Abstract
In Europe and Asia, Ixodid ticks transmit tick-borne encephalitis virus (TBEV), a flavivirus that causes severe encephalitis in humans but appears to show no virulence for livestock and wildlife. In the British Isles, where TBEV is absent, a closely related tick-borne flavivirus, named louping ill virus (LIV), is present. However, unlike TBEV, LIV causes a febrile illness in sheep, cattle, grouse and some other species, that can progress to fatal encephalitis. The disease is detected predominantly in animals from upland areas of the UK and Ireland. This distribution is closely associated with the presence of its arthropod vector, the hard tick Ixodes ricinus. The virus is a positive-strand RNA virus belonging to the genus Flavivirus, exhibiting a high degree of genetic homology to TBEV and other mammalian tick-borne viruses. In addition to causing acute encephalomyelitis in sheep, other mammals and some avian species, the virus is recognized as a zoonotic agent with occasional reports of seropositive individuals, particularly those whose occupation involves contact with sheep. Preventative vaccination in sheep is effective although there is no treatment for disease. Surveillance for LIV in Great Britain is limited despite an increased awareness of emerging arthropod-borne diseases and potential changes in distribution and epidemiology. This review provides an overview of LIV and highlights areas where further effort is needed to control this disease.
Introduction
Louping ill disease has been described in Scottish sheep since the eighteenth century and has been associated with tick-borne transmission for almost 100 years. The causative agent was recognized as a virus just over 80 years ago (Greig et al., 1931). The virus continues to cause livestock disease and death in upland areas of Great Britain (GB) and Ireland and is known to cause disease in humans. In 1991, louping ill in man was described as a forgotten disease (Davidson et al., 1991). Now, in the twenty-first century it appears to be a forgotten disease of livestock, with vaccine production faltering, serological tests that have been successfully used but remained unchanged for almost 80 years, and molecular testing very gradually being developed to improve virus detection. The decline in the profitability of sheep farming in upland areas has no doubt contributed to this neglect, and the repeated incursions of emerging/re-emerging infectious diseases such as foot-and-mouth disease virus, bluetongue virus and Schmallenberg virus have focused attention on exotic diseases rather than persistent localized endemic infections. With this in mind and with recognition of the consequences of exotic arthropod-borne disease incursion, it is a timely reminder that GB already has an existing zoonotic tick-borne virus that has persisted despite a greater understanding of virus ecology, vaccination and vector control.
Louping ill virus (LIV) is mainly detected in sheep, cattle, red grouse and ticks in upland areas of the British Isles, particularly in Scotland, Cumbria, Wales, Devon and Ireland. It has also been detected in a range of other animal species, including goats, dogs, pigs, horses, deer, llamas, alpacas and mountain hares (Table 1). Serology has been used extensively to assess LIV host-associations in the UK. However, currently there is limited surveillance for the virus in wildlife and estimates of prevalence and distribution are based on voluntary submissions from livestock with neurological signs suggestive of LIV infection. Historically, detection of LIV has been restricted almost exclusively to the British Isles, although cases have been reported in Norway and Spain.
Table 1. Species infected by LIV.
| Species | Reference (s) |
| Sheep (Ovis aries) | Smith et al. (1964a) |
| Cattle (Bos taurus) | Twomey et al. (2001) |
| Benavides et al. (2011) | |
| Pig (Sus scrofa) | Bannatyne et al. (1980) |
| Ross et al. (1994) | |
| Goat (Capra aegagrus hircus) | Gray et al. (1988) |
| Dog (Canis familiaris) | MacKenzie et al. (1973) |
| Red grouse (Lagopus scoticus) | Williams et al. (1963) |
| Reid & Boyce (1974) | |
| Hare (Lepus timidus) | Smith et al. (1964b) |
| Horse (Equus ferus caballus) | Fletcher (1937) |
| Timoney et al. (1976) | |
| Hyde et al. (2007) | |
| Roe deer (Capreolus capreolus) | Reid et al. (1976) |
| Red deer (Cervus elephus) | Reid et al. (1978a) |
| Alpaca (Vicugna pacos) | Cranwell et al. (2008) |
| Llama (Lama glama) | Macaldowie et al. (2005) |
Virus classification
LIV was first isolated in Selkirkshire (Scotland) in 1929 and was the first isolated arthropod-borne virus in Europe (Greig et al., 1931). The virus is grouped with a growing number of tick-transmitted viruses within the genus Flavivirus and family Flaviviridae (http://ictvdb.bio-mirror.cn/Ictv/fs_flavi.htm). In common with all members of this genus, the virus has a positive sense, single-stranded RNA genome approximately 11 kb in length. The sequencing of a complete LIV genome (Gritsun et al., 1997) has revealed that it has a conserved genome structure typical of the Flaviviridae. This is divided into structural [capsid (C), pre-membrane (prM), envelope (E)] and non-structural genes (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5); the coding sequences are shown schematically in Fig. 1. Following entry into the cell, the genome is uncoated and is directly translated into a single polypeptide which is proteolytically cleaved by a combination of host and virally encoded proteases. The envelope protein shares antigenic cross-reactivity with other members of the tick-borne flaviviruses (Calisher et al., 1989) and is the principal target of neutralizing antibodies. The virus, as with many viruses, is sensitive to heat, disinfectants and acidity (Gritsun et al., 2003a).
Fig. 1.
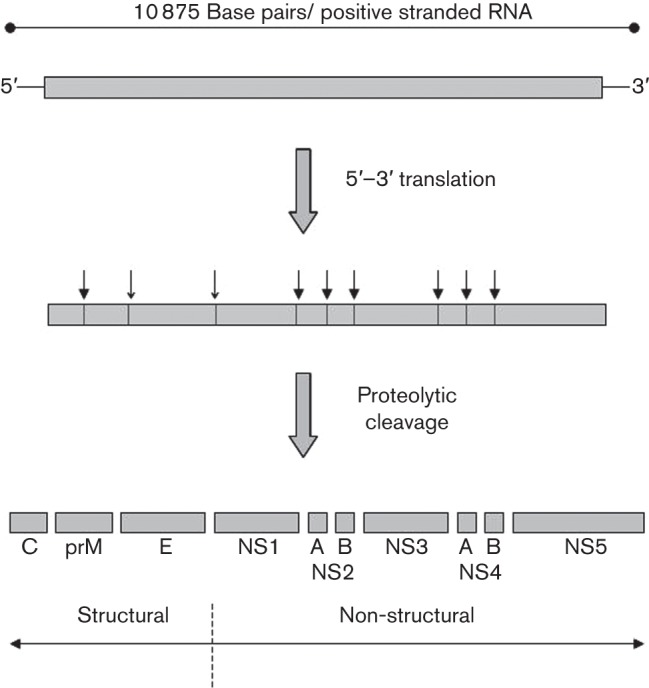
A schematic of the LIV genome, translation of the viral polyprotein and proteolytic cleavage to generate individual proteins. Closed arrowheads indicate cleavage by a virally encoded protease, whereas open arrowheads are cleavage sites mediated by host proteases such as furin (Grard et al., 2007).
