1. Summary
B-cell receptor (BCR) signaling plays a vital role in B-cell malignancies; Bruton's Tyrosine Kinase (BTK) is a critical mediator of this signaling. BCR signaling, either constitutively or following antigen binding, leads to activation of several downstream pathways involved in cell survival, proliferation, and migration. The efficacy observed in studies of the BTK inhibitor, ibrutinib, confirms that BCR signaling is critical for the growth of B-cell malignancies. Ibrutinib characteristically induces redistribution of malignant B-cells from tissues into the peripheral blood, and rapid resolution of adenopathy. Further, ibrutinib therapy results in normalization of lymphocyte counts and improvement in cytopenias. Ibrutinib has been shown to have an excellent safety profile and does not cause myelosuppression. Early data from combination studies of ibrutinib with anti-CD20 monoclonal antibodies have shown more rapid responses compared to those seen with ibrutinib monotherapy. Current data strongly support continued clinical evaluation of Ibrutinib in B-cell malignancies.
Keywords: B-cell receptor signaling, Bruton tyrosine kinase inhibitor, Ibrutinib, PCI-32765, Chronic lymphocytic leukemia
2. Introduction
2.1. Disease incidence, prevalence, unmet medical needs & treatment guidelines
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults, with approximately 15,720 men and women expected to be diagnosed with CLL in 2014 in the United States.[1] The median age at diagnosis is 72 yrs and 10% of the patients are younger than 55 yrs.[2] CLL is characterized by a clonal proliferation of CD5 positive B cells in blood, bone marrow, lymph nodes and spleen.[3,4] Only a minority of patients with CLL requires treatment at the time of diagnosis, one third of patients never require therapy, while others develop cytopenia, symptomatic lymphadenopathy/splenomegaly, and/ or disease related B symptoms warranting treatment.[4] Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) is a standard of care for patients with symptomatic disease.[4] The FCR regimen was developed at MD Anderson Cancer Center; where in a phase II trial FCR produced a high overall response rate (ORR) of 95% in previously untreated patients.[5] Hallek and colleagues conducted a randomized trial comparing FCR to FC as initial therapy for patients with CLL. FCR produced an ORR of 90% with a complete remission (CR) rate of 44%.[6] The CR rate seen with FCR was double that observed with FC chemotherapy (44% vs 22%). The median progression free survival (PFS) in the chemoimmunotherapy group was higher than observed with chemotherapy alone. [7] However, certain group of patients had suboptimal responses. Patients with deletion of the short arm of chromosome 17 (del 17p13.1), unmutated IGHV, serum beta 2 microglobulin of at least 3-5mg/L and a white blood cell count (WBC) of 50×109 per L had a shorter PFS.[6] In the relapse setting, the ORR with FCR decreases from 90% to 70% and median PFS decreases from 51 to 30 months.[8] Badoux et al conducted a phase II trial at MD Anderson Cancer Center to evaluate the safety and efficacy of FCR in patients with relapsed CLL.[9] The ORR was 74% with 30% CR. The median PFS was 21 months; in patients who achieved CR the median PFS was 60 months. Bendamustine and rituximab (BR) is a frequent salvage regimen in patients who have had prior fludarabine-based therapy. Fisher and colleagues reported an ORR of 59% and a CR rate of 9% in patients who received a median of 2 prior regimens.[10] Better responses were observed in fludarabine sensitive patients (60.5%) than those who were fludarabine resistant (46%).
Once patients relapse after chemo-immunotherapy, the treatment options are not standardized. Other agents have been used to treat patients with relapsed CLL including; lenalidomide, ofatumumab and alemtuzumab.[10-18] Ofatumumab, a humanized monoclonal antibody targeting CD20, has been approved in the United States and Europe is restricted to patients with CLL.[16] Patients refractory to fludarabine and alemtuzumab showed a response rate of 50% to ofatumumab. The treatment was well tolerated; the main side effect was infusion reactions, predominantly seen with the first dose. Responses observed with ofatumumab in the refractory CLL population were impressive but lasted for only 6 months and patients progressed soon after stopping treatment. The label for ofatumumab in the United States as well as in Europe restricted to patients refractory to alemtuzumab and fludarabine. In patients with bulky, fludarabine refractory CLL, a randomized trial is being conducted in Europe comparing ofatumumab to physician's choice (Clinical Trials.gov, NCT01313689). Ofatumumab is also being evaluated in a clinical trial as maintenance therapy after second or third remission to increase duration of remission (PROLONG; Clinical Trials.gov, NCT01039376).
Lenalidomide is an oral immunomodulator approved for the treatment of patients with multiple myeloma (MM) and myelodysplastic syndrome (MDS) with a 5q- chromosomal abnormality.[19,20] Lenalidomide has shown efficacy in patients with relapsed CLL at a dose of 10-25mg daily.[21,22] Better responses with lenalidomide were observed at a higher dose level. However, most patients are unable to tolerate more than 5-10mg daily due to neutropenia and gastrointestinal complaints. Neutropenia can be managed with colony stimulating factors; tumor lysis can be seen in patients with CLL receiving higher doses of lenalidomide and during dose escalation.[23] Tumor flare reaction (TFR) is another toxicity seen in patients with CLL receiving lenalidomide.[24] Tumor flare reaction is associated with sudden painful enlargement of lymph nodes, low grade fever, and skin rash. Lenalidomide associated TFR is seen early in the course of treatment; TFR should not be confused with tumor progression as it is an immune mediated phenomenon associated with better response.[24] Lenalidomide is being evaluated in several pivotal trials. One of them is the Continuum Trial where safety and efficacy of lenalidomide as maintenance therapy is compared to placebo in patients with CLL in second remission. (Clinical Trials.gov, NCT00774345) Lenalidomide has also been evaluated in combination with anti-CD20 monoclonal antibody in patients with relapsed CLL.[13] The ORR was 64%, higher than seen with lenalidomide alone. Similar responses were seen with ofatumumab in combination with lenalidomide in patients with relapsed CLL.[25]
2.2. B-cell receptor inhibitors and BCL-2 inhibitors, which competitor's compounds/classes of compounds are in the clinic
B-cell receptor (BCR) inhibitors are an exciting new class of agents developed to treat B-cell malignancies. BCR activating-signaling plays a vital role in the pathogenesis of CLL.[26-30] The BCR signaling pathway consists of immunoglobulin bound to cell membrane that attaches to a heterodimer consisting of CD79b and CD 70b.[31,32] Ligation of the B-cell receptor provides a strong proliferative and survival signal to both normal and malignant B-cells, through phosphorylation of spleen tyrosine kinase (SYK) and SRC family kinase (Lyn). Phosphorylation of SYK, results in the recruitment and phosphorylation of other kinases and adapter proteins including Bruton tyrosine kinase (BTK).[33,34] BTK is involved in signaling of multiple receptors that control cell migration, adhesion, proliferation and survival. The signals from chemokine receptors, such as CXCR4, are also mediated by BTK; CXCR4 binds to the chemokine CXCL12 to mediate homing and retention.[35,36] As a consequence, inhibition of BTK results in redistribution of CLL cells from tissues to peripheral blood and inhibition of re-homing.[37] (Figure 1) Several kinases in the BCR signaling pathway are now being evaluated as therapeutic targets in CLL including Lyn,[38,39] SYK,[40,41] PI3K[42-44] and BTK.[32]
Figure 1.
