Abstract
Salmonellosis is a major health problem worldwide. Salmonella enterica serovar Enteritidis (S. Enteritidis) has been a primary cause of Salmonella outbreaks in many countries. AvrA is an SPI-1 effector protein involved in the enteritis pathway, with critical roles in inhibiting inflammation and apoptosis. In this work, we constructed an AvrA-FLAG-tagged strain of S. Enteritidis to analyse the expression profile of AvrA in vitro, in cell culture and in vivo. AvrA expression and secretion were observed in vitro under culture conditions that mimicked intestinal and intracellular environments. In agreement, bacteria isolated from infected cell monolayers expressed and translocated AvrA for at least 24 h post-inoculation. For in vivo experiments, BALB/c mice were inoculated by the natural route of infection with the AvrA-FLAG strain. Infecting bacteria and infected cells were recovered from mesenteric lymph nodes (MLN). Our results showed that AvrA continues to be synthesized in vivo up to day 8 post-inoculation. Moreover, AvrA translocation was detected in the cytosol of cells isolated from MLN 8 days after infection. Interestingly, we observed that AvrA is secreted by both type three secretion system (T3SS)-1 and T3SS-2. In summary, these findings indicate that AvrA expression is not constrained to the initial host–bacteria encounter in the intestinal environment as defined previously. The AvrA effector may participate also in systemic S. Enteritidis infection.
Introduction
Salmonellosis is considered one of the most widespread foodborne diseases in the world (Bollaerts et al., 2008), and Salmonella enterica serovar Enteritidis (S. Enteritidis) is the main serotype responsible for human infections (Oliveira et al., 2006; Moore et al., 2007; Pang et al., 2007). In Argentina, for instance, the proportion of salmonellosis cases attributed to this pathogen showed a 275-fold increase in the last decades (Hogue et al., 1997; Rodrigue et al., 1990; Morales & McDowell, 1999). Infection with S. enterica occurs mainly through the consumption of contaminated food; in particular, chicken eggs are considered a major source of infection (Perales & Audicana, 1988; Bäumler et al., 2000; Hald et al., 2004). The estimated number of human infections is greater than 93.8 million cases, with 155 000 deaths per year worldwide (Boyle et al., 2007; Majowicz et al., 2010; Hendriksen et al., 2011). S. Enteritidis infection is generally confined to the intestinal mucosa and is usually self-limited, though systemic dissemination may occur in immunocompromised persons. Moreover, it is important to note that in developed countries up to 5 % of non-typhoidal Salmonella cases may be an invasive, extra-intestinal disease leading to bacteraemia and focal systemic infections.
Salmonella use a molecular ‘needle’, referred to as a type three secretion system (T3SS), to inject bacterial effector proteins into host cells. Stimulation of inflammation by effectors is crucial for Salmonella growth within the intestine (Stecher et al., 2007). Effectors, such as SipA, SopE and SopB, are known to activate inflammation in host cells (Galyov et al., 1997; Ehrbar et al., 2003; Hapfelmeier et al., 2004; Bruno et al., 2009; Broberg & Orth, 2010). Uncontrolled inflammation is harmful to the host and eventually damages the niche occupied by Salmonella during infection. However, Salmonella secreted SspH 1, SptP and AvrA reverse the activation of signalling pathways induced by inflammatory Salmonella effectors (Ye et al., 2007; McGhie et al., 2009; Sun, 2009; Wu et al., 2010). AvrA is a multiple-function protein that plays a critical role in inhibiting inflammation, regulating epithelial apoptosis and enhancing proliferation during bacterial infections in cell culture models and acutely infected mice (Ye et al., 2007; Jones et al., 2008; Liao et al., 2008; Du & Galán, 2009; Wu et al., 2012). In this regard, it has been suggested that AvrA is primarily involved in the enteritis pathway (Lawley et al., 2006).
Most S. enterica strains (approximately 80 %) contain the avrA gene; only some serovars such as S. enterica serovar Typhi (S. Typhi) and S. enterica serovar Paratyphi (S. Paratyphi) do not possess it. Some authors linked the absence of AvrA to the ability of these serovars to evade epithelial defences resulting in severe systemic disease (within macrophages) (Hardt & Galán, 1997; Prager et al., 2000, 2003).
The modulation of gene expression in SPI-1 is remarkably complex and needs further characterization (Waterman & Holden, 2003; Ellermeier & Slauch, 2007). For example, in contrast to the current model of SPI-mediated pathogenesis, SPI-1 proteins SipA, SopA, SopB, SopD and SopE2 were found to be expressed by Salmonella in infected animals at the late stages of infection (Giacomodonato et al., 2007). These results suggest that in addition to its generally recognized function in invasion, the SPI-1 factors may play an important role during systemic infection. Extensive studies have been carried out to investigate the expression of SPI-1 under different conditions in vitro (Löber et al., 2006; Ellermeier & Slauch, 2007); however, little is known about the expression of Salmonella effectors in vivo, especially during the established phase of infection. In this study, we used an S. Enteritidis strain to study the expression profile of AvrA during late stages of murine salmonellosis. We demonstrated that AvrA effector can be expressed and translocated during early and late stages of systemic infection.
Methods
Bacterial strains.
This work was carried out using strains of S. Enteritidis derived from strain SS218 (an isolate from poultry collected from an Argentine farm) and tagged with the 8 aa FLAG epitope tag peptide. S. Enteritidis strains SE1702 (avrA : : 3×FLAG cat : : FLAG), SE1703 (sipA : : 3×FLAG cat : : FLAG) and SE1704 (sseJ : : 3×FLAG cat : : FLAG) were obtained using the method described by Uzzau et al. (2001). 3×FLAG epitope tails were added to the ends of the avrA, sipA and sseJ genes. The 3×FLAG epitope is a sequence of three tandem FLAG epitopes (22 aa). A pair of primers was designed to amplify a 3×FLAG and KmR coding sequence using plasmid pSUB11 (Uzzau et al., 2001). The 3′ ends of these oligonucleotides were complementary to the first 20 nt of the pSUB11 3×FLAG coding region (GACTACAAAGACCATGACGG, forward primers) and to the 20 nt of the pSUB11 priming site 2 (CATATGAATATCCTCCTTAG, reverse primers). The 5′ ends of the oligonucleotides were designed to be homologous to the last 40 nt of each tagged gene, not including the stop codon (forward primers), and to the 40 nt immediately downstream of the gene stop codon (reverse primers). Mutants invG : : aphT (KmR) avrA : : 3×FLAG, invG : : aphT (KmR) sseJ : : 3×FLAG, invG : : aphT (KmR) sipA : : 3×FLAG, ssaK : : aphT (KmR) avrA : : 3×FLAG, ssaK : : aphT (KmR) sseJ : : 3×FLAG and ssaK : : aphT (KmR) sipA : : 3×FLAG were constructed by phage P22-mediated transduction from invG : : aphT (KmR) and ssaK : : aphT (KmR) [previously obtained in our laboratory as described by Datsenko & Wanner (2000)] strains of S. Enteritidis to strains SE1702 (avrA : : 3×FLAG cat : : FLAG), SE1703 (sipA : : 3×FLAG cat : : FLAG) and SE1704 (sseJ : : 3×FLAG cat : : FLAG) mutants. Gene deletion was verified by PCR.