Molecular epidemiology
The one area of research on LIV that has progressed in recent decades has been the increase in understanding of its relationship to other flaviviruses. In addition to antigenic relationships, comparison of the genomic sequence through phylogenetic analysis has confirmed that the virus is closely related to a small family of viruses associated with transmission by hard ticks (Ixodidae) and very closely related to the geographically dispersed tick-borne encephalitis virus (TBEV), which is endemic from Western Europe to Eastern Asia (Zanotto et al., 1995; Grard et al., 2007). The envelope glycoproteins of both viruses show a high degree of amino acid identity. Surprisingly, TBEV does not cause disease in sheep although it can cause severe disease in humans, with several thousand cases reported in Europe annually (Gritsun et al., 2003a). One subtype of TBEV has a case fatality rate approaching 40 % (Gritsun et al., 2003b; Mansfield et al., 2009). There are a number of closely related tick-borne viruses that cause neurological disease in sheep or goats, however, which have been described as louping ill-like viruses (Gao et al., 1997). These have included Turkish sheep encephalitis virus (Gao et al., 1993b; Whitby et al., 1993), Greek goat encephalitis virus (Papa et al., 2008) and Spanish sheep encephalitis virus (Marin et al., 1995). There remains some debate over the optimum classification of the different isolates of LIV and the LIV-like viruses within the tick-borne encephalitis complex (Gao et al., 1997; Norberg et al., 2013). In a manner similar to LIV, these foci of disease-causing viruses appear to be geographically limited and rare, whilst TBEV is pervasive and increasing in incidence and geographical range (Grard et al., 2007). The reason for this phenotypic dichotomy is unclear.
Molecular phylogeny based on the envelope gene of LIV isolates from the British Isles (Gao et al., 1997) suggests that there are four geographically separate lineages (genotype 1, Scotland and England; genotype 2, Scotland; genotype 3, Wales; and genotype 4, Ireland). This analysis has also suggested that a LIV isolate reported from Norway (Gao et al., 1993a) originated from GB, grouping with the genotype 1 isolates from Scotland and England (Gao et al., 1997). There is less than 5 % nucleotide divergence within the envelope coding sequence between all LIV samples studied, and geographical separation over many years has probably contributed to the genetic variation that is present, although there appears to be no variation in the manifestations of disease in sheep. Molecular clock analysis of LIV envelope gene variation has led to speculation that the virus was first introduced into Ireland approximately 800 years ago (McGuire et al., 1998), presumably from a precursor TBEV. The divergence between viruses observed in this study is considered to have occurred over the past 300 years and is intimately linked to movement of livestock within the British Isles (McGuire et al., 1998). Molecular genetic studies of a virus isolated from Spanish goats in 2011 have suggested that this isolate is 94 % identical to the LIV strain from the UK that was analysed, and 93 % identical to Spanish sheep encephalitis virus (Balseiro et al., 2012). However, this study compared short fragments of the virus genome and further comparison of the complete genome of this and other LIV strains are required to characterize the sequence of viruses completely. In addition to the full genome sequence published in 1997, which was originally isolated from an infected tick but serially passaged in cell culture prior to sequencing, a second LIV genome has been sequenced directly from the spinal cord of a sheep with neurological disease signs (Marston et al., 2013). This second genome showed 95.6 % identity with the original LIV genome and 97.5 % at the amino acid level. This information will be important in establishing the extent of LIV variation, in demarcating members of the tick-borne flavivirus group and in understanding the underlying causes for the different virulence properties within the group.
The viruses from the tick-borne encephalitis complex have traditionally been thought to evolve in a clonal manner. However, recent studies into the possibility of recombination have questioned this and it has been proposed that a LIV strain studied was a recombinant with an insertion in the NS3 gene from a strain of TBEV (Bertrand et al., 2012). A further study suggested that this recombination event was likely to have occurred in the LIV strain during in vitro or in vivo testing after isolation, rather than naturally (Norberg et al., 2013). The geographical dispersion of these viruses is thought to act as a barrier to recombination in nature, as recombination requires the viruses to co-exist (Norberg et al., 2013). The viruses from this group tend to be geographically separated; however, there has been one report of the detection of both LIV and TBEV in ticks from Bornholm Island in Denmark (Jensen et al., 2004). This raises questions about the possible future evolution of this complex of viruses should their geographical distributions overlap to a greater extent, which could have implications for virulence, species specificity and therefore virus control and vaccine development (Norberg et al., 2013).
Transmission
The natural vector is the sheep or castor bean tick, Ixodes ricinus, and the distribution of LIV reflects those areas where the ticks are most abundant, mainly upland grazing areas (Fig. 2). Initial experimental infections by Stockman (1918) used sheep ticks obtained from affected farms. Clinical signs were suggestive of LIV but definitive confirmation of causation was complicated by simultaneous infection with another tick-borne pathogen, Anaplasma phagocytophilum, the causative agent of tick-borne fever. Macleod & Gordon (1932) made the association between LIV disease and ticks through the demonstration of disease transmission by inoculation of macerated I. ricinus into susceptible sheep.
Fig. 2.