The chemical structure of the BTK inhibitor; Ibrutinib (PCI-32765).
Fostamatinib disodium, a SYK inhibitor, was evaluated in phase I/II trial in patients with relapsed or refractory B-cell lymphoid malignancies including diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), mucosa-associated lymphoid tissue lymphoma (MALT), marginal zone lymphoma (MZL) and small lymphocytic lymphoma (SLL) /CLL.[45] The dose limiting toxicities (DLT) were neutropenia, diarrhea and thrombocytopenia and 200mg twice daily was chosen for phase II studies. The response rate was 22% (5/23 patients) in DLBCL, 10% (2/21 patients) in FL, 55% (6/11 patients) in CLL and 11% (1/9 patient) with MCL. The median progression free survival was 4.2 months. The best responses were observed in patients with CLL/SLL. Fostamatinib disodium has been evaluated in clinical trials for solid malignancies and autoimmune diseases.[46,47]
Another oral agent, idelalisib is a phosphatidylinositol-3-kinase (PI3K) inhibitor recently approved in combination with rituximab for the treatment of patients with relapsed chronic lymphocytic leukemia (CLL), for whom rituximab alone would be considered appropriate therapy due to other co-morbidities.[48]Phosphatidylinositol-3-kinase p110δ serves as a central integrator for downstream signaling from cell surface receptors, promoting survival and proliferation of B-cell malignancies.[49] Idelalisib is a highly selective and potent small molecule inhibitor of the delta isoform with an EC50 of 8nM. In preclinical studies idelalisib blocked PI3K signaling, leading to decreased phosphorylation of AKT, and apoptosis of malignant B-cells.[49] Idelalisib has shown activity in a phase 1 trial for patients with heavily pre-treated relapsed B-cell malignancies.[50] Patients were treated at 6 dose levels ranging from 50-350mg once or twice oral daily. No dose limiting toxicity (DLT) was observed during the first 4 weeks of therapy and a maximum tolerated dose (MTD) was not defined. Pneumonia, neutropenic fever and diarrhea were adverse events. Elevation of transaminases was a common toxicity, which resolved on drug discontinuation and frequently didn't recur after re-challenging at a lower or the same dose of idelalisib.[50] Reduction in lymphadenopathy was observed in 81% of patients. The ORR was 72%; 39% of patients meeting the criteria for partial response (PR) as per IWCLL 2008 and 33% meeting the recently updated criteria of PR with treatment-induced lymphocytosis (PR-L).[51] The median PFS was 15.8 months and the median overall survival was not reached. The median PFS for patients treated at ≥ 150mg twice daily was 32 months compared to 7 months for patients treated at a lower dose.
In a double blind, placebo-controlled, phase III study idelalisib 150mg twice daily dose was evaluated in combination with rituximab.[48] The treatment population was patients with relapsed CLL, with short prior remission duration, cytopenias, renal insufficiency, prior therapy induced myelosuppression, or significant comorbidities rendering them unable to receive standard therapy. Patients were randomly assigned to receive rituximab and either idelalisib (150mg BID) or placebo twice daily. The median PFS was 5.5 months in the placebo group and was not reached in the idelalisib group. The hazard ratio for progression or death in the idelalisib group was 0.15; P≤0.001. The ORR in patients receiving idelalisib vs those receiving placebo was 81% vs 13% respectively; odds ratio, 29.92; P≤0.001. The overall survival at 12 months was 92% in idelalisib group compared to 80% in the placebo group (hazard ratio for death, 0.28; P=0.02). The adverse events were similar in both groups, including; fatigue, nausea, chills and diarrhea; the frequencies of elevation of transaminases was higher in the idelalisib group. The study was stopped based on an interim analysis, and the drug was approved.
Another phase I study utilized idelalisib in combination with rituximab and/or bendamustine in patients with previously treated CLL.[52] Grade ≥ 3 adverse events were as expected for chemotherapy. No new toxicity was observed with the combination. The ORR with idelalisib plus rituximab was 79%, with bendamustine 78%, and with bendamustine and rituximab 87%. Ten percent of patients had grade 3-4 elevation in transaminases.
Several pivotal trials are being conducted in Europe and the United States in patients with relapsed CLL, evaluating the safety and efficacy of idelalisib in combination with ofatumumab (Clinical Trials.gov, NCT01659021).
BCL-2 family members regulate the apoptotic process. The BCL-2 family is comprised of proapoptotic and prosurvival proteins, and shifting the balance towards the prosurvival proteins is an established mechanism of cancer proliferation. ABT-199 (GDC-0199) is a novel agent being evaluated for safety and efficacy in patients with CLL/SLL. ABT-199 is a highly selective, potent, orally bioavailable, small molecule BCL-2 inhibitor. In a phase I clinical trial, 56 patients with relapsed CLL were enrolled at doses ranging from 150mg to 1200mg.[53] The most common adverse events occurring in ≥25% of patients (all grades) were diarrhea, neutropenia, fatigue, upper respiratory tract infections (URTI) and cough. Grade 3/4 adverse events occurred in 4 patients: neutropenia, tumor lysis syndrome (TLS), anemia and febrile neutropenia. Seven dose limiting toxicities were observed up to date; 5 TLS at doses from 50mg to 200mg, 1 grade 4 neutropenia at 600mg and one death at 1200mg due to TLS. The ORR was 84%, including 20% CR/CRi. Eight patients in CR were evaluated for minimal residual disease (MRD). Four patients were MRD negative by 4 color flow cytometry and 4 patients had a low level MRD positivity. The study is currently enrolling patients with a revised dose schedule to reduce the risk of TLS.
ABT-199 was also evaluated in patients with relapsed/ refractory NHL in a phase I clinical trial.[54] Thirty two patients were enrolled; ABT-199 was given in a stepwise dose escalation (200, 300, 400, 600 and 900mg). Adverse events occurring in ≥ 20% of patients were nausea, diarrhea, vomiting, fatigue and URTI. Grade 3/4 adverse events were anemia, neutropenia and thrombocytopenia. At a target dose of 600mg 2 of 10 patients had a DLT of grade 3 febrile neutropenia and grade 4 neutropenia. Grade 3 laboratory TLS was observed in two patients; 1 with bulky MCL and 1 with DLBCL. The ORR was 53%. The responses were observed at all dose levels. Further clinical trials are being conducted with ABT-199 as monotherapy or in combination with anti-CD20 monoclonal antibodies.
Obinutuzumab (GA101) is glycoengineered, humanized type II CD20-antibody in clinical trials for patients with CLL. Pre-clinical studies showed superior activity compared to type I CD20 antibodies.[55] In older patients with CLL, not fit for standard chemoimmunotherapy, obinutuzumab in combination with chlorambucil has shown promising results.[56] Obinutuzumab-chlorambucil or rituximab-chlorambucil was compared with chlorambucil monotherapy. The PFS was 26.7 months with obinutuzumab-chlorambucil vs 16.3 months with rituximab-chlorambucil vs 11.1 months with chlorambucil alone (p=≤0.001). The obinutuzumab-chlorambucil group had higher ORR, CR and MRD negative rates as compared to those seen with rituximab-chlorambucil. Obinutuzumab and chlorambucil received approval by the FDA for previously untreated CLL patients, not fit for standard chemo-immunotherapy. Obinutuzumab as monotherapy or in combination is being evaluated in other B-cell malignancies.