Culture conditions.
For in vitro studies, bacteria were grown to exponential phase under different culture conditions. To mimic the intestinal environment (Miki et al., 2004) bacteria were grown at 37 °C without aeration in a Luria–Bertani (LB) broth containing 0.3 M NaCl. An intracellular milieu was recreated by growing bacteria in MgM minimal medium containing 0.1 % casamino acids at 37 °C with aeration at pH 6 (Miki et al., 2004).
For in vivo studies, bacterial inocula used to infect cells or animals were prepared by growing the tagged strains overnight under SPI-1 non-inducing conditions (LB at 28 °C) as previously described Giacomodonato et al. (2009). In this way, the residual expression of AvrA from in vitro bacterial growth was ruled out.
Cultures were centrifuged, diluted in sterile saline and inoculated to cultured cells or mice. Viable bacteria in the inoculum were quantified by dilution and plating onto LB agar plates.
Expression and secretion of AvrA in vitro.
For the isolation of cell-associated proteins, 1.5 ml bacterial culture was centrifuged and suspended in 100 µl of H2O and immediately mixed with 100 µl of Laemmli buffer. To isolate the proteins released into the culture supernatants (secreted proteins), bacteria were pelleted by centrifugation and 2 ml of supernatant was collected from each sample. Supernatants were then filtered (0.45 µm pore size), and the proteins were precipitated with 25 % trichloroacetic acid and sedimented by high-speed centrifugation (14 000 g for 30 min). The pellet was washed in cold acetone and suspended in PBS and Laemmli buffer. Four independent extractions for each sample were added together to minimize differences in protein recovery from sample to sample. Proteins (cell-associated and secreted proteins) were then boiled for 5–10 min, and an aliquot of each sample was separated by 10 % SDS-PAGE (Raffatellu et al., 2005). Finally, effector proteins were immunodetected as described below.
Expression and secretion of AvrA in infected eukaryotic cells.
Human laryngeal epithelial (HEp-2) cells (ATCC CCL-23), were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 10 % FBS. Infected monolayers (m.o.i. 10 : 1) were incubated for 20 min at 37 °C in 5 % CO2, washed twice with PBS, and then incubated in fresh tissue culture medium containing 100 µg gentamicin ml−1 for 1 h to remove extracellular bacteria, and finally in fresh tissue culture medium containing 10 µg gentamicin ml−1 for the remainder of the experiment. At 20 min and 24 h post-infection monolayers were washed twice with cold HBSS and lysed with 1.0 ml HBSS containing 0.1 % Triton X-100 and 1 mM PMSF as described by Kubori & Galán (2003). This procedure lyses the infected cells, but does not affect the integrity of the bacterial membrane (Collazo & Galán, 1997). An aliquot of this suspension was used to determine the number of intracellular bacteria by plating serial dilutions onto LB agar plates. Cell lysates were collected in chilled microfuge tubes, and centrifuged at 17 000 g for 15 min at 4 °C to separate the soluble fraction, containing bacterial proteins that have been translocated into the host-cell cytosol, from the insoluble fraction, which contains the internalized bacteria. The soluble fraction was filtered through a 0.45 µm pore-size filter and subjected to 10 % trichloroacetic acid precipitation and sedimented by high-speed centrifugation (14 000 g for 30 min). The pellet was washed in cold acetone and suspended in PBS and Laemmli buffer. The insoluble fraction was washed once with cold PBS and resuspended in an appropriate volume of PBS and Laemmli buffer. The protein extracts were boiled for 5–10 min, and resolved by 10 % SDS-PAGE. Finally, effector proteins were immunodetected as described below.
Subcellular fractionation of HEp-2 cells.
HEp-2 cells were infected, washed, and harvested at 20 min and 24 h post-infection as described above. Homogenization buffer [20 mM HEPES (pH 7.2), 200 mM sucrose, 0.5 mM EGTA, 1 mM PMSF, 1× protease inhibitor cocktail (Roche)] was used. Cells were disrupted by mechanical lysis with a dounce homogenizer, and lysates were centrifuged twice at 20 000 g at 4 °C for 30 min to remove bacteria and debris. The resulting supernatant was centrifuged at 100 000 g at 4 °C to separate the membrane (pellet) from the cytoplasmic (supernatant) fractions. The pellet fraction was resuspended in an appropriate volume of PBS and Laemmli buffer. The soluble fraction was filtered through a 0.45 µm pore-size filter, subjected to 10 % trichloroacetic acid precipitation and sedimented by high-speed centrifugation (14 000 g for 30 min). The pellet was washed in cold acetone and resuspended in PBS and Laemmli buffer. The protein extracts were boiled for 5–10 min and resolved by 10 % SDS-PAGE. Finally, effector proteins were immunodetected as described below.
Quantitative reverse transcriptase PCR (qRT-PCR).