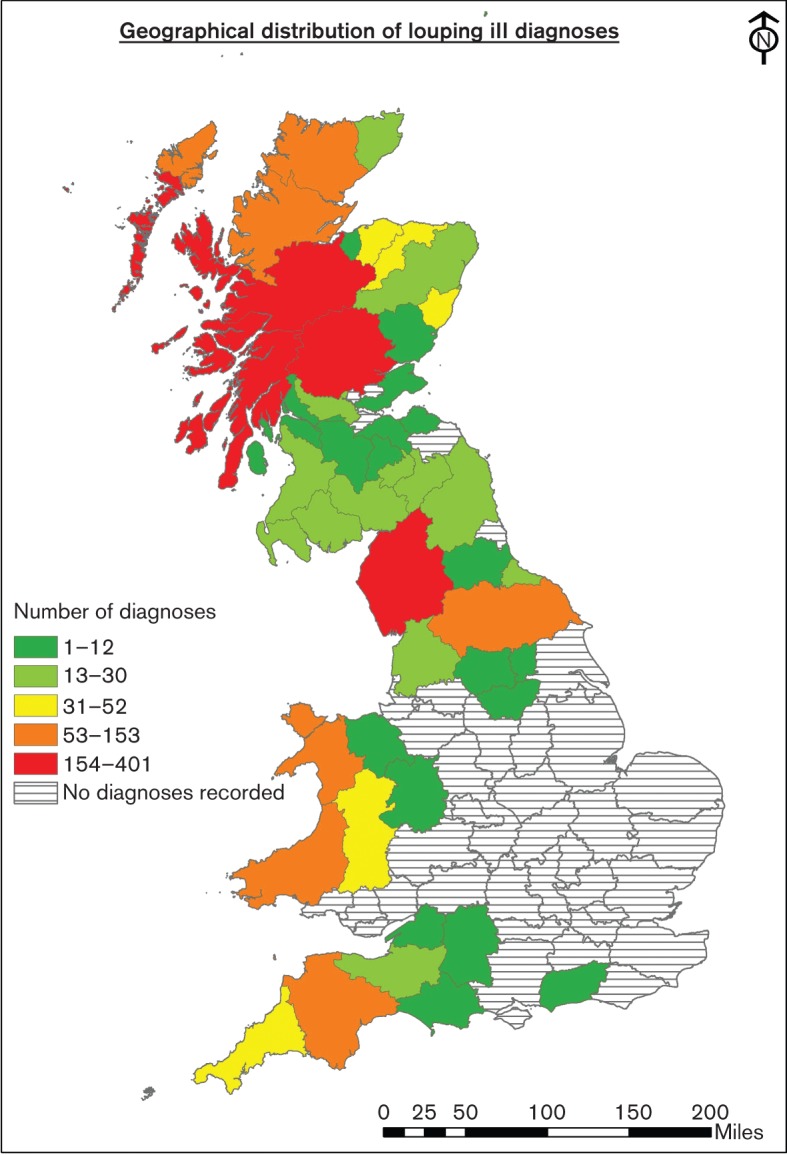
Map of the British Isles showing the distribution and number of LIV diagnoses in animals in British counties from 1975–2013.
There are three feeding stages in the life cycle of the I. ricinus tick: larva, nymph and adult (Gray, 1991). Larvae and nymphs require a blood meal before metamorphosing to the next stage, and mated females require a blood meal before egg development and oviposition can occur. In GB, ticks become active as temperatures rise above 7 °C. A single cohort of each I. ricinus life cycle stage emerges from its previous stage during late summer and autumn. These newly emerged ticks become active and feed if hosts are available in the autumn, or overwinter as unfed ticks and instead feed during the spring and early summer as temperatures rise above threshold levels. Due to the phenomenon of diapause (delay in development in response to environmental cues), ticks that feed in the autumn do not moult until the following autumn. Alternatively, those that feed during spring do not undergo diapause and emerge at the same time, during the autumn months (Randolph et al., 2002). Feeding is preceded by a behaviour called questing, where all stages will climb vegetation to exposed sites and with forelimbs held aloft await chance contact with a host. Movement, warmth and carbon dioxide exhaled by a potential host provide attractants to questing ticks. During hot, dry conditions encountered during the summer, questing ticks show a positive geotropism by moving down into the grass mat, presumably to avoid desiccation and death (MacLeod, 1935). At this time, if energy reserves are depleted the tick will die. Small mammals, birds and reptiles are suitable hosts for juvenile stages but larger mammals, particularly sheep and deer, are preferred by the adult stages (Gilbert et al., 2000). The whole life cycle takes approximately 3 years to complete, although it can take longer. There tend to be seasonal patterns of LIV incidence in spring and late summer/autumn which coincide with the months in which I. ricinus feed (Sargison, 2008). LIV actively replicates in the tick and can survive the transition between stages, known as transtadial transmission, but there is no evidence to date of transovarial transmission of LIV (Hudson et al., 1995).
Transmission by co-feeding, where uninfected ticks can become infected through feeding in close proximity to an infected tick, was initially observed in TBEV transmission (Labuda et al., 1993). A similar mechanism has been reported for LIV transmission in I. ricinus ticks feeding on mountain hares (Jones et al., 1997). There has been much speculation that mammals other than sheep, such as rodents (Gilbert et al., 2000), deer and hares (Laurenson et al., 2003), can act as mammalian reservoirs for LIV. Tick infestations of such species can be high. However, there is still no conclusive evidence that any particular species is vital to the maintenance of LIV in a particular area.
Disease in humans
The first incidence of possible human LIV infection was reported in 1934 (Rivers & Schwentker, 1934) and since then there have been a further 44 published reports of clinical disease in man (Health Protection Agency, 2011). Most historical reports of infection have occurred through occupational exposure to infected livestock. Professionals, such as stockmen, abattoir workers, butchers and veterinarians who have contact with sheep or other potentially infected species, are most at risk (Williams & Thorburn, 1962). Laboratory scientists who work with LIV are also at particular risk, with a number of well-documented cases of laboratory-acquired infection being reported (Reid et al., 1972). Human encounters with ticks in areas where LIV is endemic are unquantified at present, although they may increase in future due to changes in socio-economic and climatic factors (i.e. increased recreational use of the countryside or climate change leading to changes in tick populations) (Mansfield et al., 2009).
