Abstract
Deterioration of adaptive immunity with ageing may reflect changes in the repertoire of T cells and B cells available to respond to antigenic challenges, due to altered proportions and absolute numbers of lymphocyte subpopulations as well as changes in the repertoire of antigen receptor genes expressed by these cells. High-throughput DNA sequencing (HTS) now facilitates examination of immunoglobulin and T cell receptor gene rearrangements, and initial studies using these methods to study immune system ageing in humans have demonstrated age-related alterations in the receptor populations within lymphocyte subsets, as well as in repertoires responding to vaccination. Accurate measurement of repertoire diversity remains an experimental challenge. Studies of larger numbers of human subjects, analysis of defined lymphocyte subpopulations including antigen-specific populations, and controlling for factors such as chronic viral infections, will be important for gaining additional understanding of the impact of ageing on human lymphocyte populations.
Introduction
A highly diverse repertoire of lymphocytes facilitates adaptive immunity by providing a sufficiently varied selection of specificities to meet immunological challenges. Diversity is achieved by way of genomic rearrangements, combining variable (V) diversity (D) and joining (J) gene segments to create the antigen binding regions of immunoglobulin (Ig) heavy chains (VDJ), light chains (VJ) and T cell receptor (TCR) alpha or gamma chains (VJ) and beta or delta chains (VDJ). The junctional regions of these gene segment rearrangements are particularly diverse due to variable exonuclease shortening of segment ends, and random addition of additional junctional nucleotides by terminal deoxynu-cleotidyl transferase. These regions encode areas of the Ig or TCR that are particularly important for antigen binding and are known as complementarity determining region 3 (CDR3). Further diversity is attained by assortment of the products of rearranged genes into the final molecule; Ig/B cell receptor (BCR) consisting of 2 identical heavy chains and 2 identical light chains and the TCR consisting of either a gamma and delta chain or, more commonly, an alpha and beta chain. The gene rearrangements encoding Ig and TCR molecules represent highly informative markers of individual clonal lineages of B cells and T cells.
The lymphocyte repertoire as a whole can be characterised by sequence features (such as V, D and J gene segment usage, and CDR3 physicochemical properties) and is shaped by a number of factors. Initial gene rearrangement may not be completely random and may be affected by preferences of the recombination activation enzymes (RAG1 and RAG2) for differing recombination signal sequences (RSS) that flank the genes in their germline configuration, or by the proximity of different gene segments to each other in the germline. In addition to this there are powerful selective forces that alter repertoire composition. In early development in the bone marrow (B cells) or the thymus (T cells) there is positive selection for receptors that are functional and negative selection to remove receptors that have high affinity for self-antigen. Thus the mature naïve lymphocyte populations consist of cells with functional receptors that do not have high affinity for autoantigen. Antigen encounter and a subsequent immune response will skew the repertoire towards having cells with affinity for the challenging antigens. Hence the memory lymphocyte repertoires will reflect the antigen response history of the individual.
In old age there appears to be a breakdown of the immune system on two fronts. In the first instance there is a decreased ability to respond to challenge, as evidenced by poor responses to vaccination and increased susceptibility to infection. Secondly, there is increased evidence of inappropriate immune activation. The level of autoanti-bodies in the serum is increased and there is a general increase in inflammatory mediators. In terms of repertoire one could hypothesise that these two failures reflect changes in positive selection and negative selection respectively.
