8 | 69.67 ± 6 | 75.75 ± 9 | 74 ± 10 | 78 ± 7 |
No significant changes were observed.
3.4. Blood Chemistries (CBC and Comprehensive Metabolic Panel)
No statistically significant changes were observed in CBC compared to baseline for all markers (Table 3). There were no significant changes in liver, lung, and kidney function test. No significant changes were observed in comprehensive metabolic panel (Table 4). CBC and CMP are within normal range. Repeated measures analysis of variance for all assessments revealed no significant effect of dose on tolerability assessments and safety measures. Overall compliance was ≥91%–100%.
Table 3.
Blood chemistries at baseline and week-6 visits.
| Blood chemistries | Baseline | Week 6 |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| White blood cells (WBCs) | 6.81 ± 1.393 | 6.68 ± 1.872 |
| Red blood cells (RBCs) | 4.44 ± 0.245 | 4.43 ± 0.327 |
| Hemoglobin (Hb) | 13.28 ± 0.783 | 13.15 ± 1.028 |
| Hematocrit | 39.43 ± 2.249 | 39.08 ± 3.082 |
| Mean corpuscular volume (MCV) | 88.92 ± 3.622 | 88.33 ± 3.580 |
| Mean corpuscular hemoglobin | 29.93 ± 1.207 | 29.74 ± 1.218 |
| Red cell distribution width (RDW) | 14.13 ± 1.508 | 13.87 ± 1.034 |
| Platelet count | 235.25 ± 38.973 | 237.33 ± 48.095 |
| Mean platelet volume (MPV) | 9.53 ± 0.859 | 9.70 ± 0.966 |
| Neutrophil | 60.83 ± 11.352 | 62.00 ± 10.600 |
| Lymphocyte | 30.00 ± 10.036 | 29.50 ± 9.100 |
| Monocytes | 6.50 ± 1.679 | 5.83 ± 1.586 |
| Eosinophil | 2.67 ± 1.923 | 2.25 ± 1.545 |
| Basophil | 0.25 ± 0.452 | 0.42 ± 0.515 |
| Total protein | 7.08 ± 0.425 | 6.98 ± 0.282 |
| Albumin | 4.25 ± 0.323 | 4.20 ± 0.280 |
| Globulin | 2.83 ± 0.439 | 2.78 ± 0.359 |
| Ratio of albumin and globulin | 1.55 ± 0.332 | 1.55 ± 0.265 |
| Calcium | 9.33 ± 0.440 | 8.89 ± 0.578 |
| Chloride | 105.58 ± 2.193 | 104.50 ± 2.844 |
| Sodium | 140.00 ± 2.558 | 138.75 ± 1.960 |
| Potassium | 3.99 ± 0.334 | 3.86 ± 0.318 |
| Anion gap | 8.42 ± 1.975 | 7.58 ± 2.353 |
Table 4.
Serum markers for liver, lung, and kidney functions.