The disease in humans can present in a number of ways, and serosurveys of at-risk groups suggest that most exposures are asymptomatic or result in an influenza-like illness with fever, headache and some muscle stiffness. In some cases this is followed by a further period of raised temperature with more severe neurological signs that in one case were fatal (Williams & Thorburn, 1962). In Northern Ireland, four cases of LIV infection presented as poliomyelitis-like disease (Lawson et al., 1949).
No human cases of LIV encephalitis have been definitively diagnosed in GB over the past 20 years. However, there has been a recent case study of a patient who suffered headaches and a febrile illness, progressing to refractory status epilepticus (seizures), where serological and epidemiological evidence implicated LIV as the most likely cause. This case was fatal, but a definitive diagnosis of LIV was not made (Walkington et al., 2013). The reason for the lack of reported human cases over the past few decades is unclear, although numerous cases of encephalitis of unknown origin are reported annually (Davison et al., 2003) and some could represent cases of LIV infection. It is possible that a lack of awareness amongst clinicians or a lack of specific testing may have contributed to an absence of reported cases.
Disease in animals
Sheep are susceptible to LIV infection and develop an encephalitis which is fatal in many cases. Morbidity and mortality rates are dependent on previous exposure status, age and breed, and range from 5 to 60 % (Sargison, 2008; Reid, 2011). Of 506 cases of LIV detected between 1999 and 2012 by the Animal Health and Veterinary Laboratories Agency and the Scottish Agricultural College, 394 were sheep (78 %). The remaining cases were in cattle (n = 94), birds (n = 13) and miscellaneous species (n = 5). The majority of cases in sheep are reported in weaned lambs where maternal-derived antibody has waned and the lambs have been moved to hill pasture. Subsequent tick bites may lead to infection that in sheep manifests as neurological signs, and which in extreme cases causes the ‘leaping’ behaviour from which the virus derives its name from the old Scottish word to ‘loup’ or leap. The disease is characterized by a biphasic fever. Initial signs are non-specific and are often not detected in livestock. The first phase is associated with viraemia whilst the second is correlated with neuroinvasion that initially manifests as depression, panting and nibbling (Reid, 1991) and then into the characteristic signs of disease including muscle tremors, incoordination, circling and ataxia. Animals appear depressed, do not eat and become recumbent, finally developing paralysis leading to death. Those surviving the encephalitic stage may show signs of torticollis (twisted neck syndrome) and paraplegia. Experimentally infected lambs exposed to LIV directly by intracranial inoculation invariably develop neurological disease and die within 6 days (Reid & Doherty, 1971). Inoculations by peripheral routes such as subcutaneous infection show a range of outcomes, from mild fever, recumbency, panting and depression to the full spectrum of neurological disease (Doherty & Reid, 1971a; Sheahan et al., 2002). Disease usually results after an incubation period of between 8 and 13 days. Experimental studies of concurrent infection of LIV with A. phagocytophilum in sheep suggest that pathogenicity is enhanced (Reid et al., 1986). An extensive survey by Reid (1984) of viraemias in a range of animals demonstrated that only sheep can support a sufficiently high viraemia to infect both larval and nymph tick stages.
In addition to sheep, cattle are also occasionally infected with LIV and present with neurological signs. Three cases were reported from Devon with signs of lateral recumbency, convulsions and hyperaesthesia, with one dying within hours of disease onset (Twomey et al., 2001). A number of reports have recorded louping ill disease in horses in Scotland (Fletcher, 1937), Ireland (Timoney et al., 1976) and most recently in England (Hyde et al., 2007). Common features of disease include ataxia, muscle tremors and hyperexitability. In all cases, the affected horses recovered with high titres of anti-LIV antibodies.
Most wildlife species do not show overt disease, although seroprevalence studies suggest that infections do take place; for example, serosurveys have shown the presence of LIV antibodies in Scottish red deer (Adam et al., 1977). Deer were sampled from 16 locations, with seroprevalence for LIV being 31 % in males and 26 % in females. Occasionally cases of disease are observed (Table 1) but these are rarely reported, so it is unclear what the true burden of disease is in wild ungulates.
In addition to livestock losses, a further economic effect of LIV infection is its impact on red grouse (Lagopus scoticus) populations in areas where estates are managed for commercial grouse shooting. Red grouse are highly susceptible to infection, developing high levels of viraemia and thus contributing to the persistence of the virus, providing they do not die, as infection also results in high mortality (Reid et al., 1978b). In addition to tick bites, it is possible that infection results from grouse eating ticks. Experimental infection of red grouse with LIV caused approximately 80 % mortality (Reid, 1975). A later report confirmed this finding and suggested that ingestion of infected ticks might increase transmission rates (Gilbert et al., 2004).
Laboratory mice are also particularly susceptible to louping ill infection. Subcutaneous inoculation leads to disease, which develops within 6 days as ruffled fur, reduced movement and depression associated with viraemia (Sheahan et al., 2002; unpublished data). This then develops into more severe neurological disease and results ultimately in death.
Diagnosis
Observation of clinical signs, together with the knowledge of the presence of ticks on grazing land and a history of the disease within the area, will give a strong suggestion of LIV infection. However, confirmation of the diagnosis by laboratory-based methods is strongly recommended due to the ambiguity of clinical signs. These consist of neurological dysfunction including ataxia, incoordination and posterior paralysis, though sudden deaths have also been reported (Reid, 1991; Reid & Chianini, 2007).
In the field, serology plays a key role in confirming infection. Antibodies produced against the virus have haemagglutination inhibiting properties and the haemagglutination inhibition test can be used as a simple means of monitoring seroconversion in man and livestock (Williams & Thorburn, 1962; Brotherston & Boyce, 1970; Laurenson et al., 2007). Because LIV is cytolytic in tissue culture, a plaque reduction neutralization test using LIV can also be used to measure antiviral titres. Commonly in GB, the presence of IgM in sheep from areas where the disease is endemic is used as an indicator of a recent infection (Reid & Chianini, 2007).