Changes in lymphocyte subpopulations with ageing
Changes in the proportions and absolute numbers of different lymphocyte subsets could account for some of the age-related impairments of the immune system. In particular, reduction in naïve lymphocyte output accompanied by increases in clonally expanded memory cells could skew the repertoire in favour of cells that have been positively selected by antigen, decreasing the ability to respond to new antigenic challenges. During ageing, thymic involution occurs, resulting in altered tissue architecture, decreased tissue mass and a reduction in CD3+ T cell production [1-5]. Similarly, it has been shown in mice that there is a reduced output of B cells from the bone marrow in old mice [6]. However, the situation may not be so simple. The thymus involutes at a very early age and yet the size and quality of the naïve T cell repertoire can be maintained until well past the 5th decade in life [7]. Some maintenance is provided by homeostatic turnover, but a recent TREC analysis of a large number of blood samples to measure recent thymic emigrants did not show any significant decrease until the 9th decade of life [2]. Data on B cell production from human bone marrow are limited, but output is sufficient to reconstitute the repertoire within a year of B cell depletion by Rituximab in patients as old as 80 [8]. That said, it is clear that the older repertoire contains more memory cells. Despite there being different ways of measuring T cell memory [9], an age related increase in effector memory T cells is well established [10-12]. There is a significant decrease in CD3+CD45RA+CCR7+ naive cells and an increase in CD3+CD45RA− and CCR7− effector memory cells [12], changes that are more apparent in CD8+ than CD4+ T cells. CD4+ T cells seem to maintain their number and diversity to a much later age before changing suddenly in the 7th decade [7]. CD8+ T cell populations are dysre-gulated earlier, with an increase in numbers (likely exacerbated by chronic infection with viruses such as cytomegalovirus (CMV) and Epstein–Barr virus (EBV)) resulting in a decreased CD4+:CD8+ ratio [4,10,13-15]. Within the CD4 and CD8 populations there are further subpopulations that are changed with age, for example with respect to relative proportions of TCRαβ and TCRγδ [12,14,15], and an age related increase in CD4+FoxP3+ Tregs [15-18]. T cell populations in ageing rhesus macaques show similar features, with decreased naïve CD4 and CD8 T cell numbers and persistent oligoclonal T cell expansions correlated with poor vaccination responses measured by CD8 T cell and antibody response [15].
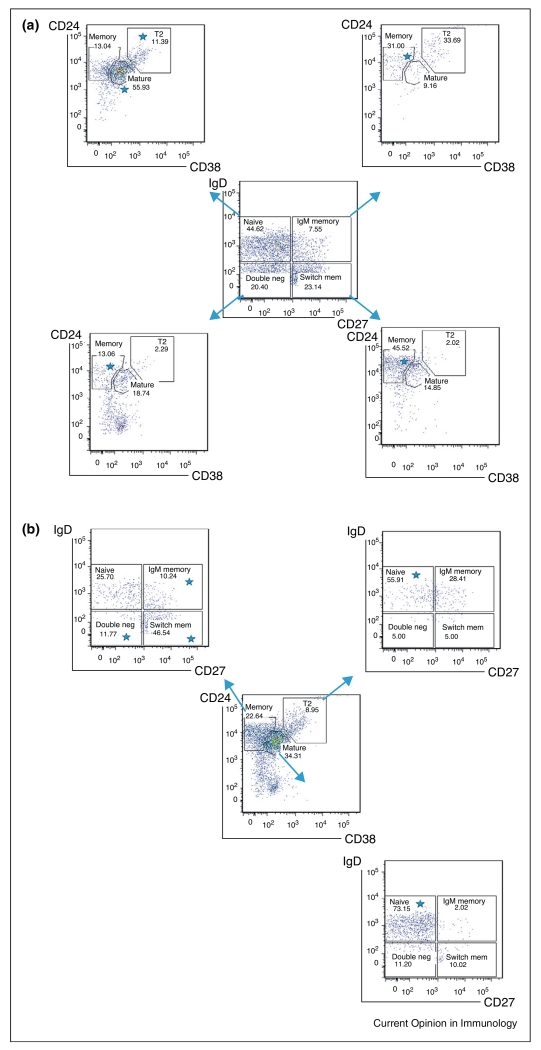
Within aged B cell populations, it is also thought that naive B cells decrease in proportion to memory cells. However, there are conflicting reports in the literature, potentially related to the markers used to define B cell subsets [15,19-22]. Two distinct combinations of markers, CD27/IgD and CD24/CD38, have been used to define B cell populations, but the populations defined by these markers do not easily map onto each other (Figure 1). To complicate this further, different isotypes, for example IgM, IgG, and IgA, exist within many of the memory populations and older papers defining memory by class switching therefore will have missed a large proportion of IgM+ memory cells. Furthermore, CD27 was until recently considered to be a memory B cell marker but reliance on this alone would fail to take account of CD27− cells with memory characteristics [23]. Although they only comprise a small subset of total B cells, the proportion of CD27−IgD− memory cells increases with age [24]. CD27+IgD+ (IgM memory) cells have been reported to decrease, or remain unchanged, with age. Some groups have also reported a decrease in CD27+IgD− (Switched Memory) cells [20,25-27]. A decrease in the absolute number and proportion of plasma cells (IgG, IgM and IgA) [28] as well as a decrease in the generation of plasmablasts in response to influenza vaccine [29] has also been reported with age. Standardized methods of determining lymphocyte memory populations would facilitate comparisons of the reports in the literature, but the general consensus does seem to favour the increase of memory B cell subsets with age.