3. Introduction of ibrutinib; chemistry, pharmacodynamics, pharmacokinetics & metabolism
Ibrutinib, formerly known as PCI-32765, is the first BTK inhibitor studied in clinical trials; it was initially developed by Celera Genomic and later acquired by Pharmacyclics (Fig 1). Ibrutinib is a small molecule inhibitor of BTK, which inactivates BTK through an irreversible covalent bond with Cys-481 in the ATP binding domain.[57] Ibrutinib also inhibits interlukin-2-inducible kinase (ITK), a member of the TEC kinase family. ITK has critical role in T-cell signaling, contributing to infectious processes, autoimmune and neoplastic diseases.[58] Ibrutinib was initially designed as a novel therapeutic target for rheumatoid arthritis (RA) and tested in vitro models.[59] Ibrutinib is a potent and selective inhibitor (50% inhibitory concentration; 0.5nM) of BTK.[59] It has good oral bioavailability and is rapidly absorbed. Peak plasma concentrations are reached after 1 to 2 hours of dosing. Full occupancy of the BTK binding site occurred at 2.5mg/kg per day and dose escalation was continued to 12.5mg/kg without reaching a maximum tolerated dose (MTD). The mean terminal half-life ranged from 4 to 8 hours. There was no evidence of drug accumulation after repeated daily dosing. [60] In spite of rapid clearance from plasma, BTK remained covalently bound to ibrutinib for at least 24hrs. In CLL the drug is given at a dose of 420mg to induce full BTK-target occupancy, based on a fluorescent-tagged derivative of ibrutinib (BTK probe) assay.[60] However, in MCL the approved dose of ibrutinib is 560mg daily.[61]
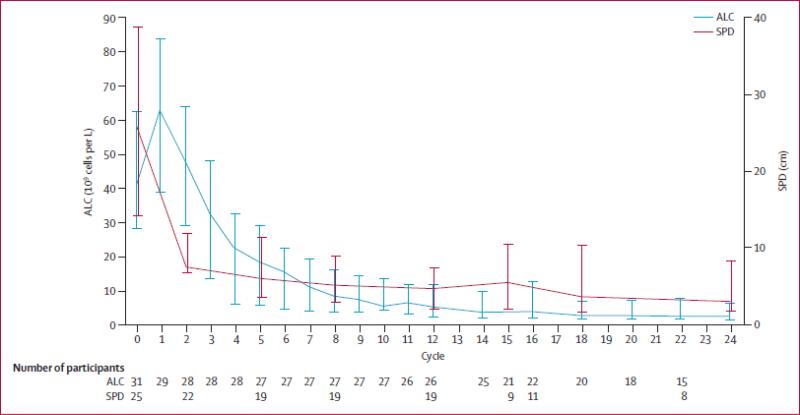
Ibrutinib causes transient, lymphocytosis during the first few weeks of therapy due to redistribution of CLL cells from the tissue to peripheral blood.[62] Normally CLL cells circulate in the peripheral blood, where they are attracted by chemokine gradients established by tissue stromal cells. Critical chemokines for lymph node homing are CXCL 12, CXCL 13, and CCL 19/21, which bind to CXCR4, CXCR5 and CCR7 chemokine receptors on CLL cells, respectively.[26] The CXCR4-CXCL 12 axis is the predominant one for marrow homing. CLL adhesion molecules (integrins, selectins, CD44) co-operate with chemokine receptors during tissue homing. Pharmacologic inhibition of these homing mechanisms by ibrutinib leads to exit of tissue CLL cells into blood, causing increase in lymphocytosis. The lymphocytosis resolves with continued therapy.[37,60] When ibrutinib was given intermittently, 21 days on and 7 days off each month, the absolute lymphocyte count (ALCs) dropped during off- drug period, and rose again when ibrutinib was restarted. The lymphocytosis occurs concomitantly with reduction in lymph node size (figure 2).[60,63] This transient lymphocytosis should not be confused with disease progression and should not lead to discontinuation of therapy.[51]Woyach et al [62] reported the molecular characterization of patients with persistent lymphocytosis beyond 12 months. Peripheral blood flow cytometry of cells from patients with persistent lymphocytosis did not show any change in kappa and lambda expression, immunoglobulin heavy chain variable region (IGHV) sequencing, or Zap-70 methylation from baseline to the time of evaluation. This demonstrates that the persistent lymphocytosis in patients on ibrutinib do not represent clonal evolution. Furthermore, the PFS was not different in patients with persistent lymphocytosis compare to those with a PR. A similar redistribution phenomenon in patients with CLL is also seen with the SYK inhibitor (fostamatinib)[45] and the PI3Kδ inhibitor (idelalisib).[64] This prompted CLL experts to re-define response guidelines in view of the therapy related lymphocytosis with these inhibitors.[51]
Figure 2.
Median absolute lymphocyte counts (ALC) and the sum of the products of lymph node diameters (SPD). (Reprinted from [63] with permission from Elsevier)
Ibrutinib has produced durable remission in patients with CLL, however resistance has occurred. A mutation in BTK and clonal evolution are two mechanisms identified as causes of resistance to ibrutinib.[65,66] Woyach et al [67] reported about mutations in the BTK binding site of ibrutinib in 6 patients who acquired resistance during ibrutinib therapy. Five patients had mutations in cysteine-to-serine in BTK at position 481(C481S), the sixth patient had an arginine to tryptophan mutation in PLCγ2 (a downstream kinase) at position 665 (R665W). Whether the second generation BTK inhibitors currently in clinical trials [68,69], will be active in the setting of these mutations is unclear but may be unlikely given they have the same binding site as ibrutinib. Another mechanism of resistance to ibrutinib is clonal evolution; Landau et al[66] presented data on three patients who didn't achieve an optimal response with ibrutinib; one patient acquired a new clonal mutation in SF3B1 (K666T), 2 other patients had clonal deletions in chromosome 8p. Further studies are needed to evaluate the mechanism of resistance in patients who progress after ibrutinib therapy.
4. Clinical Efficacy
4.1 Phase I and II studies
Advani et al [60] reported results from phase I clinical trial of ibrutinib in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), CLL, or Waldenström macroglobulinemia (WM). Patients received oral ibrutinib capsule once daily at 1.25, 2.5, 5, 8.3, or 12.5mg/kg/day on a 28 days on, 7 days off schedule (35 day cycle), or continuous daily dosing of 8.3mg/kg or 560mg until disease progression, intolerable toxicity, patient or investigator decision to discontinue ibrutinib. No dose limiting toxicity was observed. Full BTK site occupancy was achieved at 2.5mg/kg/day; the dose was escalated to 12.5mg/kg/day without reaching a maximum tolerated dose (MTD). Two cases of dose limiting toxicity (DLT) were observed, 1 case of grade 3 allergic hypersensitivity and 1 case of dose interruption for more than a week due to grade 2 neutropenia. Adverse events occurring in more than 10% of patients were diarrhea, nausea/vomiting, fatigue, rash, muscle spasms, cough and edema. Adverse events were mostly grade I-II and self-limiting. Grade 3 and grade 4 events were uncommon and not related to the dose. The ORR was 60%, 16% of patients had a CR. Response were observed in all B-cell malignancies. Of 16 patients with CLL, 11 had rapid reduction in lymphadenopathy during the first cycle of ibrutinib, along with transient increase in the ALC. Durable responses were achieved with a median progression free survival (PFS) of 13.6 months.