To confirm that the disease observed is caused by viral infection, it is necessary to carry out a pathological examination. Usually, gross lesions are not detected; however, minimal hyperaemia of the meningeal blood vessels can sometimes be observed (Reid & Chianini, 2007). Lesions are restricted to the central nervous system and consist of a non-suppurative polioencephalomyelitis characterized by a combination of neuronal degeneration and necrosis, neuronophagia, multifocal gliosis and perivascular cuffing (Fig. 3a). Various groups of researchers found the most severe lesions in the medulla, pons, cerebellum, midbrain and thalamus of naturally infected sheep (Brownlee & Wilson, 1932; Doherty & Reid, 1971b; Simpson et al., 2003). Lesions can also be observed in the spinal cord, though their distribution has not been studied in detail (Doherty & Reid, 1971b). This type of polioencephalitis is typical for a neurotropic viral infection, but can also be caused by other viruses. Therefore, immunological techniques are commonly used to confirm LIV infection in pathological material (Krueger & Reid, 1994). In British field cases, viral antigen can be observed in the cytoplasm of morphologically normal and degenerated neurons and neuronal processes throughout the brain (Fig. 3b). In protracted cases, immunoreactivity can also be observed in phagocytes in areas of neuronophagia (Sheahan et al., 2002). In a detailed analysis of field cases (Simpson et al., 2003), labelling with mouse monoclonal antibody LM3.3 was observed in a wide range of neurones throughout the brain. Whilst labelling was abundant in most infected cases, in 6 of the 28 cases the labelling was limited and in a very few cases absent. In these chronic cases, loss of Purkinje cells was a consistent finding. In other mammals, the pathology is similar to that observed in sheep (Table 1).
Fig. 3.
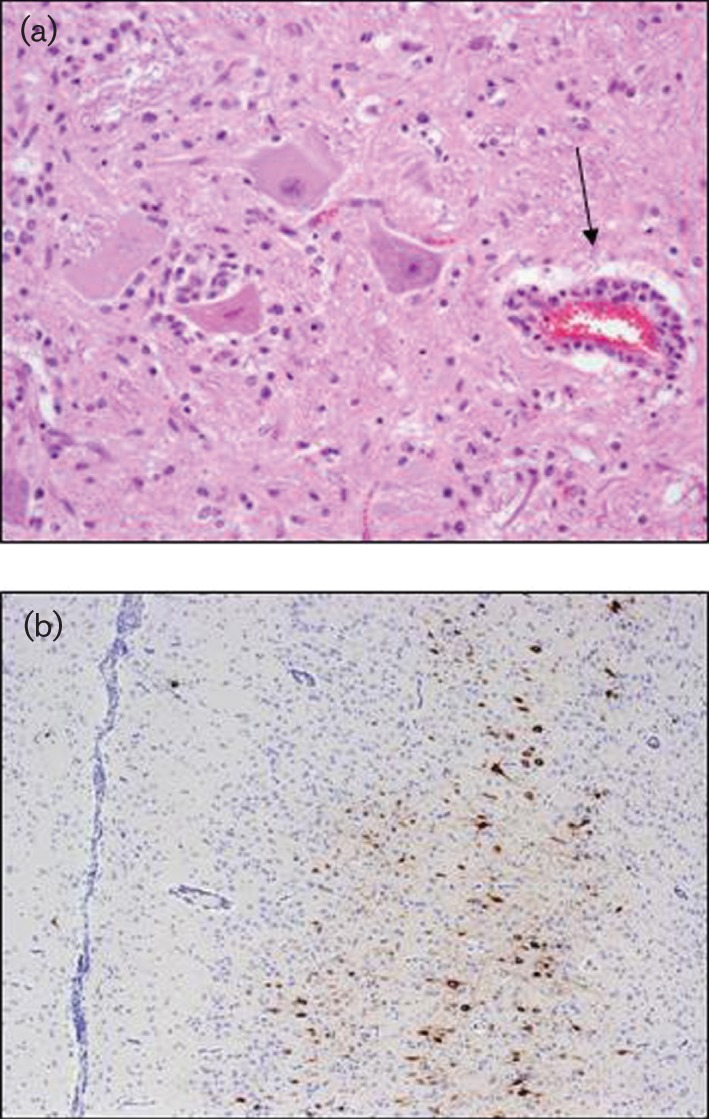
Pathology associated with infection with LIV. (a) Subacute polioencephalitis observed in the brain of a British sheep. Note the perivascular cuff (arrow), neuronophagia, vacuolation of the neuronal cytoplasm and gliosis. Image shows a formalin-fixed section of the red nucleus stained with haematoxylin and eosin (original magnification ×200). (b) Detection of LIV antigen in the cerebral cortex of a sheep with neurological disease. Note the distinct brown labelling of the cortical neurones associated with a subtle perivascular and menigeal response. The staining was obtained using monoclonal antibody LM3.3 kindly provided by Moredun Research Institute (original magnification ×20).
These diagnostic methods have been corroborated by experimental studies in sheep. Animals inoculated subcutaneously to mimic natural infection by ticks show similar lesion distribution to that observed in natural cases, with the most severe lesions being observed in the pons and the ventral horn of the spinal cord (Doherty & Reid, 1971a). Marked cerebellar lesions were observed only in a minority of these cases. Experimental studies using routes other than subcutaneous inoculation tend to result in different antigen distribution (Brownlee & Wilson, 1932; Sheahan et al., 2002). For example, intranasal infection of lambs resulted in marked involvement of the forebrain, including the anterior olfactory nucleus and prepiriform and enterorhinal cortex (Sheahan et al., 2002). In this experiment, viral antigen labelling was more extensive in clinically affected lambs than in apparently clinically normal lambs surviving for 21 days post-infection.
Detection of virus can be achieved by isolation from brain or spinal cord homogenates of suspect animals that have died or undergone euthanasia, though this can be relatively costly. This can either be through virus isolation in susceptible cell lines, such as Vero cells, where the virus is cytopathic, or by intra-cerebral inoculation of mice. Virus isolation from serum is rare due to the early development of antiviral antibodies, often before the appearance of encephalitic signs (Reid & Doherty, 1971).
In recent years, laboratory diagnostic tests have been augmented by molecular detection assays (Johnson et al., 2012). Gaunt et al. (1997) reported a reverse transcriptase (RT)-PCR assay that amplified fragments of the LIV envelope-coding gene. This approach was used to detect LIV in I. ricinus ticks collected from an endemic area in Scotland. A further development has been the publication of a one-step TaqMan RT-PCR (Marriott et al., 2006) that also targets a region of the virus envelope gene. The assay showed similar sensitivity to virus isolation, is rapid and could be applied in cases where virus can not be isolated from tissue due to sample decomposition. Additionally, this method has been used to detect LIV in blood samples from red grouse chicks (Moseley et al., 2007). Various pan-flavivirus detection assays also detect LIV. Confirmation can be made through sequencing the amplicons generated by the PCR (Johnson et al., 2010; Patel et al., 2013).