RNA was isolated using TRIzol (Invitrogen), according to the manufacturer’s instructions, from bacteria culture (exponential phase) and from infected HEp-2 cells in six-well plates. Contaminating DNA was digested with RNase-free DNase I (Epicentre Biotechnologies), and the purity of all RNA preparations was confirmed by subjecting them to PCR analysis using primers specific for the gene encoding the 16S rRNA. After inactivation of DNase, RNA was used as a template for qRT-PCR. Complementary cDNA was synthesized using random hexamer primers (Invitrogen), deoxynucleoside triphosphates and Moloney murine leukaemia virus (M-MLV) reverse transcriptase (Invitrogen). Relative quantitative real-time PCR was performed with an appropriate primer set, cDNAs and Mezcla Real (Biodynamics), which contained nucleotides, polymerase, reaction buffer and Green dye, using a Rotor-Gene 6000 real-time PCR machine (Corbett Research). The primer sequences were 16S rRNA forward 5′-GCCGCAAGGTTAAAACTCAA-3′ and reverse 5′-AAGGCACCAATCCATCTCTG-3′, avrA forward 5′-TGTTGAGCGTCTGGAAAGTG-3′ and reverse 5′-CAGATTCAACGCCTTCCATT-3′, sseJ forward 5′-GCCGATGCATTTAAGGTGAT-3′ and reverse 5′-TTTTCTGTCCACCGCTATCC-3′, and sipA forward 5′-CGTGACCACCTTTCCATCTT-3′ and reverse 5′-CCATTCGACTAACAGCAGCA-3′.
The amplification program consisted of an initial incubation for 3 min at 95 °C, followed by 40 cycles of 95 °C for 20 s, 60 °C for 30 s and 72 °C 20 s. A no-template control was included for each primer set. Melting curve analysis verified that each reaction contained a single PCR product. All samples were analysed in the same run for 16S expression for normalization. The number of copies of each sample transcript was determined with the aid of the Rotor-Gene 6000 Series Software Version 1.7. Quantification of gene expression was calculated using the comparative threshold cycle (Ct) method, normalized to the 16S control and efficiency of the reverse transcriptase reaction (relative quantity 2−ΔΔCt). The replicates were then averaged and fold induction was determined (Livak & Schmittgen, 2001).
Mice.
Six to eight-week old BALB/c mice were obtained from our vivarium, maintained under standard conditions and provided with food and water ad libitum. At the end of the experiment mice were killed with carbon dioxide. All experimental protocols were approved by the Animal Ethics Committee, University of Buenos Aires, Buenos Aires, Argentina.
Expression and translocation of AvrA in vivo.
Mice were inoculated intragastrically with 106 c.f.u. per mouse of the tagged Salmonella strains and were euthanized 8 days post-inoculation. Mesenteric lymph nodes (MLN) were aseptically removed and incubated for 20 min in 3 ml HBSS containing 100 mg gentamicin ml−1, followed by three washes in 10 ml HBSS without antibiotic, before single cell suspensions were prepared using an iron mesh sieve. Then, the isolated cells were processed as described above to analyse the expression and translocation of AvrA in HEp-2 cells.
Western blot analysis.
The gels were blotted onto a Hybond-P membrane (GE Health-care). The 3×FLAG fusion proteins were immunodetected using mouse anti-FLAG M2-horseradish peroxidase mAbs (Sigma). The reacting bands were detected by enhanced chemiluminescence (Luminol; Santa Cruz Biotechnology) in an Image Quant 300 cabinet (GE Healthcare) following the manufacturer’s instructions.
Statistical analysis.
Data are represented as the mean±sd from triplicates. One-way ANOVA was employed to assess the significance of the differences between the mean values of experimental groups using GraphPad Prism software version 5.
Results
AvrA is synthesized and secreted in vitro under different culture conditions
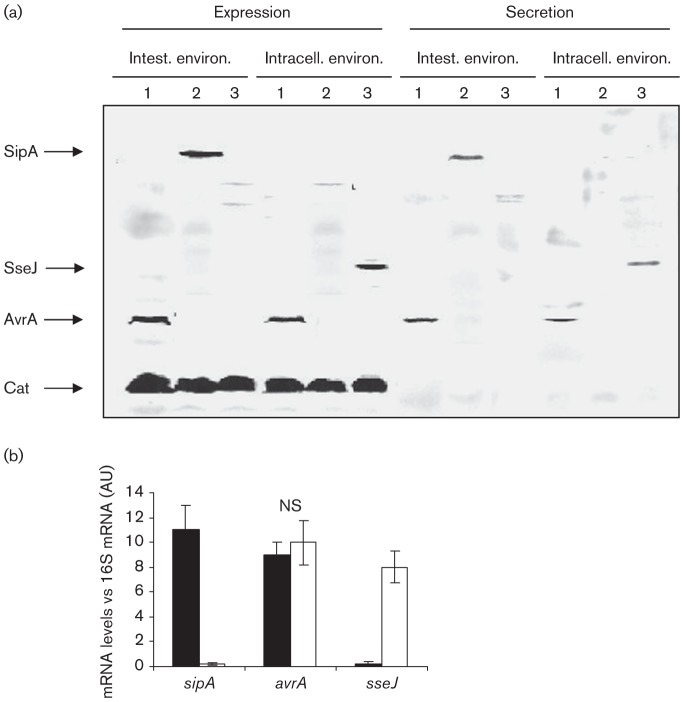
Upon ingestion, Salmonella encounters different environments such as high osmolarity, basic pH and hypoxia in the intestine, and low nutrient levels and acidity inside the eukaryotic cell. To investigate the capacity of a tagged Salmonella strain to synthesize and secrete AvrA, bacteria were grown under different conditions resembling early and late stages of Salmonella infection as described in Methods. Results are shown in Fig. 1(a). We observed that AvrA was synthesized and secreted at the same level by bacteria grown under both culture conditions, whereas SipA expression and secretion were evident only under conditions that mimicked the intestinal milieu (Fig. 1a). This result was corroborated by qRT-PCR. As shown in Fig. 1(b), no differences were observed in the transcript levels of avrA of bacteria cultured under intestinal or intracellular conditions. Taken together these results suggest that AvrA can be synthesized and secreted not only by Salmonella located in the intestinal environment but also by intracellular bacteria.
Fig. 1.
(a) Analysis of AvrA expression and secretion in vitro by Western blot. AvrA-tagged, SipA-tagged and SseJ-tagged strains of S. Enteritidis were grown under different culture conditions that mimicked the intestinal environment and the intracellular environment. Expression and secretion were investigated in whole bacterial extracts and supernatants, respectively. Samples were subjected to SDS-PAGE and tagged proteins were detected by anti-FLAG antibodies. Each lane was loaded with material from approximately 106 c.f.u. bacteria. Lanes: 1, SE1702 (avrA : : 3×FLAG cat : : FLAG); 2, SE1703 (sipA : : 3×FLAG cat : : FLAG); 3, SE1704 (sseJ : : 3×FLAG cat : : FLAG). Data are representative from three independent experiments. (b) Analysis of avrA expression under different culture conditions by qRT-PCR. The relative mRNA amount was determined by real-time qRT-PCR and related to 16S mRNA levels. Values are means±sd of three independent mRNA extractions performed in triplicate. AU, Arbitrary unit; NS, no significant difference between the two culture conditions (ANOVA). Black bars, intestinal environment; white bars, intracellular environment.