Byrd et al [70] conducted a phase Ib/II multicenter study, evaluating the safety and efficacy of ibrutinib in patients with relapsed or refractory CLL/SLL. Two fixed daily dose schedules of Ibrutinib, 420mg daily and 840mg daily were administered. The toxicities were mainly grade I or II. Adverse events occurring in ≥ 20% of patients included transient diarrhea (47%), upper respiratory tract infections (URTI) 33%, fatigue (28%), cough (31%), arthralgia (27%), rash (27%), pyrexia (27%) and peripheral edema (21%). The ORR was 71%; 2 CR and 34 PR were seen in the 420mg daily cohort and 24 PR were noted in the 840mg daily cohort. Ten patients (20%) in the 420mg cohort and 5 (15%) patients in the 840mg cohort achieved a PR with persistent lymphocytosis (PR-L). Responses were identical in the two cohorts. An increase in lymphocytosis was observed on day 7 of therapy and peaked at 4 weeks. In 79% (50/63) of patients, lymphocyte counts normalized or decreased by ≥ 50% from baseline. Interestingly, in patients with unmutated immunoglobulin genes, lymphocyte counts normalized more rapidly compared to patients with mutated immunoglobulin genes (median, 6.4 vs. 14.8 months). Responses were independent of the stage of disease, number of prior therapies and the presence of a del 17p13.1. The response rate among the patients with del 17p13.1 was 68% (1 CR) compared to 71% among those without the deletion. The responses observed were durable, at 26 months follow up; 75% of patients were progression free, and the overall survival (OS) was 83%. The rate of PFS among 28 patients with del 17p13.1at 26 months follow-up was 57% and the rate of OS was 70%.
Ibrutinib was also evaluated as initial therapy for older patients with CLL/SLL.[63] Patients 65 years or older with symptomatic CLL/SLL received 420mg of ibrutinib daily, in a 28 day cycle. Only one patient required dose modification for nausea (grade1), and was maintained at lower dose of 280mg daily. Two patients discontinued treatment due to grade 3 fatigue and grade 2 viral infection respectively. Symptomatic adverse effects were mainly grade 1-2. Adverse events that occurred in 10% or more of patients were similar to those seen in earlier studies including: diarrhea, nausea, fatigue, hypertension, arthralgia and edema. Four patients had grade 3 diarrhea and 1 patient had grade 4 thrombocytopenia. The overall response rate was 71% (22/31 patients). Four patients (13%) achieved a CR. The median PFS was not reached; one patient with a del 17p13.1 had an initial response and then progressed to Richter's transformation at 9.6 months.
The combination of ibrutinib and rituximab resulted in full occupancy of BTK at day 7 and 14 of treatment. In vivo, serial blood samples of patients with CLL treated with the rituximab and ibrutinib combination were tested to explore the effect of this combination in the migration of CLL cells.[71] The ibrutinib and rituximab combination showed significant reduction in CLL cell chemotaxis towards chemokines; CXCL12 and CXCL13.[71] Subsequently, a phase II study of ibrutinib in combination with rituximab in patients with high risk CLL (del 17p13.1 or TP53 mutation [treated or untreated]), PFS <36 months after frontline chemo-immunotherapy, or relapsed CLL with del 11q was conducted.[71] Patients received ibrutinib dose of 420 mg daily, in combination with weekly rituximab (375 mg/m2) for weeks 1–4 (cycle 1), then monthly rituximab until cycle 6, followed by continuous single-agent ibrutinib. The ORR was 95% (8% CR and 87% PR). One patient with CR became MRD negative by flow cytometry. The ORR in 20 patients with del 17p13.1 or TP53 mutation was 90% (2 CR and 16 PR). At 18-months PFS in all patients was 78% (95% CI 60·6–88·5), whereas in those with a del (17p) or TP53 mutation the PFS was 72·4% (95% CI 45·6–87·6). The treatment was well tolerated, 9 patients had infectious complication (6 cases of pneumonia and 3 URTI). One patient had grade 3 mucositis and one had grade 3 peripheral neuropathy.
The redistribution lymphocytosis peaked at lower levels and resolved more quickly in comparison to results seen with single agent ibrutinib. This combination showed promising early results in patients with high risk relapsed CLL.
Barrientos et al[72] described results from a phase Ib study of ibrutinib in combination with bendamustine and rituximab (BR) in patients with relapsed/refractory CLL. Patients received up to 6 cycles of BR with ibrutinib 420mg daily. Ibrutinib was continued after completion of BR. Bendamustine was administered at 70mg/m2 on day 1 and day 2 combined with rituximab 375mg/m2 on day 1 for cycle 1 and 500mg/m2 on day 1 of subsequent courses. The adverse events were those expected with BR. The most frequent treatment related adverse events were diarrhea, nausea, fatigue, neutropenia and upper respiratory tract infections. The ORR was 93% (18% CR and 14% PR). The responses were independent of high risk features. This regime is being further evaluated in a phase 3 trial (ClinicalTrials.gov, NCT01611090).
The good safety profile and pronounced efficacy of ibrutinib have opened the doors for combination studies with ibrutinib. Phase I and phase II studies of ibrutinib are summarized in table 1.
Table 1.
Phase I and Phase II studies of ibrutinib in B cell malignancies.
| Study phase | Phase I | Phase 1b/2 | Phase II | Phase II |
|---|---|---|---|---|
| Title | Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies[60] | Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia[70] | Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma[61] | The Btk Inhibitor Ibrutinib (PCI-32765) in Combination with Rituximab Is Well Tolerated and Displays Profound Activity in High-Risk Chronic Lymphocytic Leukemia (CLL) Patients[71] |
| Primary objective | Maximum tolerated dose (MTD) | Safety of two fixed doses of 420mg and 840mg daily | ORR | ORR |
| Secondary Objective | Adverse events (AE) and overall response rate (ORR) and progression free survival (PFS) | ORR, PFS, PK and PD | Response duration, PFS, OS and safety | Safety and efficacy |
| No of patients in the study | 56 | 85 | 111 | 40 |
| Results | MTD not reached. Most common AE; G1 and G2. ORR 60%. Median PFS 13.6 months | Median Tmax 2hrs, or the terminal half-life (7.8±3.4hrs with 420mg and 8.1±3.4 hrs with 840mg). Most AE G1 and G2. ORR 71%. ORR was similar in both dose regimen | ORR 68% (CR 21% and PR 47%). Median response duration was 17.5 months, median PFS 13.9 months. Median OS not reached | ORR 95% (8% CR and 87% PR). Treatment was well tolerated; 6 cases of PNA and 3 cases of URTI |
PK; pharmacokinetics, PD; pharmacodynamics, PNA; pneumonia and URTI; upper respiratory tract infections, Tmax; time to maximum plasma concentration, CR; complete remission and PR; partial remission.
4.2 Phase III studies
Two large intergroup studies are being conducted in previously untreated patients with CLL; ibrutinib and rituximab combination compared with FCR in patients with untreated CLL requiring treatment (ClinicalTrials.gov, NCT02048813). The primary end point is PFS; secondary end points include OS and safety. Another phase III study is underway for older patients with CLL; this is the rituximab and bendamustine combination compared to ibrutinib and rituximab or ibrutinib alone in previously, untreated patients with CLL (ClinicalTrials.gov, NCT01886872).