Figure 1.
Identification of B cells using CD27, IgD, CD38 and CD24, and showing how the two methods of separating B cells relate to each other. (a) CD27/IgD-gated B cell populations are mapped onto (blue arrows) CD24/CD38 gating and (b) visa versa. If the markers for different memory/naïve populations mapped exactly between the two different methods the cells would fall exclusively in the gates marked by ◇ in the outer FACS plots.
Studies of lymphocyte repertoires using high-throughput DNA sequencing (HTS) of antigen receptor genes
Of particular interest in studies of human ageing is the diversity of the B cell and T cell populations, most often considered to be the number of distinct species present in the repertoire, a quantity known as ‘richness’ in the ecology literature [30]. Decreases in repertoire diversity would be expected to leave ‘holes in the repertoire’ that could prevent recognition of novel antigens and would therefore contribute to decreased vaccine responses and increased vulnerability to pathogens. Before the advent of high throughput DNA sequencing (HTS) methods, the quantitative analysis of B and T cell repertoires was severely limited by experimental constraints. PCR-based spectratyping analyses of the lengths of CDR3 regions have been employed to assess repertoire diversity, and have shown age-related changes in the relative proportions of large clonal populations and rarer populations for both T cells and B cells [7,31•,32], but provide no information on gene usage. Heroic efforts at Sanger sequencing could produce on the order of 200 sequences, but with an average human blood volume containing approximately 1–2 billion B cells, and several-fold more T cells, only overt differences between samples can be detected using such small numbers. The true number of distinct rarer sequences in a population cannot be determined in this way, and only very conservative estimates of the lower limit of diversity are feasible, not the upper limit or the most likely value. Even in high-throughput DNA sequencing experiments, where thousands to millions of sequences are determined and the B cells or T cells from relatively large blood volumes are analyzed, the fraction of the circulating repertoire actually measured is on the order of 0.1–1% of the total. Nor would this include lymphocytes at other tissue sites, which probably represent additional tens of billions of cells. Recent work estimating lower limits of B cell repertoire diversity from Ig heavy chain HTS data from healthy blood donors showed a dependence on the depth at which each sample was sequenced, and yielded minimum diversity values up to 6 million, but did not show any age-related decreases in repertoire diversity in individuals up to 79 years of age [33]. HTS studies of T cell receptor beta chain diversity have not yet examined age-related changes, but the repertoire sizes for two young adults were estimated to be 3-4 million, several-fold greater than prior estimates, and with 10–20-fold greater diversity in antigen-experienced CD45RO+ T cells compared to previous estimates [34]. Experimental designs generating many independent replicate libraries from each individual can facilitate data analysis, by giving an indication of whether a sequence seen in many copies in one library is a result of preferential PCR amplification, or instead corresponds to an expanded cellular clonal population. Overall, given the very large number of B cells and T cells in the human body, HTS approaches applied to the fraction of an individual’s blood typically available for study are still somewhat underpowered for estimation of population diversity. Substituting empirical B cell HTS data from healthy individuals into the variance estimator from the population richness lower-bound estimator known as ‘Chao2’ in the ecology literature confirms our intuition that the variance in diversity estimates is still very high with current data sets [35].
In addition to diversity studies, there is also interest in the changing quality of the lymphocyte repertoire, since this may reflect age-related changes in the selective forces in lymphocyte development and activation. The research is in its infancy but older B cell repertoires appear to show increased numbers of IgM B cells with long Ig heavy chain CDR3 regions [31•]. Other recent HTS-enabled studies of lymphocyte repertoires have expanded upon prior observations that different lymphocyte subsets show distinctive features, such as V gene usage frequencies and CDR3 lengths [36•,37,38]. In the light of the age-related changes in lymphocyte populations mentioned above, these factors should be taken into account when studying potential age-related repertoire changes in mixed lymphocyte populations.