Control
There is no treatment for louping ill disease in humans or animals. Sedation can be applied to affected animals although this has not been shown to change the outcome of infection and, due to the low value of individual animals that are usually infected, it is often not an economically viable option. Methods available for controlling the disease are principally by vaccination of sheep or control of the tick vector. Land use management strategies such as the rotation of grazing areas to reduce tick build-up are also practised.
The currently available vaccine for animal use is produced commercially and consists of inactivated virus grown in tissue culture mixed with liquid paraffin/montanide as adjuvant. A single subcutaneous injection is sufficient to induce protection for 2 years (Shaw & Reid, 1981). Lambs born to vaccinated ewes acquire passive immunity via the colostrum for the first few weeks of life (MSD Animal Health, Louping ill vaccine data sheet). There are a number of published studies on recombinant Semliki Forest virus particles expressing LIV antigens as new vaccine candidates which demonstrated promising safety and efficacy, and which induced a better protective response in experimentally infected mice (Fleeton et al., 1999, 2000). However, these candidates are not currently commercially available. There is no commercial LIV vaccine licensed for human use; however, neutralizing antibodies produced following immunization with human TBEV vaccines have shown some protective neutralizing capacity against some closely related viruses (Mansfield et al., 2011; Orlinger et al., 2011). This may mean that human TBEV vaccines could provide some cross-protection against LIV infection, although this requires further investigation.
Control of tick infestation is achieved through treatment of sheep with acaricides, although there is increasing evidence for resistance of ticks to commonly used products. Treatment of grouse with acaricides has also been proposed as a method of controlling ticks and therefore LIV burden in these game birds, with a recent modelling study concluding that the efficacy of this control method is likely to be dependent on the densities of deer and grouse in the area (Porter et al., 2013). Treatments can be augmented with land management to reduce the mat of plant material available, which ticks need to survive periods of low humidity.
Conclusions
In GB, LIV is a fatal disease of sheep and red grouse and is detected almost exclusively in upland grazing areas of the British Isles. The exception to this is Northern Ireland and Eire, where environmental conditions are more conducive to tick survival and ticks are encountered more frequently (Gould et al., 2004). Discrete outbreaks of LIV have also been reported in sheep in Norway and goats in Spain, in addition to detection of LIV-like viruses in several European countries, including Turkey, Greece and Spain. LIV is closely related to TBEV which is endemic throughout Eurasia, although not present in the British Isles. The reason for the geographical separation is unclear, as is the basis of the contrasting ability of LIV to cause disease in sheep and other animals, whereas TBEV causes disease in humans but not livestock. Further investigations on the genomes of viruses from the tick-borne flavivirus complex in future should yield explanations for this latter observation. Although a confirmed case of louping ill disease in humans has not been definitively diagnosed for two decades, the disease in sheep and other animals persists and control of LIV remains an important problem confronting upland landowners where sheep and grouse are managed. Vaccination successfully protects livestock but does not appear to eliminate the virus due to persistence in ticks with the involvement of wildlife hosts, and thus remains an ongoing economic burden. The instigation of active surveillance linked to the introduction of validated rapid detection methods would increase understanding of the disease and may provide a basis for establishing more effective control programmes. Strategies that both protect livestock and reduce the persistence of virus through tick control will be of benefit to the farming industry and minimize the impact of disease.
Acknowledgements
This review was funded by the Department of Environment, Food and Rural Affairs (Defra) through grant SE0530.
References
- Adam K. M., Beasley S. J., Blewett D. A. (1977). The occurrence of antibody to Babesia and to the virus of louping-ill in deer in Scotland. Res Vet Sci 23, 133–138. [PubMed] [Google Scholar]
- Balseiro A., Royo L. J., Martínez C. P., Fernández de Mera I. G., Höfle Ú., Polledo L., Marreros N., Casais R., Marín J. F. (2012). Louping ill in goats, Spain, 2011. Emerg Infect Dis 18, 976–978. 10.3201/eid1806.120220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne C. C., Wilson R. L., Reid H. W., Buxton D., Pow I. (1980). Louping-ill virus infection of pigs. Vet Rec 106, 13. 10.1136/vr.106.1.13 [DOI] [PubMed] [Google Scholar]
- Benavides J., Willoughby K., Underwood C., Newman B., Mitchell G., Carty H. (2011). Encephalitis and neuronal necrosis in a 3-month-old suckled beef calf. Vet Pathol 48, E1–E4. 10.1177/0300985810396515 [DOI] [PubMed] [Google Scholar]
- Bertrand Y., Töpel M., Elväng A., Melik W., Johansson M. (2012). First dating of a recombination event in mammalian tick-borne flaviviruses. PLoS ONE 7, e31981. 10.1371/journal.pone.0031981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherston J. G., Boyce J. B. (1970). Development of a non-infective protective antigen against louping-ill (arbovirus group B). Laboratory experiments. J Comp Pathol 80, 377–388. 10.1016/0021-9975(70)90068-X [DOI] [PubMed] [Google Scholar]