Ibrutinib monotherapy is also being compared with monoclonal antibodies and alkylating agents in phase III studies for relapsed/refractory CLL and in an older treatment naive population with CLL. These include a phase 3 study of Ibrutinib versus ofatumumab in patients with relapsed or refractory CLL.[73] The primary end point was PFS and secondary endpoints were OS and ORR. The median duration of PFS was not reached in the ibrutinib group (PFS 88% at 6 months) compared to 8.1 months in ofatumumab group (hazard ratio (HR) for progression or death in the ibrutinib group, 0.22; P<0.001). The rate of OS at 12 months was 90% in the ibrutinib group compare to 81% in ofatumumab group. The ORR with ibrutinib was 42.6% compare to 4.1% with ofatumumab (p= <0.001). In high risk patients with a del 17p13.1, the median PFS was not reached in the ibrutinib group compared to 5.8 months with ofatumumab (HR for progression or death, 0.25; 95% CI, 0.14 to 0.45).
Ibrutinib has also moved into phase III clinical trials in MCL. One of them is a randomized, double-blind, placebo controlled study of ibrutinib with bendamustine and rituximab (BR) in patient 65 years or older with newly diagnosed MCL (ClinicalTrials.gov, NCT01776840).
Phase III clinical trials are also being conducted in previously treated indolent non-Hodgkin lymphoma (iNHL) comparing ibrutinib in combination with BR or rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (ClinicalTrials.gov, NCT01974440).
5. Safety & tolerability; safety outcomes in clinical trial
Ibrutinib is well tolerated; the most common side effects are grade 1-2 diarrhea, fatigue, upper respiratory tract infection, rash and edema. These are transient and often not requiring therapeutic intervention or discontinuation of therapy.[60,61,63,70] In patients with persistent diarrhea, anti-motility agents like loperamide hydrochloride, can be useful. Most grade 3-4 toxicities were infectious, such as pneumonia.[61,70] In the phase 2 study [70] 2 patients (4%) in the 420mg cohort and 4 patients (12%) in the 840mg cohort discontinued treatment due to adverse events. In this study infectious complications were more common in the first 6 months of therapy and reduced by half after 6 months of therapy. The average rate of infection was 7.1 per 100 patient-months during the first 6 months and 2.6 per patients-months after 6 months of therapy. Treatment delays or discontinuation due to side effects of ibrutinib were infrequent. IgG and IgM levels remained stable throughout ibrutinib treatment, and IgA levels increased at 3, 6 and 12 months of therapy.[61,70] Overall the rate of grade 3-4 infections in patients with relapsed CLL treated with ibrutinib was around 40% [70], when ibrutinib was given as frontline therapy in older patients with CLL the rate of infectious complication was approximately 10%[63]. This suggests that the infectious complications observed in the studies are due to the immunocompromised state of the patients rather than solely ibrutinib therapy.
Ibrutinib is also well tolerated in older patients. In a phase 1b/2 multicenter study of ibrutinib as initial therapy in patients with CLL/SLL ≥ 65 years old, adverse events were often self-limited and didn't require discontinuation of therapy.[63] The most common adverse event was diarrhea which occurred in 68% of patients (all grades). Four patients (13%) had grade 3 diarrhea, none of the patients had grade 4 diarrhea. Adverse events observed in ≥ 20% of patients were hypertension, fatigue, nausea, edema and URTI.
Bleeding is another adverse event noted in ibrutinib studies. In a phase II study of ibrutinib in relapsed or refractory patients with MCL, four patients had a subdural hematoma.[61] All the patients had a history of trauma and were on warfarin. Only one patient had a low platelet count. Warfarin was excluded in subsequent clinical trials of ibrutinib. Only one more case of subdural hematoma was reported in the phase II study of ibrutinib in combination with rituximab in patients with high risk CLL.[71] Apart from B cell receptor signaling, BTK is also involved in the signaling of the glycoprotein (GP) VI and GPVI von Willebrand (vW) receptor.[74] Ibrutinib might increase the risk of bleeding by interfering in thrombus formation. Farooqui et al[74] evaluated platelet function in patients receiving 420mg of ibrutinib daily;16% of patients had abnormalities in some platelet function tests but none of these patient developed ecchymosis or bleeding. Further studies are needed to determine the possibility of an association of bleeding with ibrutinib therapy.
In contrast to conventional chemo-immunotherapy, ibrutinib doesn't cause myelosuppression. In fact, in patients with disease related cytopenias, ibrutinib led to significant improvement in hematopoiesis.[70,71,74] Improvement in thrombocytopenia was observed in 32/41 (78%) patients, anemia in 27/33 (82%) patients and neutropenia in 24/31 (77%) patients in a phase 1b/2 study of ibrutinib in relapsed/refractory CLL.[70]
6. Characteristics of drug with competitors and FDA approval
6.1 Characteristics of drug with competitors
Characteristics of ibrutinib with competitor compounds are summarized in table 3.
Table 3.
Comparing the characteristics of drug with competitors
| Name of Drug | Mechanism of action | Adverse events | Efficacy | Phase |
|---|---|---|---|---|
| Ibrutinib[70] | Irreversible BTK inhibitor | Diarrhea, fatigue, URTI and Pneumonia | ORR 71% (CR+PR) in relapsed ant treatment naïve patients with CLL | Approved for relapse/refractory CLL |
| ONO-4059[77] | 2nd generation irreversible BTK inhibitor | Diarrhea, skin rash, febrile neutropenia | ORR 70% (PR; 29%, PRL; 71%) in relapsed refractory CLL patients | Phase 1/ 2 clinical trial |
| CC-292[68] | 2nd generation irreversible BTK inhibitor | Diarrhea, fatigue, neutropenia, thrombocytopenia, headache, and URTIs | PR 40% and nodal response 64.5%. | Phase I clinical trial |
| Idelalisib[50] | PI3K-delta inhibitor | Diarrhea, fatigue, neutropenia, transaminases, pneumonitis and lymphocytic colitis. | ORR 72% and PFS 15.8 months in relapsed CLL, PFS improved in those treated at doses of ≥ 150 mg BID | Approved for relapsed CLL, in combination with rituximab, for whom rituximab alone would be considered appropriate therapy due to other co-morbidities |
| AMG 319[78] | PI3K-delta inhibitor | Colitis, hemolysis, anemia, infection and leucocytosis | PR 7%, 75% had nodal response | Phase 1 trial in relapsed or refractory lymphoid malignancies |
| Fostamatinib[45] | Spleen tyrosine kinase inhibitor | Diarrhea, fatigue, cytopenia and hypertension | ORR 22% and PFS 4.2 months in relapse/refractory B-NHL | Phase 1/ 2 clinical trial |
| ABT-199[53] | Bcl-2 inhibitor | Diarrhea, neutropenia, fatigue, URTIs and tumor lysis syndrome | ORR 84% (CR; 21% +PR; 63%) in relapsed refractory patients with NHL | Phase 1/ 2 clinical trial |
6.2 Countries where Ibrutinib is approved
Ibrutinib is approved in the USA as second line therapy in CLL and mantle cell lymphoma. The drug is not yet approved in Europe for CLL/ B cell lymphoma; however several pivotal trials are underway for approval in Europe.