HTS has also provided initial observations of age-related repertoire changes in vaccine responses. One study of combined influenza and pneumococcal vaccination in 6 young adults (aged 19–45) and 6 older individuals (aged 70–89) showed that the vaccine-induced expansion of B cells with short and hydrophilic Ig heavy chain CDR3 regions at day 7 post-vaccination was lower in older individuals. The older subjects had impaired IgM and IgA anti-pneumococcal antibody responses, which correlated with features of the spectratypes for their IgM and IgA-expressing B cells [31•]. In a second recent study of influenza vaccination, younger subjects showed a decrease in the proportion of IgM sequences amplified in multiplex PCRs in day 28 post-vaccination B cell repertoires compared to pre-vaccination repertoires, likely as a result of upregulation of IgG and IgA transcript levels in vaccine-responsive B cells. This feature of the vaccine response was reduced in 4 older subjects (aged 70–100) included in the study [39•]. Two of the four older subjects also showed vaccination responses with fewer and larger responding clones than were seen in young individuals, raising the possibility that greater oligoclon-ality of vaccine-stimulated cells could be correlated with age. These features could be relevant to impaired vaccine responses in the elderly, although relationships between the features described and serological responses were not reported [39•].
Conclusions
The new experimental possibilities for ageing research enabled by high-throughput DNA sequencing of lymphocyte antigen receptor genes have not yet been fully exploited. Studies to date have examined small numbers of individuals, but have generated hypotheses and evidence supporting age-related alterations in lymphocyte selection, repertoire diversity, and functional responses to antigenic exposures. Interesting avenues for further work will include application of repertoire HTS to understand changes with ageing in T cell populations, more detailed measurement of deficits in phenotypically distinct B cell and T cell subsets, defining alterations in antigen-specific lymphocytes for important pathogens as a function of age, understanding the impact of chronic viral infections such as CMV and EBV on both B cell and T cell repertoires with age, and validation of current and future findings in larger patient cohorts.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell WA, Lang PO, Aspinall R. Tracing thymic output in older individuals. Clin Exp Immunol. 2010;161:497–503. doi: 10.1111/j.1365-2249.2010.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surh CD, Sprent J. Regulation of naive and memory T-cell homeostasis. Microbes Infect. 2002;4:51–56. doi: 10.1016/s1286-4579(01)01509-x. [DOI] [PubMed] [Google Scholar]
- 6.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 7.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 9.Alam I, Goldeck D, Larbi A, Pawelec G. Aging affects the proportions of T and B cells in a group of elderly men in a developing country – a pilot study from Pakistan. Age (Dordr) 2012 doi: 10.1007/s11357-012-9455-1. PMID:22810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong MS, Dan JM, Choi JY, Kang I. Age-associated changes in the frequency of naive, memory and effector CD8+ T cells. Mech Ageing Dev. 2004;125:615–618. doi: 10.1016/j.mad.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Yan J, Greer JM, Hull R, O’Sullivan JD, Henderson RD, Read SJ, McCombe PA. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, Clementi M, Chieco-Bianchi L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–1283. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 14.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ongradi J, Kovesdi V. Factors that may impact on immunosenescence: an appraisal. Immun Ageing. 2010;7:7. doi: 10.1186/1742-4933-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25 high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simone R, Zicca A, Saverino D. The frequency of regulatory CD3+CD8+CD28− CD25+ T lymphocytes in human peripheral blood increases with age. J Leukoc Biol. 2008;84:1454–1461. doi: 10.1189/jlb.0907627. [DOI] [PubMed] [Google Scholar]