Synthesis and secretion of AvrA by intracellular bacteria
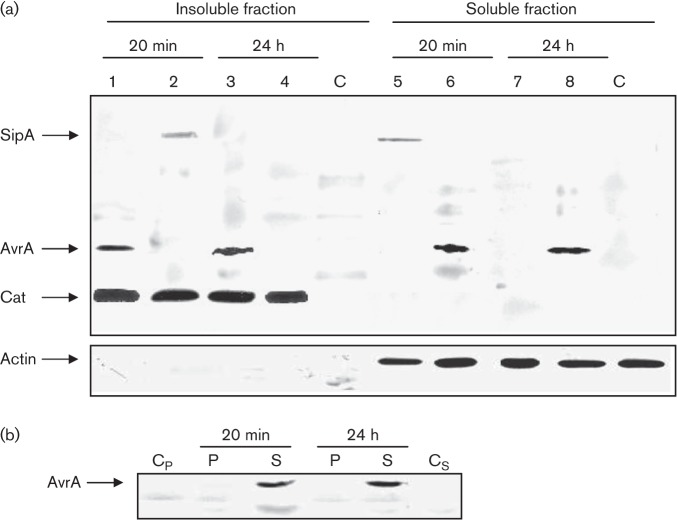
We investigated the synthesis and secretion of AvrA in bacteria infecting HEp-2 cells. Confluent HEp-2 cells were inoculated with an AvrA-FLAG-tagged Salmonella strain. At 20 min and 24 h post-infection cells were mechanically disrupted and centrifuged at low speed to separate cell lysates into a soluble fraction (containing bacterial proteins that have been translocated into the host-cell cytosol) and an insoluble fraction (containing internalized bacteria). Western blot analysis of the insoluble fraction revealed that S. Enteritidis expressed similar levels of AvrA at both time points analysed (Fig. 2a). In the same way, immunoblotting analysis of the soluble fraction showed that AvrA is translocated at early and late stages of Salmonella infection (Fig. 2a). SipA was expressed at 20 min post-infection and its translocation was observed only at early stages of infection. To analyse AvrA subcellular localization, we mechanically fractionated HEp-2 cells at 20 min and 24 h post-infection to separate the pellet containing plasma membranes from the cytoplasm. No AvrA was observed in the plasma membranes, but it was clearly present in the soluble fraction containing host cytoplasm at early and late stages of Salmonella infection Fig. 2(b). These results show the persistence of AvrA in the host cytosol after initial translocation. To expand upon these results we used qRT-PCR to directly measure avrA mRNA levels in intracellular bacteria, as described in Methods. As predicted from other studies (Kerrinnes et al., 2009), we found that avrA mRNA levels were detectable for at least 10 h post-infection overlapping with the maximal induction of sseJ (Fig. 3). Again, we observed that transcript levels of avrA mRNA were similar at early (20 min) and late (10 h) stages of infection (Fig. 3).
Fig. 2.
Analysis of AvrA expression, translocation (a) and localization (b) in HEp-2 cells by Western blot. Epithelial cells were infected with SipA-tagged and AvrA-tagged strains of S. Enteritidis for 20 min and 24 h. (a) Post-infection cells were processed as indicated in Methods to obtain an insoluble fraction containing intact bacteria and a soluble fraction containing translocated effectors. Both fractions were analysed by immunoblotting using anti-FLAG antibodies. Lanes: 1, 3, 6 and 8, SE1702 (avrA : : 3×FLAG cat : : FLAG); 2, 4, 5 and 7, SE1703 (sipA : : 3×FLAG cat : : FLAG); C, control uninfected cell cultures. As a control for the host-cell cytosolic fraction some blots were reprobed with polyclonal antibodies to actin. (b) Subcellular localization of AvrA. Epithelial cells were infected with an AvrA-tagged strain of S. Enteritidis for 20 min and 24 h, and were fractionated into membrane insoluble (P) or soluble fractions (S) as described in Methods. Both fractions were analysed by immunoblotting using anti-FLAG antibodies. Cp and Cs, negative controls (pellet and soluble fraction from uninfected cell cultures, respectively). Each lane was loaded with material from approximately 106 c.f.u. bacteria. Data are representative from three independent experiments.
Fig. 3.
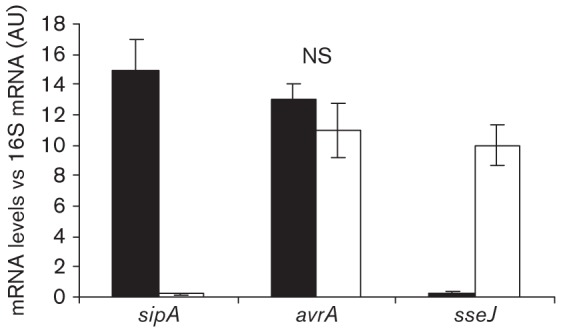
Analysis of avrA expression in HEp-2 cells by qRT-PCR. Epithelial cells were infected with a wt strain of S. Enteritidis for 20 min (black bars) or 10 h (white bars). Post-infection cells were processed as indicated in Methods to obtain total mRNA. sipA, avrA and sseJ mRNA levels from bacteria colonizing HEp-2 cells were measured by qRT-PCR at the indicated times. sipA and sseJ were used as controls for SPI-1 and SPI-2 expression, respectively. The mRNA amount was related to 16S mRNA levels. Values are means±sd of three independent mRNA extractions performed in triplicate. AU, Arbitrary unit; NS, no significant difference between the two times post-infection (ANOVA).
AvrA is synthesized and translocated during murine salmonellosis
Next we determined the length of time that AvrA is synthesized in infecting bacteria and for how long it is translocated into the cytosol of infected cells. To this purpose mice were infected by the natural route with the tagged strain of Salmonella. Animals received a high dose of Salmonella that, in turn, yielded a sufficient number of infecting bacteria (to be recovered for AvrA expression analysis), and also provided an adequate amount of infected cells (to be isolated to determine AvrA translocation). Previously, we demonstrated that 24 h after inoculation, SopB continues to be expressed by wild-type infecting bacteria recovered from MLN (Giacomodonato et al., 2011). As shown in Fig. 4, the expression of AvrA was still evident on day 8 following intragastric inoculation. However, at day 8 after infection SipA was weakly expressed (Fig. 4, lane 1, insoluble fraction), but was not translocated since it was not detected in the soluble fraction. In contrast, as shown in Fig. 4 (lane 2, insoluble and soluble fractions) translocation of AvrA during murine salmonellosis was evident for at least 8 days, coincident with the AvrA expression (Fig. 4).