- Brownlee A., Wilson D. R. (1932). Studies of the histopathology of louping ill. J Comp Pathol Ther 45, 67–92. 10.1016/S0368-1742(32)80006-8 [DOI] [Google Scholar]
- Calisher C. H., Karabatsos N., Zeller H., Digoutte J. P., Tesh R. B., Shope R. E., Travassos da Rosa A. P., St George T. D. (1989). Antigenic relationships among rhabdoviruses from vertebrates and hematophagous arthropods. Intervirology 30, 241–257. [DOI] [PubMed] [Google Scholar]
- Cranwell M. P., Josephson M., Willoughby K., Marriott L. (2008). Louping ill in an alpaca. Vet Rec 162, 28. 10.1136/vr.162.1.28 [DOI] [PubMed] [Google Scholar]
- Davidson M. M., Williams H., Macleod J. A. J. (1991). Louping ill in man: a forgotten disease. J Infect 23, 241–249. 10.1016/0163-4453(91)92756-U [DOI] [PubMed] [Google Scholar]
- Davison K. L., Crowcroft N. S., Ramsay M. E., Brown D. W. G., Andrews N. J. (2003). Viral encephalitis in England, 1989–1998: what did we miss? Emerg Infect Dis 9, 234–240. 10.3201/eid0902.020218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Reid H. W. (1971a). Experimental louping-ill in sheep and lambs: II. Neuropathology. J Comp Pathol 81, 331–337. 10.1016/0021-9975(71)90020-X [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Reid H. W. (1971b). Louping-ill encephalomyelitis in the sheep: II. Distribution of virus and lesions in nervous tissue. J Comp Pathol 81, 531–536. 10.1016/0021-9975(71)90081-8 [DOI] [PubMed] [Google Scholar]
- Fleeton M. N., Sheahan B. J., Gould E. A., Atkins G. J., Liljeström P. (1999). Recombinant Semliki Forest virus particles encoding the prME or NS1 proteins of louping ill virus protect mice from lethal challenge. J Gen Virol 80, 1189–1198. [DOI] [PubMed] [Google Scholar]
- Fleeton M. N., Liljeström P., Sheahan B. J., Atkins G. J. (2000). Recombinant Semliki Forest virus particles expressing louping ill virus antigens induce a better protective response than plasmid-based DNA vaccines or an inactivated whole particle vaccine. J Gen Virol 81, 749–758. [DOI] [PubMed] [Google Scholar]
- Fletcher J. M. (1937). Louping-ill in the horse. Vet Rec 49, 17–18. [Google Scholar]
- Gao G. F., Hussain M. H., Reid H. W., Gould E. A. (1993a). Classification of a new member of the TBE flavivirus subgroup by its immunological, pathogenetic and molecular characteristics: identification of subgroup-specific pentapeptides. Virus Res 30, 129–144. 10.1016/0168-1702(93)90002-5 [DOI] [PubMed] [Google Scholar]
- Gao G. F., Jiang W. R., Hussain M. H., Venugopal K., Gritsun T. S., Reid H. W., Gould E. A. (1993b). Sequencing and antigenic studies of a Norwegian virus isolated from encephalomyelitic sheep confirm the existence of louping ill virus outside Great Britain and Ireland. J Gen Virol 74, 109–114. 10.1099/0022-1317-74-1-109 [DOI] [PubMed] [Google Scholar]
- Gao G. F., Zanotto P. M. de A., Holmes E. C., Reid H. W., Gould E. A. (1997). Molecular variation, evolution and geographical distribution of louping ill virus. Acta Virol 41, 259–268. [PubMed] [Google Scholar]
- Gaunt M. W., Jones L. D., Laurenson K., Hudson P. J., Reid H. W., Gould E. A. (1997). Definitive identification of louping ill virus by RT-PCR and sequencing in field populations of Ixodes ricinus on the Lochindorb estate. Arch Virol 142, 1181–1191. 10.1007/s007050050151 [DOI] [PubMed] [Google Scholar]
- Gilbert L., Jones L. D., Hudson P. J., Gould E. A., Reid H. W. (2000). Role of small mammals in the persistence of louping-ill virus: field survey and tick co-feeding studies. Med Vet Entomol 14, 277–282. 10.1046/j.1365-2915.2000.00236.x [DOI] [PubMed] [Google Scholar]
- Gilbert L., Jones L. D., Laurenson M. K., Gould E. A., Reid H. W., Hudson P. J. (2004). Ticks need not bite their red grouse hosts to infect them with louping ill virus. Proc Biol Sci 271 (Suppl. 4), S202–S205. 10.1098/rsbl.2003.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. A., Moss S. R., Turner S. L. (2004). Evolution and dispersal of encephalitic flaviviruses. Arch Virol Suppl. 18, 65–84. [DOI] [PubMed] [Google Scholar]
- Grard G., Moureau G., Charrel R. N., Lemasson J.-J., Gonzalez J.-P., Gallian P., Gritsun T. S., Holmes E. C., Gould E. A., de Lamballerie X. (2007). Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology 361, 80–92. 10.1016/j.virol.2006.09.015 [DOI] [PubMed] [Google Scholar]
- Gray D., Webster K., Berry J. E. (1988). Evidence of louping ill and tick-borne fever in goats. Vet Rec 122, 66. 10.1136/vr.122.3.66 [DOI] [PubMed] [Google Scholar]
- Gray J. S. (1991). The development and seasonal activity of the tick Ixodes ricinus: vector of Lyme borreliosis. Rev. Med. Vet. Entomol. 79, 323–333. (http://www.cabdirect.org/abstracts/19910506476.html) [Google Scholar]
- Greig J. R., Brownlee A., Wilson D. R., Gordon W. S. (1931). The nature of louping ill. Vet Rec 11, 325–333. [Google Scholar]
- Gritsun T. S., Venugopal K., Zanotto P. M., Mikhailov M. V., Sall A. A., Holmes E. C., Polkinghorne I., Frolova T. V., Pogodina V. V. & other authors (1997). Complete sequence of two tick-borne flaviviruses isolated from Siberia and the UK: analysis and significance of the 5′ and 3′-UTRs. Virus Res 49, 27–39. 10.1016/S0168-1702(97)01451-2 [DOI] [PubMed] [Google Scholar]
- Gritsun T. S., Lashkevich V. A., Gould E. A. (2003a). Tick-borne encephalitis. Antiviral Res 57, 129–146. 10.1016/S0166-3542(02)00206-1 [DOI] [PubMed] [Google Scholar]
- Gritsun T. S., Nuttall P. A., Gould E. A. (2003b). Tick-borne flaviviruses. Adv Virus Res 61, 317–371. 10.1016/S0065-3527(03)61008-0 [DOI] [PubMed] [Google Scholar]