| Markers | Baseline | Week 6 |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Serum glutamic-oxaloacetic transaminase (SGOT) | 18.58 ± 5.178 | 20.50 ± 7.657 |
| Serum glutamic-pyruvic transaminase (SGPT) | 24.33 ± 12.630 | 26.00 ± 14.610 |
| Alkaline phosphatase | 72.17 ± 18.556 | 76.92 ± 26.387 |
| Bilirubin | 0.46 ± 0.202 | 0.53 ± 0.336 |
| Carbon dioxide | 26.00 ± 2.296 | 26.67 ± 3.085 |
| Blood urea nitrogen (BUN) | 11.67 ± 3.143 | 11.08 ± 3.343 |
| Creatinine | 0.72 ± 0.083 | 0.77 ± 0.078 |
| Blood urea nitrogen | 16.33 ± 4.163 | 14.42 ± 3.554 |
4. Discussion
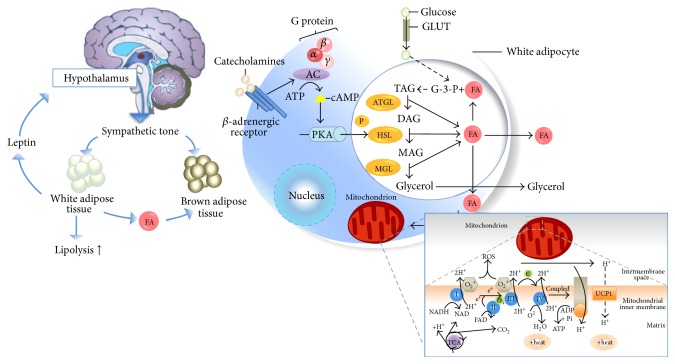
Capsaicinoids (CAPs) serve as agonists of the transient receptor potential vanilloid subfamily member 1 (TRPV1). TRPV1 releases substance P, which activates the postsynaptic receptor of substance P, neurokinin-1 [29]. CAPs are found to have weight loss properties based on their potential mechanism of action (Figure 3). Activation of neurokinin-1 results in an increased activation of the sympathetic nervous system, leading to the release of epinephrine (EPI) and norepinephrine/noradrenaline (NA). Recent systematic review and met analysis [30, 31] reported that consumption of 2 mg capsaicinoids increases energy expenditure (50 kcal/day) and would produce clinically significant levels of weight loss in 1-2 years. It was also observed that regular consumption reduced much abdominal adipose tissue levels and reduced appetite and energy intake as part of a weight management program [32].
Figure 3.
Potential mechanism of action; capsaicinoids promote lipolysis of fat and stimulate thermogenesis in adipose tissue.
In vitro dissolution data (unpublished) for 500 mg Capsimax beadlets is equal to 10 mg CAPs tested in acidic medium similar to stomach pH. No release of CAPs is observed under acidic conditions. In addition, CAPs from Capsimax were released in alkaline medium, which is similar to conditions in the upper intestine. The release of CAPs in alkaline medium was gradual, over the period of 4 hours. At 4 hours, 75% of the CAPs were released [33]. Results from this study showed that 6-week consumption of the study product in increasing doses (2–10 mg/d) has no significant effect on change in skin color, bowel movements, hair, digestion, mouth or throat, and breathing including urination. There were no significant changes in anthropometric measures, clinical chemistries, CBC, metabolic measures, and vital signs compared to baseline measurements. Lastly, there were no serious adverse events related to the study product. Overall, the results of the study indicated that CAPs supplemented dosages were safe and well tolerated.
The current data supports that higher dose of CAPs was safe and tolerable for human consumption. The results reinforce animal and human studies on the acute effects of CAPs on metabolic measures such as white blood cells (WBCs), neutrophils, eosinophils, basophils, monocytes, lymphocytes, T lymphocytes, B lymphocytes, and NK cells. Capsaicinoids decreased the levels of acquired immunity cells and increased the number of total WBCs and neutrophils without changing the number of monocytes, eosinophils, or basophils [34]. This indicates that intake of CAPs does not elicit harmful immune response.
In vitro and in vivo studies further support its safety and tolerability [35–38]. No gross or microscopic lesions were observed in any of the rats. The rats showed oral irritation when fed with normal Capsicum extract during pilot experiments, because no irritation was noted with CAPs (Capsimax beadlets). CAPs and CAPs plus formulations did not show any adverse effects on body weights or on organs as evaluated by necropsy and histopathological examination. The CAPs (Capsimax) and CAPs containing formulations were tolerable in experimental animals and without any behavior changes. In mutagenesis test, no real increase in revertant colony numbers in any of the five tester strains was observed following CAPs treatment at any concentration, regardless of metabolic activation (S9 mix). There was also no tendency for higher mutation rates with increasing concentrations in the range below the border of biological relevance. The results of these investigations revealed that CAPs did not induce gene mutations by base pair changes or frameshifts in the genome of the strains. CAPs supplementation does not cause mutagenesis. The results of these investigations suggest that CAPs were nonmutagenic in both the presence and the absence of metabolic activation. In the chromosomal aberration test, CAPs were tested in human peripheral blood lymphocyte cultures. CAPs did not induce gene mutations by base pair changes or frameshifts in the genome of the strains used [37]. The results suggest that CAPs supplementation does not cause mutagenesis. CAPs were well tolerated, with no observed side effects related to gastric upset or discomfort. No difference in heart rate and systolic or diastolic blood pressure was noted as compared to placebo at up to 4 hours after dosing [39]. The current findings suggest that daily consumption of capsaicinoids up to 10 mg was well tolerated and no significant changes in blood chemistries were observed. The limitations of the study were only up to 10 mg capsaicinoids for 7-day intervention for tolerability and safety of the product. This study is only trying to find the dose of tolerability to use in efficacy studies.