Fig. 4.
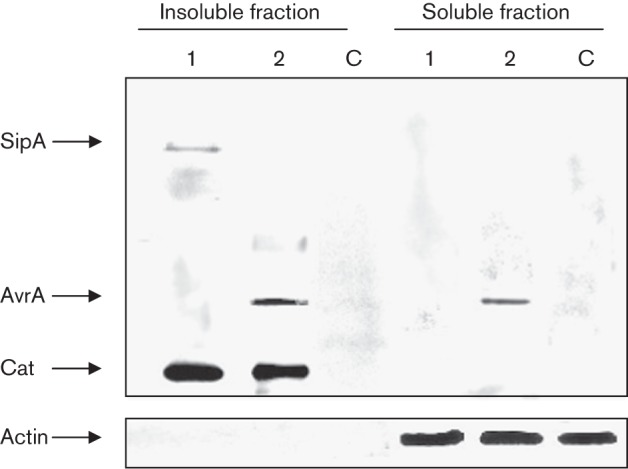
Analysis of AvrA expression (insoluble fraction) and translocation (soluble fraction) in MLN by Western blot. Groups of mice were inoculated intragastrically with 106 c.f.u. of the AvrA- and SipA-tagged strains of S. Enteritidis, and euthanized at day 8 post-infection. MLN were removed and processed as indicated in Methods to obtain an insoluble fraction containing intact bacteria and a soluble fraction containing translocated effectors. Both fractions were analysed by immunoblotting using anti-FLAG antibodies. Lanes: 1, MLN protein extracts from mice inoculated with SE1703 (sipA : : 3×FLAG cat : : FLAG); 2, MLN protein extracts from mice inoculated with SE1702 (avrA : : 3×FLAG cat : : FLAG); C, MLN protein extracts from control uninfected mice. Pooled MLN protein extracts from two mice were used in the experiments. Each lane was loaded with material from approximately 106 c.f.u. bacteria. As a control for the host-cell cytosolic fraction some blots were reprobed with polyclonal antibodies to actin. Data are representative from three independent experiments.
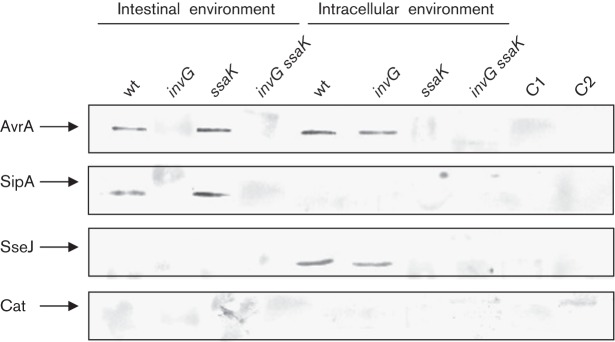
To find out whether – under intracellular conditions – AvrA is translocated via T3SS-1, T3SS-2 or independently of both T3SSs, we analysed its secretion in three genetic backgrounds, invG, ssaK and invG ssaK. These mutations render the bacteria defective in secretion through T3SS-1, T3SS-2 or both T3SS-1/2, respectively (Kubori et al., 2000; Geddes et al., 2005; Niemann et al., 2011). In vitro analysis by Western blot confirmed that AvrA is secreted through T3SS-1 under intestinal conditions and through T3SS-2 under intracellular conditions; in the absence of both T3SSs the effector is not translocated (Fig. 5). This fact demonstrates that translocation of AvrA under intracellular conditions requires a functional T3SS-2.
Fig. 5.
Analysis of T3SS-1- and T3SS-2-dependent secretion of AvrA in vitro by Western blot. wt, invG (T3SS-1 defective), ssaK (TTSS-2 defective) and invG ssaK (lacking both T3SSs) strains of S. Enteritidis containing AvrA, SipA and SseJ tagged with FLAG were grown under culture conditions that mimicked the intestinal niche and the intracellular environment as indicated in Methods. The secretion of the effectors was investigated in bacterial supernatants. SipA and SseJ were used as controls for T3SS-1- and TTSS-2-dependent secretion, respectively. C1 and C2, negative controls (culture medium used for SPI-1 and SPI-2 inducing conditions, respectively). Samples were subjected to SDS-PAGE and tagged proteins were detected by anti-FLAG antibodies. Each lane was loaded with material from approximately 106 c.f.u. bacteria. Data are representative from three independent experiments.
Discussion
The virulence-associated effector protein AvrA of S. enterica, which interferes with the first line of immune response of the target organism (Collier-Hyams et al., 2002), is an important partner in the virulence phenotype of this pathogen (Streckel et al., 2004; Ben-Barak et al., 2006). Our study reveals that S. Enteritidis express AvrA at early and late stages of infection in the murine model.
Lawley et al. (2006) suggested that the avrA gene product lacks an obvious role during long-term systemic infection, and that AvrA must be regarded as an effector protein involved in the enteritis pathway. It has been demonstrated that some SPI-1 effectors persist in host cells for several hours – or even days – after initial translocation, and play roles during systemic infection (Marcus et al., 2002; Kubori & Galán, 2003; Drecktrah et al., 2005; Giacomodonato et al., 2007, 2009, 2011; Patel et al., 2009; Gong et al., 2010). Likewise, our results provide direct evidence that AvrA is expressed in both early and late stages of Salmonella infection.
Furthermore, this study demonstrates that SipA and AvrA are differentially expressed in Salmonella colonizing the epithelial cells and MLN. These results further suggest that different SPI-1 proteins are expressed by S. Enteritidis in specific tissues. Differential expression of these effector proteins may be important for bacterial pathogenesis in certain organs, such as gastroenterititis in the intestinal epithelium and typhoid fever during systemic infection in the MLN. In concurrence with our findings, Lu et al. (2012) demonstrated that AvrA has long-term effects in the Salmonella-infected intestine emphasizing the importance of AvrA in chronic bacterial infection.