7. Expert commentary
Improvement the drug has over current available therapies
FCR is the frontline standard of care for fit patients with CLL requiring treatment.[4] In a phase II trial FCR produced an ORR of 95% in patients with untreated CLL.[5] When FCR was compared to FC in a randomized control trial, FCR produced an ORR of 90% and a CR rate of 44%. The CR rate seen with FCR was doubled that observed with FC chemotherapy, 44% and 22% respectively.[6] The treatment for relapsed CLL is less standardized; repeated therapy with FCR or the use of BR is commonly employed in the relapse setting.[10,11] In the phase II trial of FCR in patients with relapsed CLL the ORR was 74%, with 30% CR.[9] The estimated median OS was 47 months and median PFS was 21 months. Bendamustine and rituximab as a salvage regimen in CLL showed an ORR of 59% and a CR rate of 9% in patients with a median of two prior therapies.[10] Once a patient is refractory to chemo-immunotherapy, options are limited and include ofatumumab and alemtuzumab.[14,15,17,75] In patients who are refractory to fludarabine or alemtuzumab, ofatumumab showed an ORR of 50%.[16] Responses observed with ofatumumab in patients with relapsed CLL were impressive but lasted only 6 months and patients progressed after stopping treatment. Lenalidomide has activity in CLL but is not FDA approved for this disease. In patients with relapsed CLL, ibrutinib showed an ORR of 71% with an additional 20% and 15% who achieve PR with lymphocytosis in two groups receiving 420mg and 840mg of ibrutinib respectively.[70] The median PFS was not reached and at 26 months the PFS was 75% and the OS was 83%.[70] In comparison, the median PFS was 5.7months and OS 13.7 months with single agent ofatumumab in patients with fludarabine refractory CLL.[16] In patients with relapsed CLL, ibrutinib has shown better responses than with other available therapies with an excellent safety profile. The enthusiasm generated by B-cell receptor inhibitors has led to consideration of incorporating them into frontline therapies for CLL.
Patients with CLL older than 70 years have less tolerance to fludarabine-based therapy, increased toxicity, and are often unable to complete 6 cycles.[5] Ibrutinib has emerged as a novel therapy with good tolerance, and little toxicity. In fact, most patients have shown improvement in cytopenias with Ibrutinib.[74] In the phase 1b/2 trial of Ibrutinib as initial therapy in older patients with CLL/SLL, only 2 patients required treatment discontinuation due to adverse events.[63] At the median follow up of 22.1 months, the ORR was 71%; 13% CR, 55% PR and 3% (1 patient) achieved nodular PR. Ibrutinib does not have a label yet as frontline therapy in older patients with CLL but an ongoing randomized trial of ibrutinib versus chlorambucil in patients 65 years or older with treatment naïve CLL will likely lead to a label in that group. In conclusion, ibrutinib has shown better results with less toxicity than all other treatment options in patients with relapsed CLL.
Traditionally allogeneic stem cell transplant is a therapeutic option for patients with a p53 abnormality, in patients who fail to achieve CR, or are refractory to fludarabine. Now with the advent of highly effective BCR inhibitors with excellent safety profiles, the therapeutic options have increased in patients with relapsed or refractory CLL. Even in high risk patients including those with del 17p13.1, the median PFS with ibrutinib was far better than that seen with chemoimmunotherapy or single agent monoclonal antibodies. However, in relapsed patients with CLL and a del 17p13.1 the median PFS with ibrutinib was 28 months. Since these are not long term remissions the use of allogeneic stem cell transplant is still a strong consideration in that group.
Five-year view
Ibrutinib has a favorable therapeutic index with little myelosuppression, making it easy to use in combination with other agents for the treatment of CLL. The combination of Ibrutinib with rituximab in patients with relapsed CLL has led to a high response rate (95%; 87% PR and 8% CR) and earlier resolution of lymphocytosis compared to Ibrutinib alone.[71] The ORR in 20 patients with chromosome 17 abnormalities was 90%; 80% PR and 10%CR. Treatment in combination with chemotherapy may provide higher response rates than ibrutinib alone but will not leverage the highly favorable safety profile seen with single agent ibrutinib. Combination of different TKIs involved in BCR signaling is of interest. Pre-clinical data combining idelalisib and fostamatinib in primary peripheral blood and bone marrow CLL samples have shown reduced CLL cell survival, synergistic growth inhibition, and disruption of chemokine signaling at nanomolar concentration, even in high risk CLL cells.[76] This has not been evaluated in clinical trials.
The risk of disease transformation, mechanism of resistance, efficacy of second generation BTK inhibitors compare to ibrutinib, as well as long term side effects still needs to be explored.
Table 2.
Ongoing phase III clinical trials of ibrutinib in B cell malignancies
| ClinicalTrials.gov identifier | Title | Study phase | Primary objectives | Secondary objectives | Status/ Results |
|---|---|---|---|---|---|
| NCT01886872 | A Randomized Phase III Study of Bendamustine Plus Rituximab versus Ibrutinib Plus Rituximab versus Ibrutinib Alone in Untreated Older Patients (≥ 65 Years of Age) With Chronic Lymphocytic Leukemia (CLL) | 3 | PFS | OS, Time to progression (TTP), duration of response. | Study ongoing. |
| NCT02048813 | Ibrutinib and Rituximab Compared With Fludarabine Phosphate, Cyclophosphamide, and Rituximab in Treating Patients with Untreated CLL or SLL | 3 | PFS | OS, Incidence of toxicity, impact of CLL on QOL. | Study ongoing. |
| NCT01578707 | A Phase 3 Study of Ibrutinib (PCI-32765) Versus Ofatumumab in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (RESONATE™)[73] | 3 | PFS | OS, hematological improvement and improvement of disease related symptoms | Median PFS not reached. PFS 88% at 6 mths compared to 8.1 mths in Ofatumumab. At 12 mths OS 90% in ibrutinib and 81% in Ofatumumab. |
| NCT01722487 | A Multicenter, Open-label, Phase 3 Study of the BTK Inhibitor Versus Chlorambucil in Patients 65 Years or Older with Treatment-naive CLL or SLL | 3 | PFS | Safety, ORR, CR with MRD negativity | Study ongoing, but not recruiting participants. |
| NCT01776840 | A Randomized, Double-blind, Placebo-controlled Phase 3 Study of the Ibrutinib, in Combination With Bendamustine and Rituximab (BR) in Subjects With Newly Diagnosed Mantle Cell Lymphoma | 3 | PFS | ORR, OS, MRD negative and duration of response. | Study ongoing. |
| NCT01974440 | A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study of the Ibrutinib, in Combination with Either Bendamustine and Rituximab (BR) or R-CHOP in Subjects with Previously Treated iNHL | 3 | PFS | OS, CR rate, ORR and duration of response. | Study ongoing. |
SLL; small lymphocytic lymphoma, iNHL; indolent non-Hodgkin lymphoma, QOL; quality of life, PFS; progression free survival, OS; overall survival, ORR; overall response rate, CR; complete remission.
8. Key issues.
B-cell receptor (BCR) signaling plays a vital role in the pathogenesis of B-cell malignancies.
Numerous kinases in the BCR signaling pathway are being explored as therapeutic targets; PI3K, SYK, Lyn and BTK.
Ibrutinib is a selective, irreversible, oral BTK inhibitor.
Ibrutinib produces high response rate in patients with relapsed/refractory CLL, including those with a 17p13.1 del.