Capsaicinoids (CAPs) from Capsicum extract blended with other ingredients. Recent studies suggest that capsaicinoids from Capsicum extract alone and/or with other ingredients reported no adverse events and further confirmed that CAPs are safe [26, 39–45]. The results of these clinical trials show that 2 mg CAPs (Capsimax) were safe and tolerable for human consumption. Furthermore, no serious adverse effects were reported, and the regimen was well tolerated. The current findings suggest that daily consumption of 2 mg CAPs and up to 10 mg CAPs were well tolerated and no significant changes in liver and kidney functions. Further long-term safety and efficacy studies are warranted.
5. Conclusions
The escalating dose levels of CAPs from Capsimax product, a highly concentrated natural Capsicum fruit extract, were found tolerable and safe for human consumption.
Acknowledgments
The authors would like to thank the subjects who participated in this study. Additionally, they thank resources from Dr. Jay Udani and his team for completion of the study.
Conflict of Interests
The authors are employees of OmniActive Health Technologies Inc.
References
- 1. http://www.cdc.gov/obesity/
- 2.Consitt L. A., Bell J. A., Houmard J. A. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life. 2009;61(1):47–55. doi: 10.1002/iub.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Low S., Chin M. C., Deurenberg-Yap M. Review on epidemic of obesity. Annals of the Academy of Medicine, Singapore. 2009;38(1):57–59. [PubMed] [Google Scholar]
- 4.Smeets A. J., Westerterp-Plantenga M. S. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. European Journal of Nutrition. 2009;48(4):229–234. doi: 10.1007/s00394-009-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang B. C., Nahm S. H., Huh J. H., et al. An interspecific (Capsicum annuum × C. chinese) F2 linkage map in pepper using RFLP and AFLP markers. Theoretical and Applied Genetics. 2001;102:531–539. [Google Scholar]
- 6.US Government Publishing Office (GPO) 21 CFR 182.20; 582.20; 21 CFR 73.340, July 2016, https://www.gpo.gov/fdsys/granule/CFR-2011-title21-vol3/CFR-2011-title21-vol3-sec182-10.
- 7.EFSA Panel on Dietetic Products. Scientific opinion on the substantiation of health claims related to capsaicin and maintenance of body weight after weight loss (ID 2039, 2041, 2042), increase in carbohydrate oxidation (ID 2040), and contribution to normal hair growth (ID 2044) pursuant to article 13(1) of regulation (EC) no 1924/2006. European Food Safety Authority Journal. 2011;9(6, article 2210) [Google Scholar]
- 8.Bode A. M., Dong Z. The two faces of capsaicin. Cancer Research. 2011;71(8):2809–2814. doi: 10.1158/0008-5472.CAN-10-3756. [DOI] [PubMed] [Google Scholar]
- 9.Burden D. Bell and Chili Peppers Profile. Agricultural Marketing Resource Center, Iowa State University; 2014. [Google Scholar]
- 10.Yoshioka M., Lim K., Kikuzato S., et al. Effects of red-pepper diet on the energy metabolism in men. Journal of Nutritional Science and Vitaminology. 1995;41(6):647–656. doi: 10.3177/jnsv.41.647. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill J., Brock C., Olesen A. E., Andresen T., Nilsson M., Dickenson A. H. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacological Reviews. 2012;64(4):939–971. doi: 10.1124/pr.112.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludy M.-J., Moore G. E., Mattes R. D. The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chemical Senses. 2012;37(2):103–121. doi: 10.1093/chemse/bjr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W.-L., Zhu L., Jiang J.-G. Active ingredients from natural botanicals in the treatment of obesity. Obesity Reviews. 2014;15(12):957–967. doi: 10.1111/obr.12228. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka M., St-Pierre S., Drapeau V., et al. Effects of red pepper on appetite and energy intake. British Journal of Nutrition. 1999;82(2):115–123. [PubMed] [Google Scholar]