Culture cell experiments allowed us to analyse the expression and translocation of Salmonella AvrA effector protein in vivo, at early and late time points. We found that AvrA is expressed and translocated within 20 min and 24 h following cell invasion. We observed that upon translocation from the bacteria and 24 h post-infection, AvrA localizes to the cytoplasm of infected cells. Shortly after its translocation AvrA is rapidly phosphorylated by the ERK pathway and in this way remains within the cell for an extended period of time (Du & Galán, 2009). Experiments performed in vitro and in cultured cells strongly suggest that persistence of AvrA during infection is due – in part – to de novo synthesis. These results are consistent with those obtained by Kerrinnes et al. (2009). They found that avrA transcription takes place constitutively in all S. enterica strains, but that avrA translation is regulated positively in a post-transcriptional manner by CsrA/CsrB of the Csr regulatory system.
Interestingly, we demonstrated that AvrA secretion in S. Enteritidis is dependent on both T3SS-1 and T3SS-2 systems. The fact that AvrA is translocated by both syringes supports the speculation that this effector protein participates in both intestinal and systemic phases of Salmonella infection. The involvement of AvrA in late phases of murine infection has been recently investigated using Salmonella mutants (Wu et al., 2012). The authors observed that the loss of AvrA increases apoptosis in epithelial cells and macrophages during Salmonella infection. Moreover, the lack of AvrA results in a simultaneous loss of intracellular Salmonella carriage and an increase in microbial parenchymal burden in systemic lymphoid tissues, increasing mortality during late stages of infection. This finding that S. Enteritidis AvrA is secreted by both T3SSs is consistent with studies performed earlier in S. enterica serovar Typhimurium (S. Typhimurium) (Brumell et al., 2003; Geddes et al., 2005). In this regard, S. Typhimurium AvrA, SptP, SlrP, SteA, SteB and SopD, originally identified as SPI-1-secreted proteins, are now considered as dual effectors.
We demonstrated as well that AvrA is expressed in S. Enteritidis recovered from MLN 8 days after infection. This result is in complete concurrence with those revealed by in vitro and cell culture experiments. Furthermore, we were able to demonstrate that AvrA is translocated for at least 8 days following intragastric infection. It is important to note that similar to AvrA, SopB has an anti-apoptotic effect through its phosphatase activity (Knodler et al., 2005). We have previously shown that in S. Typhimurium SopB is also a dual effector. In a murine model of infection, the expression of SopB was detected for at least 5 days after a maximum expression on day 1 post-inoculation (Giacomodonato et al., 2011). Here we show that, unlike SopB, the expression of AvrA during Salmonella infection is sustained for at least 8 days. Altogether, our results clearly show that these two dual effectors are differentially expressed in vivo by infecting S. Enteritidis. It is interesting to note that despite the anti-inflammatory effect of AvrA, its presence during late stages of S. Enteritidis infection prevented neither the systemic infection nor the death of infected mice.
In conclusion, our results indicate that the expression of AvrA and its function during murine infection are not constrained to the intestinal environment as previously defined. The avrA gene is prevalent in the majority of S. enterica serovars (80 %); however, only a small number of them usually express the protein. Significantly, S. Typhi and S. Paratyphi are strains that evade epithelial defences and result in severe systemic disease. These strains invariably lack an avrA allele (Prager et al., 2000). However, the expression and translocation of AvrA in S. Enteritidis did not impede the development of a systemic infection in the murine model. Our findings do not rule out a role for AvrA in avirulence (or virulence) as detection of this putative function will require the identification of a host in which this protein may be able to exert such an effect. Our work stresses the significance of analysing protein expression and translocation in vivo in the context of infection. Further investigation on kinetics, lifespan and function of SPI-1 effectors in vivo would provide a significant approach for analysing the function of these proteins in Salmonella pathogenesis, and would confirm the complementary behaviour of SPI-1 and SPI-2 effectors functions.
Acknowledgements
We are very grateful to Ms María Isabel Bernal for her excellent technical assistance and to Dr Bacciu Donatella for providing strains. This work was supported in part by grants from Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (PIP 2012-2014 GI 11220110100911) and Secretaría de Ciencia y Técnica de la Universidad de Buenos Aires, Argentina (UBACyT 20020110200087, 20020100100541 y 20020120200021BA).
Abbreviations:
- MLN
mesenteric lymph nodes
- qRT-PCR
quantitative reverse transcriptase PCR
- T3SS
type three secretion system
Edited by: E. Hartland
References
- Bäumler A. J., Hargis B. M., Tsolis R. M. (2000). Tracing the origins of Salmonella outbreaks. Science 287, 50–52. 10.1126/science.287.5450.50 [DOI] [PubMed] [Google Scholar]
- Ben-Barak Z., Streckel W., Yaron S., Cohen S., Prager R., Tschäpe H. (2006). The expression of the virulence-associated effector protein gene avrA is dependent on a Salmonella enterica-specific regulatory function. Int J Med Microbiol 296, 25–38. 10.1016/j.ijmm.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Bollaerts K., Aerts M., Faes C., Grijspeerdt K., Dewulf J., Mintiens K. (2008). Human salmonellosis: estimation of dose-illness from outbreak data. Risk Anal 28, 427–440. 10.1111/j.1539-6924.2008.01038.x [DOI] [PubMed] [Google Scholar]
- Boyle E. C., Bishop J. L., Grassl G. A., Finlay B. B. (2007). Salmonella: from pathogenesis to therapeutics. J Bacteriol 189, 1489–1495. 10.1128/JB.01730-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg C. A., Orth K. (2010). Tipping the balance by manipulating post-translational modifications. Curr Opin Microbiol 13, 34–40. 10.1016/j.mib.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell J. H., Kujat-Choy S., Brown N. F., Vallance B. A., Knodler L. A., Finlay B. B. (2003). SopD2 is a novel type III secreted effector of Salmonella typhimurium that targets late endocytic compartments upon delivery into host cells. Traffic 4, 36–48. 10.1034/j.1600-0854.2003.40106.x [DOI] [PubMed] [Google Scholar]