Ibrutinib has a good safety profile; the majority adverse effects are grade1-2 and don't require discontinuation of the drug.
In phase I/II ibrutinib studies occasionally patients developed subdural hematomas and other serious bleeding episodes. Although there were multiple risk factors contributing to the incidence of subdural hematoma, use of warfarin excluded patients from subsequent ibrutinib trials and the package insert recommends against the use of ibrutinib in patients on warfarin. Further investigations on the risk of bleeding need to be performed.
In patients with CLL, ibrutinib induces transient lymphocytosis which may persist for months during therapy; this should not be mistaken for disease progression.
In contrast to chemo-immunotherapy, Ibrutinib does not cause myelosuppression. In fact, most patients will have improvement in cytopenias with ibrutinib.
The combination of Ibrutinib with monoclonal antibodies has shown favorable early results.
Mutation in BTK and clonal evolution are identified as possible causes of ibrutinib resistance in patients who progress on ibrutinib therapy.
Footnotes
Financial and competing interests disclosure
S O'Brien has received research support from Pharmacyclics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
* Of interest
** Of considerable interest
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ghielmini M, Vitolo U, Kimby E, et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(3):561–576. doi: 10.1093/annonc/mds517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rozman C, Montserrat E. Chronic lymphocytic leukemia. The New England journal of medicine. 1995;333(16):1052–1057. doi: 10.1056/NEJM199510193331606. [DOI] [PubMed] [Google Scholar]
- 4.Hallek M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. American journal of hematology. 2013;88(9):803–816. doi: 10.1002/ajh.23491. [DOI] [PubMed] [Google Scholar]
- 5.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(18):4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 7.Fischer K, Bahlo J, Fink A-M, et al. Extended Follow up of the CLL8 Protocol, a Randomized Phase-III Trial of the German CLL Study Group (GCLLSG) Comparing Fludarabine and Cyclophosphamide (FC) to FC Plus Rituximab (FCR) for Previously Untreated Patients with Chronic Lymphocytic Leukemia (CLL): Results On Survival, Progression-Free Survival, Delayed Neutropenias and Secondary Malignancies Confirm Superiority of the FCR Regimen. ASH Annual Meeting Abstracts. 2012;120(21):435. [Google Scholar]
- 8.Robak T, Dmoszynska A, Solal-Celigny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(10):1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 9.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117(11):3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(26):3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 11.Visco C, Finotto S, Pomponi F, et al. The combination of rituximab, bendamustine, and cytarabine for heavily pretreated relapsed/refractory cytogenetically high-risk patients with chronic lymphocytic leukemia. American journal of hematology. 2013;88(4):289–293. doi: 10.1002/ajh.23391. [DOI] [PubMed] [Google Scholar]
- 12.Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118(13):3489–3498. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badoux XC, Keating MJ, Wen S, et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(5):584–591. doi: 10.1200/JCO.2012.42.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Osterborg A. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118(19):5126–5129. doi: 10.1182/blood-2011-04-348656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wierda WG, Kipps TJ, Durig J, et al. Chemoimmunotherapy with O-FC in previously untreated patients with chronic lymphocytic leukemia. Blood. 2011;117(24):6450–6458. doi: 10.1182/blood-2010-12-323980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(10):1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elter T, Gercheva-Kyuchukova L, Pylylpenko H, et al. Fludarabine plus alemtuzumab versus fludarabine alone in patients with previously treated chronic lymphocytic leukaemia: a randomised phase 3 trial. The Lancet. Oncology. 2011;12(13):1204–1213. doi: 10.1016/S1470-2045(11)70242-X. [DOI] [PubMed] [Google Scholar]
- 18.Fiegl M, Stauder R, Steurer M, et al. Alemtuzumab in chronic lymphocytic leukemia: final results of a large observational multicenter study in mostly pretreated patients. Ann Hematol. 2014;93(2):267–277. doi: 10.1007/s00277-013-1966-z. [DOI] [PubMed] [Google Scholar]
- 19.List A. Lenalidomide--a transforming therapeutic agent in myelodysplastic syndromes. Clinical lymphoma & myeloma. 2009;9(Suppl 3):S302–304. doi: 10.3816/CLM.2009.s.028. [DOI] [PubMed] [Google Scholar]
- 20.Jasielec JK, Jakubowiak AJ. Current approaches to the initial treatment of symptomatic multiple myeloma. International journal of hematologic oncology. 2013;2(1) doi: 10.2217/ijh.13.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strati P, Keating MJ, Wierda WG, et al. Lenalidomide induces long-lasting responses in elderly patients with chronic lymphocytic leukemia. Blood. 2013;122(5):734–737. doi: 10.1182/blood-2013-04-495341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CI, Bergsagel PL, Paul H, et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(9):1175–1181. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chanan-Khan A, Miller KC, Lawrence D, et al. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukemia predicts clinical response. Cancer. 2011;117(10):2127–2135. doi: 10.1002/cncr.25748. [DOI] [PubMed] [Google Scholar]
- 25.Badoux X, O'Brien S, Wierda WG, et al. Combination of Ofatumumab and Lenalidomide In Patients with Relapsed Chronic Lymphocytic Leukemia: Initial Results of a Phase II Trial. ASH Annual Meeting Abstracts. 2010;116(21):2464. [Google Scholar]
- 26.Burger JA. Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:96–103. doi: 10.1182/asheducation-2011.1.96. [DOI] [PubMed] [Google Scholar]
- 27.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34(12):592–601. doi: 10.1016/j.it.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woyach JA, Bojnik E, Ruppert AS, et al. Bruton's tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123(8):1207–1213. doi: 10.1182/blood-2013-07-515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones JA, Byrd JC. How will B-cell-receptor-targeted therapies change future CLL therapy? Blood. 2014;123(10):1455–1460. doi: 10.1182/blood-2013-09-453092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120(24):4684–4691. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120(6):1175–1184. doi: 10.1182/blood-2012-02-362624. [This study suggested potential target; Bruton tyrosine kinase (BTK), for BTK inhibitors development.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satterthwaite AB, Witte ON. The role of Bruton's tyrosine kinase in B-cell development and function: a genetic perspective. Immunol Rev. 2000;175:120–127. [PubMed] [Google Scholar]
- 34.Khan WN. Regulation of B lymphocyte development and activation by Bruton's tyrosine kinase. Immunol Res. 2001;23(2-3):147–156. doi: 10.1385/IR:23:2-3:147. [DOI] [PubMed] [Google Scholar]
- 35.de Gorter DJ, Beuling EA, Kersseboom R, et al. Bruton's tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26(1):93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood. 2013;121(9):1501–1509. doi: 10.1182/blood-2012-08-452607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ten Hacken E, Scielzo C, Bertilaccio MT, et al. Targeting the LYN/HS1 signaling axis in chronic lymphocytic leukemia. Blood. 2013;121(12):2264–2273. doi: 10.1182/blood-2012-09-457119. [DOI] [PubMed] [Google Scholar]