- 15.Lv J., Qi L., Yu C., et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. The British Medical Journal. 2015;351, article h3942 doi: 10.1136/bmj.h3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang J.-H., Tsuyoshi G., Le Ngoc H., et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. Journal of Medicinal Food. 2011;14(3):310–315. doi: 10.1089/jmf.2010.1367. [DOI] [PubMed] [Google Scholar]
- 17.Kang J.-H., Tsuyoshi G., Han I.-S., Kawada T., Kim Y. M., Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity. 2010;18(4):780–787. doi: 10.1038/oby.2009.301. [DOI] [PubMed] [Google Scholar]
- 18.Chularojmontri L., Suwatronnakorn M., Wattanapitayakul S. K. Influence of capsicum extract and capsaicin on endothelial health. Journal of the Medical Association of Thailand. 2010;93(2):S92–S101. [PubMed] [Google Scholar]
- 19.Ahuja K. D. K., Ball M. J. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. British Journal of Nutrition. 2006;96(2):239–242. doi: 10.1079/bjn20061788. [DOI] [PubMed] [Google Scholar]
- 20.Ito K., Nakazato T., Yamato K., et al. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Research. 2004;64(3):1071–1078. doi: 10.1158/0008-5472.can-03-1670. [DOI] [PubMed] [Google Scholar]
- 21.Mori A., Lehmann S., O'Kelly J., et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Research. 2006;66(6):3222–3229. doi: 10.1158/0008-5472.can-05-0087. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic B., Loblaw D. A., Nam R. Capsaicin may slow PSA doubling time: case report and literature review. Journal of the Canadian Urological Association. 2010;4(1):E9–E11. doi: 10.5489/cuaj.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung F. W. Capsaicin as an anti-obesity drug. Progress in Drug Research. 2014;68:171–179. doi: 10.1007/978-3-0348-0828-6-7. [DOI] [PubMed] [Google Scholar]
- 24.Yoshioka M., Doucet E., Drapeau V., Dionne I., Tremblay A. Combined effects of red pepper and caffeine consumption on 24 h energy balance in subjects given free access to foods. British Journal of Nutrition. 2001;85(2):203–211. doi: 10.1079/bjn2000224. [DOI] [PubMed] [Google Scholar]
- 25.Ohyama K., Nogusa Y., Suzuki K., Shinoda K., Kajimura S., Bannai M. A combination of exercise and capsinoid supplementation additively suppresses diet-induced obesity by increasing energy expenditure in mice. American Journal of Physiology: Endocrinology and Metabolism. 2015;308(4):E315–E323. doi: 10.1152/ajpendo.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim K., Yoshioka M., Kikuzato S., et al. Dietary red pepper ingestion increases carbohydrate oxidation at rest and during exercise in runners. Medicine and Science in Sports and Exercise. 1997;29(3):355–361. doi: 10.1097/00005768-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka M., St-Pierre S., Suzuki M., Tremblay A. Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. British Journal of Nutrition. 1998;80(6):503–510. doi: 10.1017/s0007114598001597. [DOI] [PubMed] [Google Scholar]
- 28.Inoue N., Matsunaga Y., Satoh H., Takahashi M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids) Bioscience, Biotechnology and Biochemistry. 2007;71(2):380–389. doi: 10.1271/bbb.60341. [DOI] [PubMed] [Google Scholar]
- 29.Szallasi A., Cortright D. N., Blum C. A., Eid S. R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nature Reviews Drug Discovery. 2007;6(5):357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 30.Whiting S., Derbyshire E., Tiwari B. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite. 2012;59(2):341–348. doi: 10.1016/j.appet.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Whiting S., Derbyshire E. J., Tiwari B. Could capsaicinoids help to support weight management? A systematic review and meta-analysis of energy intake data. Appetite. 2014;73:183–188. doi: 10.1016/j.appet.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka M., St-Pierre S., Drapeau V., et al. Effects of red pepper on appetite and energy intake. British Journal of Nutrition. 1999;82:115–123. [PubMed] [Google Scholar]
- 33. OAHT Dissolution Test, Internal Report, 2009.