- Bruno V. M., Hannemann S., Lara-Tejero M., Flavell R. A., Kleinstein S. H., Galán J. E. (2009). Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog 5, e1000538. 10.1371/journal.ppat.1000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo C. M., Galán J. E. (1997). The invasion-associated type-III protein secretion system in Salmonella – a review. Gene 192, 51–59. 10.1016/S0378-1119(96)00825-6 [DOI] [PubMed] [Google Scholar]
- Collier-Hyams L. S., Zeng H., Sun J., Tomlinson A. D., Bao Z. Q., Chen H., Madara J. L., Orth K., Neish A. S. (2002). Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-κB pathway. J Immunol 169, 2846–2850. [DOI] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D., Knodler L. A., Galbraith K., Steele-Mortimer O. (2005). The Salmonella SPI1 effector SopB stimulates nitric oxide production long after invasion. Cell Microbiol 7, 105–113. 10.1111/j.1462-5822.2004.00436.x [DOI] [PubMed] [Google Scholar]
- Du F., Galán J. E. (2009). Selective inhibition of type III secretion activated signaling by the Salmonella effector AvrA. PLoS Pathog 5, e1000595. 10.1371/journal.ppat.1000595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrbar K., Friebel A., Miller S. I., Hardt W. D. (2003). Role of the Salmonella pathogenicity island 1 (SPI-1) protein InvB in type III secretion of SopE and SopE2, two Salmonella effector proteins encoded outside of SPI-1. J Bacteriol 185, 6950–6967. 10.1128/JB.185.23.6950-6967.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier J. R., Slauch J. M. (2007). Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10, 24–29. 10.1016/j.mib.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Galyov E. E., Wood M. W., Rosqvist R., Mullan P. B., Watson P. R., Hedges S., Wallis T. S. (1997). A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol 25, 903–912. 10.1111/j.1365-2958.1997.mmi525.x [DOI] [PubMed] [Google Scholar]
- Geddes K., Worley M., Niemann G., Heffron F. (2005). Identification of new secreted effectors in Salmonella enterica serovar Typhimurium. Infect Immun 73, 6260–6271. 10.1128/IAI.73.10.6260-6271.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomodonato M. N., Uzzau S., Bacciu D., Caccuri R., Sarnacki S. H., Rubino S., Cerquetti M. C. (2007). SipA, SopA, SopB, SopD and SopE2 effector proteins of Salmonella enterica serovar Typhimurium are synthesized at late stages of infection in mice. Microbiology 153, 1221–1228. 10.1099/mic.0.2006/002758-0 [DOI] [PubMed] [Google Scholar]
- Giacomodonato M. N., Sarnacki S. H., Llana M. N., García Cattaneo A. S., Uzzau S., Rubino S., Cerquetti M. C. (2009). Impaired synthesis and secretion of SopA in Salmonella Typhimurium dam mutants. FEMS Microbiol Lett 292, 71–77. 10.1111/j.1574-6968.2008.01473.x [DOI] [PubMed] [Google Scholar]
- Giacomodonato M. N., Sarnacki S. H., Llana M. N., Cerquetti M. C. (2011). SopB effector protein of Salmonella Typhimurium is translocated in mesenteric lymph nodes during murine salmonellosis. FEMS Microbiol Lett 317, 100–106. 10.1111/j.1574-6968.2011.02217.x [DOI] [PubMed] [Google Scholar]
- Gong H., Vu G.-P., Bai Y., Yang E., Liu F., Lu S. (2010). Differential expression of Salmonella type III secretion system factors InvJ, PrgJ, SipC, SipD, SopA and SopB in cultures and in mice. Microbiology 156, 116–127. 10.1099/mic.0.032318-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald T., Vose D., Wegener H. C., Koupeev T. (2004). A Bayesian approach to quantify the contribution of animal-food sources to human salmonellosis. Risk Anal 24, 255–269. 10.1111/j.0272-4332.2004.00427.x [DOI] [PubMed] [Google Scholar]
- Hapfelmeier S., Ehrbar K., Stecher B., Barthel M., Kremer M., Hardt W.-D. (2004). Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun 72, 795–809. 10.1128/IAI.72.2.795-809.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt W. D., Galán J. E. (1997). A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc Natl Acad Sci U S A 94, 9887–9892. 10.1073/pnas.94.18.9887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen R. S., Vieira A. R., Karlsmose S., Lo Fo Wong D. M. A., Jensen A. B., Wegener H. C., Aarestrup F. M. (2011). Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis 8, 887–900. 10.1089/fpd.2010.0787 [DOI] [PubMed] [Google Scholar]
- Hogue A., White P., Guard-Petter J., Schlosser W., Gast R., Ebel E., Farrar J., Gomez T., Madden J. & other authors (1997). Epidemiology and control of egg-associated Salmonella Enteritidis in the United States of America. Rev Sci Tech 16, 542–553. [DOI] [PubMed] [Google Scholar]
- Jones R. M., Wu H., Wentworth C., Luo L., Collier-Hyams L., Neish A. S. (2008). Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host Microbe 3, 233–244. 10.1016/j.chom.2008.02.016 [DOI] [PubMed] [Google Scholar]
- Kerrinnes T., Zelas Z. B., Streckel W., Faber F., Tietze E., Tschäpe H., Yaron S. (2009). CsrA and CsrB are required for the post-transcriptional control of the virulence-associated effector protein AvrA of Salmonella enterica. Int J Med Microbiol 299, 333–341. 10.1016/j.ijmm.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Knodler L. A., Finlay B. B., Steele-Mortimer O. (2005). The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem 280, 9058–9064. 10.1074/jbc.M412588200 [DOI] [PubMed] [Google Scholar]
- Kubori T., Galán J. E. (2003). Temporal regulation of Salmonella virulence effector function by proteasome-dependent protein degradation. Cell 115, 333–342. 10.1016/S0092-8674(03)00849-3 [DOI] [PubMed] [Google Scholar]