- 18.Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. 2004;112:258–267. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–206. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 20.Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol. 2008;180:2741–2746. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- 21.Jentsch-Ullrich K, Koenigsmann M, Mohren M, Franke A. Lymphocyte subsets’ reference ranges in an age- and gender-balanced population of 100 healthy adults – a monocentric German study. Clin Immunol. 2005;116:192–197. doi: 10.1016/j.clim.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005;175:3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- 23.Wu YC, Kipling D, Dunn-Walters DK. Age-related changes in human peripheral blood IGH repertoire following vaccination. Front Immunol. 2012;3:193. doi: 10.3389/fimmu.2012.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buffa S, Bulati M, Pellicano M, Dunn-Walters DK, Wu YC, Candore G, Vitello S, Caruso C, Colonna-Romano G. B cell immunosenescence: different features of naive and memory B cells in elderly. Biogerontology. 2011;12:473–483. doi: 10.1007/s10522-011-9353-4. [DOI] [PubMed] [Google Scholar]
- 25.Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: composition and function. Biogerontology. 2010;11:125–137. doi: 10.1007/s10522-009-9256-9. [DOI] [PubMed] [Google Scholar]
- 26.Bulati M, Buffa S, Candore G, Caruso C, Dunn-Walters DK, Pellicano M, Wu YC, Colonna Romano G. B cells and immunosenescence: a focus on IgG+IgD−CD27− (DN) B cells in aged humans. Ageing Res Rev. 2011;10:274–284. doi: 10.1016/j.arr.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Dunn-Walters DK, Ademokun AA. B cell repertoire and ageing. Curr Opin Immunol. 2010;22:514–520. doi: 10.1016/j.coi.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, Bos NA, Johnsen HE, Orfao A, Perez-Andres M. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138− and CD138+ plasma cells. Haematologica. 2010;95:1016–1020. doi: 10.3324/haematol.2009.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, Nishtala M, Wrammert J, Smith K, James JA, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heltshe JF, Forrester NE. Estimating species richness using the jackknife procedure. Biometrics. 1983;39:1–11. [PubMed] [Google Scholar]
- 31•.Ademokun A, Wu YC, Martin V, Mitra R, Sack U, Baxendale H, Kipling D, Dunn-Walters DK. Vaccination-induced changes in human B-cell repertoire and pneumococcal IgM and IgA antibody at different ages. Aging Cell. 2011;10:922–930. doi: 10.1111/j.1474-9726.2011.00732.x. The authors present the first data generated using high-throughput DNA sequencing to study age-related immunoglobulin repertoire changes in vaccine responses. There was less induction of IgM or IgA sequences with hydrophilic and/or short CDR3 regions in the elderly subjects.
- 32.Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson BO, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd SD, Marshall EL, Merker JD, Maniar JM, Zhang LN, Sahaf B, Jones CD, Simen BB, Hanczaruk B, Nguyen KD, et al. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci Transl Med. 2009;1:12ra23. doi: 10.1126/scitranslmed.3000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43:783–791. [PubMed] [Google Scholar]
- 36•.Glanville J, Kuo TC, von Budingen HC, Guey L, Berka J, Sundar PD, HuertaG Mehta GR, Oksenberg JR, Hauser SL, et al. Naive antibody gene-segment frequencies are heritable and unaltered by chronic lymphocyte ablation. Proc Natl Acad Sci U S A. 2011;108:20066–20071. doi: 10.1073/pnas.1107498108. High-throughput sequencing of immunoglobulin rearrangements in identical twin pairs highlighted the degree to which gene segment usage in immunoglobulin repertoires is genetically determined, and relatively resistant to environmental influences. This effect should be taken into consideration in the design of other studies of human immune repertoires.
- 37.Wu YC, Kipling D, Leong HS, Martin V, Ademokun AA, Dunn-Walters DK. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations. Blood. 2010;116:10701078. doi: 10.1182/blood-2010-03-275859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosner K, Winter DB, Tarone RE, Skovgaard GL, Bohr VA, Gearhart PJ. Third complementarity-determining region of mutated VH immunoglobulin genes contains shorter V, D, J, P, and N components than non-mutated genes. Immunology. 2001;103:179–187. doi: 10.1046/j.1365-2567.2001.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Jiang N, He J, Weinstein JA, Penland L, Sasaki S, He XS, Dekker CL, Zheng NY, Huang M, Sullivan M, et al. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5:171ra119. doi: 10.1126/scitranslmed.3004794. The authors report features of immunoglobulin repertoires in human subjects ranging in age from infants to the very elderly, in response to influenza vaccination.