- 34.Akimoto S., Tanihata J., Kawano F., et al. Acute effects of dihydrocapsaicin and capsaicin on the distribution of white blood cells in rats. Journal of Nutritional Science and Vitaminology. 2009;55(3):282–287. doi: 10.3177/jnsv.55.282. [DOI] [PubMed] [Google Scholar]
- 35.Kauthale R. R., Jeyakodi S., Deshpande J. J., Nalawade P. B., Gante M. M. Repeated dose effect of Capsimax® and Capsimax® plus blend formulations in Wistar rats. Proceedings of the 14th Annual Conference of Indian Society of Veterinary Pharmacology and Toxicology; December 2014; Guwahati, India. [Google Scholar]
- 36.Gante M. M. Repeated dose effect of Capsimax® and Capsimax® plus formulations in wistar rats [Study] Mumbai, India: Department of Pharmacology and Toxicology, Bombay Veterinary College; 2014. [Google Scholar]
- 37.Rusia S. Study Number. 2451. RCC Laboratories India Private; 2011. Bacterial reverse mutation assay with Capsimax® capsicum extract. [Google Scholar]
- 38.Narayanasamy D. Study Number. 2450. RCC Laboratories India Private; 2011. In vitro mammalian chromosome aberration test using human peripheral blood lymphocyte with Capsimax® capsicum extract beadlets. [Google Scholar]
- 39.Bloomer R. J., Canale R. E., Shastri S., Suvarnapathki S. Effect of oral intake of capsaicinoid beadlets on catecholamine secretion and blood markers of lipolysis in healthy adults: a randomized, placebo controlled, double-blind, cross-over study. Lipids in Health and Disease. 2010;9(1):p. 72. doi: 10.1186/1476-511x-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan E. D., Beck T. W., Herda T. J., et al. Acute effects of a thermogenic nutritional supplement on energy expenditure and cardiovascular function at rest, during low-intensity exercise, and recovery from exercise. The Journal of Strength & Conditioning Research. 2009;23(3):807–817. doi: 10.1519/jsc.0b013e3181a30fb8. [DOI] [PubMed] [Google Scholar]
- 41.Lopez H. L., Ziegenfuss T. N., Hofheins J. E., et al. Eight weeks of supplementation with a multi-ingredient weight loss product enhances body composition, reduces hip and waist girth, and increases energy levels in overweight men and women. Journal of the International Society of Sports Nutrition. 2013;10, article 22 doi: 10.1186/1550-2783-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloomer R. J., Canale R. E., Fisher-Wellman K. H. The potential role of capsaicinoids in weight management. Agro Food Industry Hi-Tech. 2009;20:60–62. [Google Scholar]
- 43.Rondanelli M., Opizzi A., Perna S., et al. Improvement in insulin resistance and favourable changes in plasma inflammatory adipokines after weight loss associated with two months’ consumption of a combination of bioactive food ingredients in overweight subjects. Endocrine. 2013;44(2):391–401. doi: 10.1007/s12020-012-9863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergstrom H. C., Housh T. J., Traylor D. A., et al. Physiologic responses to a thermogenic nutritional supplement at rest, during low-intensity exercise, and during recovery from exercise in college-aged women. Applied Physiology, Nutrition, and Metabolism. 2013;38(9):988–995. doi: 10.1139/apnm-2013-0029. [DOI] [PubMed] [Google Scholar]
- 45.Bergstrom H. C., Housh T. J., Traylor D. A., et al. Metabolic, cardiovascular, and perceptual responses to a thermogenic nutritional supplement at rest, during exercise, and recovery in men. The Journal of Strength & Conditioning Research. 2014;28(8):2154–2163. doi: 10.1519/jsc.0000000000000369. [DOI] [PubMed] [Google Scholar]