- Kubori T., Sukhan A., Aizawa S. I., Galán J. E. (2000). Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc Natl Acad Sci U S A 97, 10225–10230. 10.1073/pnas.170128997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T. D., Chan K., Thompson L. J., Kim C. C., Govoni G. R., Monack D. M. (2006). Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog 2, e11. 10.1371/journal.ppat.0020011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao A. P., Petrof E. O., Kuppireddi S., Zhao Y., Xia Y., Claud E. C., Sun J. (2008). Salmonella type III effector AvrA stabilizes cell tight junctions to inhibit inflammation in intestinal epithelial cells. PLoS ONE 3, e2369. 10.1371/journal.pone.0002369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Löber S., Jäckel D., Kaiser N., Hensel M. (2006). Regulation of Salmonella pathogenicity island 2 genes by independent environmental signals. Int J Med Microbiol 296, 435–447. 10.1016/j.ijmm.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Lu R., Liu X., Wu S., Xia Y., Zhang Y. G., Petrof E. O., Claud E. C., Sun J. (2012). Consistent activation of the β-catenin pathway by Salmonella type-three secretion effector protein AvrA in chronically infected intestine. Am J Physiol Gastrointest Liver Physiol 303, G1113–G1125. 10.1152/ajpgi.00453.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majowicz S. E., Musto J., Scallan E., Angulo F. J., Kirk M., O’Brien S. J., Jones T. F., Fazil A., Hoekstra R. M., International Collaboration on Enteric Disease ‘Burden of Illness’ Studies (2010). The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50, 882–889. 10.1086/650733 [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Knodler L. A., Finlay B. B. (2002). Salmonella enterica serovar Typhimurium effector SigD/SopB is membrane-associated and ubiquitinated inside host cells. Cell Microbiol 4, 435–446. 10.1046/j.1462-5822.2002.00202.x [DOI] [PubMed] [Google Scholar]
- McGhie E. J., Brawn L. C., Hume P. J., Humphreys D., Koronakis V. (2009). Salmonella takes control: effector-driven manipulation of the host. Curr Opin Microbiol 12, 117–124. 10.1016/j.mib.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Okada N., Danbara H. (2004). Two periplasmic disulfide oxidoreductases, DsbA and SrgA, target outer membrane protein SpiA, a component of the Salmonella pathogenicity island 2 type III secretion system. J Biol Chem 279, 34631–34642. 10.1074/jbc.M402760200 [DOI] [PubMed] [Google Scholar]
- Moore G., Blair I. S., McDowell D. A. (2007). Recovery and transfer of Salmonella Typhimurium from four different domestic food contact surfaces. J Food Prot 70, 2273–2280. [DOI] [PubMed] [Google Scholar]
- Morales R. A., McDowell R. M. (1999). Economic consequences of Salmonella enterica serovar Enteritidis infection in humans and the U. S. egg industry. In Salmonella Enterica Serovar Enteritidis in Humans and Animals, pp. 271–290. Edited by Saeed A. M., Gast R. K., Potter M. E., Wall P. G. Ames, IA: Iowa State University Press. [Google Scholar]
- Niemann G. S., Brown R. N., Gustin J. K., Stufkens A., Shaikh-Kidwai A. S., Li J., McDermott J. E., Brewer H. M., Schepmoes A. & other authors (2011). Discovery of novel secreted virulence factors from Salmonella enterica serovar Typhimurium by proteomic analysis of culture supernatants. Infect Immun 79, 33–43. 10.1128/IAI.00771-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira K., Oliveira T., Teixeira P., Azeredo J., Henriques M., Oliveira R. (2006). Comparison of the adhesion ability of different Salmonella Enteritidis serotypes to materials used in kitchens. J Food Prot 69, 2352–2356. [DOI] [PubMed] [Google Scholar]
- Pang J.-C., Chiu T.-H., Helmuth R., Schroeter A., Guerra B., Tsen H.-Y. (2007). A pulsed field gel electrophoresis (PFGE) study that suggests a major world-wide clone of Salmonella enterica serovar Enteritidis. Int J Food Microbiol 116, 305–312. 10.1016/j.ijfoodmicro.2006.05.024 [DOI] [PubMed] [Google Scholar]
- Patel J. C., Hueffer K., Lam T. T., Galán J. E. (2009). Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell 137, 283–294. 10.1016/j.cell.2009.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales I., Audicana A. (1988). Salmonella Enteritidis and eggs. Lancet 332, 1133. 10.1016/S0140-6736(88)90542-9 [DOI] [PubMed] [Google Scholar]
- Prager R., Mirold S., Tietze E., Strutz U., Knüppel B., Rabsch W., Hardt W. D., Tschäpe H. (2000). Prevalence and polymorphism of genes encoding translocated effector proteins among clinical isolates of Salmonella enterica. Int J Med Microbiol 290, 605–617. 10.1016/S1438-4221(00)80009-0 [DOI] [PubMed] [Google Scholar]
- Prager R., Rabsch W., Streckel W., Voigt W., Tietze E., Tschäpe H. (2003). Molecular properties of Salmonella enterica serotype Paratyphi B distinguish between its systemic and its enteric pathovars. J Clin Microbiol 41, 4270–4278. 10.1128/JCM.41.9.4270-4278.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M., Wilson R. P., Chessa D., Andrews-Polymenis H., Tran Q. T., Lawhon S., Khare S., Adams L. G., Bäumler A. J. (2005). SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect Immun 73, 146–154. 10.1128/IAI.73.1.146-154.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue D. C., Tauxe R. V., Rowe B. (1990). International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect 105, 21–27. 10.1017/S0950268800047609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B., Robbiani R., Walker A. W., Westendorf A. M., Barthel M., Kremer M., Chaffron S., Macpherson A. J., Buer J. & other authors (2007). Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5, 2177–2189. 10.1371/journal.pbio.0050244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streckel W., Wolff A.-C., Prager R., Tietze E., Tschäpe H. (2004). Expression profiles of effector proteins SopB, SopD1, SopE1, and AvrA differ with systemic, enteric, and epidemic strains of Salmonella enterica. Mol Nutr Food Res 48, 496–503. 10.1002/mnfr.200400035 [DOI] [PubMed] [Google Scholar]
- Sun J. (2009). Pathogenic bacterial proteins and their anti-inflammatory effects in the eukaryotic host. Antiinflamm Antiallergy Agents Med Chem 8, 214–227. 10.2174/187152309789151986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzau S., Figueroa-Bossi N., Rubino S., Bossi L. (2001). Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A 98, 15264–15269. 10.1073/pnas.261348198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman S. R., Holden D. W. (2003). Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol 5, 501–511. 10.1046/j.1462-5822.2003.00294.x [DOI] [PubMed] [Google Scholar]
- Wu S., Ye Z., Liu X., Zhao Y., Xia Y., Steiner A., Petrof E. O., Claud E. C., Sun J. (2010). Salmonella typhimurium infection increases p53 acetylation in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 298, G784–G794. 10.1152/ajpgi.00526.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Jones R. M., Neish A. S. (2012). The Salmonella effector AvrA mediates bacterial intracellular survival during infection in vivo. Cell Microbiol 14, 28–39. 10.1111/j.1462-5822.2011.01694.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Petrof E. O., Boone D., Claud E. C., Sun J. (2007). Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am J Pathol 171, 882–892. 10.2353/ajpath.2007.070220 [DOI] [PMC free article] [PubMed] [Google Scholar]