- 39.Wang YH, Fan L, Wang L, et al. Expression levels of Lyn, Syk, PLCgamma2 and ERK in patients with chronic lymphocytic leukemia, and higher levels of Lyn are associated with a shorter treatment-free survival. Leukemia & lymphoma. 2013;54(6):1165–1170. doi: 10.3109/10428194.2012.736983. [DOI] [PubMed] [Google Scholar]
- 40.Suljagic M, Longo PG, Bennardo S, et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Emu- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 2010;116(23):4894–4905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 41.Hoellenriegel J, Coffey GP, Sinha U, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia. 2012;26(7):1576–1583. doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrd JC, Woyach JA, Johnson AJ. Translating PI3K-Delta Inhibitors to the Clinic in Chronic Lymphocytic Leukemia: The Story of CAL-101 (GS1101). Am Soc Clin Oncol Educ Book. 2012;32:691–694. doi: 10.14694/EdBook_AM.2012.32.75. [DOI] [PubMed] [Google Scholar]
- 43.Burger JA, Hoellenriegel J. Phosphoinositide 3′-kinase delta: turning off BCR signaling in Chronic Lymphocytic Leukemia. Oncotarget. 2011;2(10):737–738. doi: 10.18632/oncotarget.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang JE, Kahl BS. PI3-Kinase Inhibitors in Chronic Lymphocytic Leukemia. Current hematologic malignancy reports. 2014;9(1):33–43. doi: 10.1007/s11899-013-0189-7. [DOI] [PubMed] [Google Scholar]
- 45.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SR, Speranza G, Piekarz R, et al. A multi-histology trial of fostamatinib in patients with advanced colorectal, non-small cell lung, head and neck, thyroid, and renal cell carcinomas, and pheochromocytomas. Cancer chemotherapy and pharmacology. 2013;71(4):981–990. doi: 10.1007/s00280-013-2091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genovese MC, Kavanaugh A, Weinblatt ME, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis and rheumatism. 2011;63(2):337–345. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- 48*.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [This study proven to be a pivotal trial for approval of idelalisib combination with rituximab in USA for patients with relapsed or refractroy CLL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(23):2820–2822. doi: 10.1200/JCO.2012.43.3748. [This was important study, identifying need to redefine response criteria in patients with BTK inhibitor associated lymphocytosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharman J, de Vos S, Leonard JP, et al. A Phase 1 Study of the Selective Phosphatidylinositol 3-Kinase-Delta (PI3K{delta}) Inhibitor, CAL-101 (GS-1101), in Combination with Rituximab and/or Bendamustine in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL). ASH Annual Meeting Abstracts. 2011;118(21):1787. [Google Scholar]
- 53.Davids MS, Pagel JM, Kahl BS, et al. Bcl-2 Inhibitor ABT-199 (GDC-0199) Monotherapy Shows Anti-Tumor Activity Including Complete Remissions In High-Risk Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL) and Small Lymphocytic Lymphoma (SLL). Blood. 2013;122(21):872. [Google Scholar]
- 54.Seymour JF, Gerecitano JF, Kahl BS, et al. The Single-Agent Bcl-2 Inhibitor ABT-199 (GDC-0199) In Patients With Relapsed/Refractory (R/R) Non-Hodgkin Lymphoma (NHL): Responses Observed In All Mantle Cell Lymphoma (MCL) Patients. Blood. 2013;122(21):1789. [Google Scholar]
- 55.Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. The New England journal of medicine. 2014;370(12):1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 57.Singh J, Petter RC, Kluge AF. Targeted covalent drugs of the kinase family. Curr Opin Chem Biol. 2010;14(4):475–480. doi: 10.1016/j.cbpa.2010.06.168. [DOI] [PubMed] [Google Scholar]
- 58.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60**.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(1):88–94. doi: 10.1200/JCO.2012.42.7906. [This is the initial phase I study, showing safety and efficacy of ibrutinib in patients with relapsed or refractory CLL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. The New England journal of medicine. 2013;369(6):507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–1817. doi: 10.1182/blood-2013-09-527853. [This trial suggested that the persistent lymphocytosis did not represent clonal evolution and PFS was not different in patients with persistent lymphocytosis compare to those with a response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.O'Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. The Lancet. Oncology. 2014;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [This is the initial phase Ib/II trial which showed safety and efficacy of ibruitnib in elderly patients with CLL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furman RR, Cheng S, Lu P, et al. A Novel Mutation In Bruton Tyrosine Kinase Confers Acquired Resistance To Ibrutinib (PCI-32765) In CLL. Blood. 2013;122(21):4914. [Google Scholar]
- 66.Landau D, Hoellenriegel J, Sougnez C, et al. Clonal Evolution In Patients With Chronic Lymphocytic Leukemia (CLL) Developing Resistance To BTK Inhibition. Blood. 2013;122(21):866. [Google Scholar]
- 67*.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. The New England journal of medicine. 2014;370(24):2286–2294. doi: 10.1056/NEJMoa1400029. [This an improtant study identifying mechanism to resistance to ibrutinib therapy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harb WA, Hill BT, Gabrilove J, et al. Phase 1 Study Of Single Agent CC-292, a Highly Selective Bruton's Tyrosine Kinase (BTK) Inhibitor, In Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL). ASH Annual Meeting Abstracts. 2013;122(21):1630–1630. [Google Scholar]
- 69.Karlin L, Rule S, Shah N, et al. A Phase I Study Of The Oral Btk Inhibitor ONO-4059 In Patients With Relapsed/Refractory and High Risk Chronic Lymphocytic Leukaemia (CLL). ASH Annual Meeting Abstracts. 2013;122(21):676–676. [Google Scholar]
- 70.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. The Lancet. Oncology. 2014;15(10):1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [This is important study showing better responses with ibrutinib and rituximab combination compare to ibrutinib alone with good safety profile.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrientos JC, Barr PM, Flinn I, et al. Ibrutinib In Combination With Bendamustine and Rituximab Is Active and Tolerable In Patients With Relapsed/Refractory CLL/SLL: Final Results Of a Phase 1b Study. ASH Annual Meeting Abstracts. 2013;122(21):525–525. [Google Scholar]
- 73.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. The New England journal of medicine. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farooqui M, Lozier JN, Valdez J, et al. Ibrutinib (PCI 32765) Rapidly Improves Platelet Counts in Chronic Lymphocytic Leukemia / Small Lymphocytic Lymphoma (CLL/SLL) Patients and Has Minimal Effects On Platelet Aggregation. ASH Annual Meeting Abstracts. 2012;120(21):1789. [Google Scholar]
- 75.Gilbert JA. Lenalidomide as first-line therapy for elderly CLL patients. The Lancet. Oncology. 2013;14(9):e345. doi: 10.1016/s1470-2045(13)70330-9. [DOI] [PubMed] [Google Scholar]
- 76.Burke RT, Meadows S, Loriaux MM, et al. A potential therapeutic strategy for chronic lymphocytic leukemia by combining Idelalisib and GS-9973, a novel spleen tyrosine kinase (Syk) inhibitor. Oncotarget. 2014;5(4):908–915. doi: 10.18632/oncotarget.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karlin L, Rule S, Shah N. A Phase I Study Of The Oral Btk Inhibitor ONO-4059 In Patients With Relapsed/Refractory and High Risk Chronic Lymphocytic Leukaemia (CLL) 2013;122(21):676–676. [Google Scholar]
- 78.Glenn M, Mato AR, Allgood SD, et al. First-In-Human Study Of AMG 319, a Highly Selective, Small Molecule Inhibitor Of PI3Kδ, In Adult Patients With Relapsed Or Refractory Lymphoid Malignancies. ASH Annual Meeting Abstracts. 2013;122(21):678–678. [Google Scholar]