- Health Protection Agency (now Public Health England) . (2011). Louping ill virus general information data sheet. [Last updated 14 June 2011]. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/LoupingIll/GeneralInformation/ Accessed 18 April 2013. [Google Scholar]
- Hudson P. J., Norman R., Laurenson M. K., Newborn D., Gaunt M., Jones L., Reid H., Gould E., Bowers R., Dobson A. (1995). Persistence and transmission of tick-borne viruses: Ixodes ricinus and louping-ill virus in red grouse populations. Parasitology 111 (Suppl.), S49–S58. 10.1017/S0031182000075818 [DOI] [PubMed] [Google Scholar]
- Hyde J., Nettleton P., Marriott L., Willoughby K. (2007). Louping ill in horses. Vet Rec 160, 532. 10.1136/vr.160.15.532 [DOI] [PubMed] [Google Scholar]
- Jensen P. M., Skarphedinsson S., Semenov A. (2004). [Densities of the tick (Ixodes ricinus) and coexistence of the louping ill virus and tick borne encephalitis virus on the island of Bornholm]. Ugeskr Laeger 166, 2563–2565 [in Danish]. [PubMed] [Google Scholar]
- Johnson N., Wakeley P. R., Mansfield K. L., McCracken F., Haxton B., Phipps L. P., Fooks A. R. (2010). Assessment of a novel real-time pan-flavivirus RT-polymerase chain reaction. Vector Borne Zoonotic Dis 10, 665–671. 10.1089/vbz.2009.0210 [DOI] [PubMed] [Google Scholar]
- Johnson N., Voller K., Phipps L. P., Mansfield K., Fooks A. R. (2012). Rapid molecular detection methods for arboviruses of livestock of importance to northern Europe. J Biomed Biotechnol 2012, 719402. 10.1155/2012/719402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. D., Gaunt M., Hails R. S., Laurenson K., Hudson P. J., Reid H., Henbest P., Gould E. A. (1997). Transmission of louping ill virus between infected and uninfected ticks co-feeding on mountain hares. Med Vet Entomol 11, 172–176. 10.1111/j.1365-2915.1997.tb00309.x [DOI] [PubMed] [Google Scholar]
- Krueger N., Reid H. W. (1994). Detection of louping ill virus in formalin-fixed, paraffin wax-embedded tissues of mice, sheep and a pig by the avidin-biotin-complex immunoperoxidase technique. Vet Rec 135, 224–225. 10.1136/vr.135.10.224 [DOI] [PubMed] [Google Scholar]
- Labuda M., Danielova V., Jones L. D., Nuttall P. A. (1993). Amplification of tick-borne encephalitis virus infection during co-feeding of ticks. Med Vet Entomol 7, 339–342. 10.1111/j.1365-2915.1993.tb00702.x [DOI] [PubMed] [Google Scholar]
- Laurenson M. K., Norman R. A., Gilbert L., Reid H. W., Hudson P. J. (2003). Identifying disease reservoirs in complex systems: mountain hares as reservoirs of ticks and louping-ill virus, pathogens of red grouse. J Anim Ecol 72, 177–185. 10.1046/j.1365-2656.2003.00688.x [DOI] [Google Scholar]
- Laurenson M. K., McKendrick I. J., Reid H. W., Challenor R., Mathewson G. K. (2007). Prevalence, spatial distribution and the effect of control measures on louping-ill virus in the Forest of Bowland, Lancashire. Epidemiol Infect 135, 963–973. 10.1017/S0950268806007692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J. H., Manderson W. G., Weston Hurst E. (1949). Louping-ill meningo-encephalitis: a further case and a serological survey. Lancet 254, 696–699. 10.1016/S0140-6736(49)91328-8 [DOI] [PubMed] [Google Scholar]
- Macaldowie C., Patterson I. A. P., Nettleton P. F., Low H., Buxton D. (2005). Louping ill in llamas (Lama glama) in the Hebrides. Vet Rec 156, 420–421. [DOI] [PubMed] [Google Scholar]
- MacKenzie C. P., Lewis N. D., Smith S. T., Muir R. W. (1973). Louping-ill in a working collie. Vet Rec 92, 354–356. 10.1136/vr.92.14.354 [DOI] [PubMed] [Google Scholar]
- MacLeod J. (1935). Ixodes ricinus in relation to its physical environment: II. The factors governing survival and activity. Parasitology 27, 123–144. 10.1017/S0031182000015006 [DOI] [Google Scholar]
- Macleod J., Gordon W. S. (1932). Studies in louping ill: II. Transmission by the sheep tick, Ixodes ricinus L. J Comp Pathol Ther 45, 240–252. 10.1016/S0368-1742(32)80022-6 [DOI] [Google Scholar]
- Mansfield K. L., Johnson N., Phipps L. P., Stephenson J. R., Fooks A. R., Solomon T. (2009). Tick-borne encephalitis virus – a review of an emerging zoonosis. J Gen Virol 90, 1781–1794. 10.1099/vir.0.011437-0 [DOI] [PubMed] [Google Scholar]
- Mansfield K. L., Horton D. L., Johnson N., Li L., Barrett A. D. T., Smith D. J., Galbraith S. E., Solomon T., Fooks A. R. (2011). Flavivirus-induced antibody cross-reactivity. J Gen Virol 92, 2821–2829. 10.1099/vir.0.031641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M. S., McKenzie J., Gao G. F., Reid H. W., Antoniadis A., Gould E. A. (1995). The virus causing encephalomyelitis in sheep in Spain: a new member of the tick-borne encephalitis group. Res Vet Sci 58, 11–13. 10.1016/0034-5288(95)90081-0 [DOI] [PubMed] [Google Scholar]
- Marriott L., Willoughby K., Chianini F., Dagleish M. P., Scholes S., Robinson A. C., Gould E. A., Nettleton P. F. (2006). Detection of Louping ill virus in clinical specimens from mammals and birds using TaqMan RT-PCR. J Virol Methods 137, 21–28. 10.1016/j.jviromet.2006.05.025 [DOI] [PubMed] [Google Scholar]
- Marston D. A., Mansfield K. L., Mearns R., Ellis R. J., Fooks A. R., Johnson N. (2013). Louping ill virus genome sequence derived from the spinal cord of an infected lamb. Genome Announc 1, e00454-e13. 10.1128/genomeA.00454-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire K., Holmes E. C., Gao G. F., Reid H. W., Gould E. A. (1998). Tracing the origins of louping ill virus by molecular phylogenetic analysis. J Gen Virol 79, 981–988. [DOI] [PubMed] [Google Scholar]
- Moseley M. H., Marriott L., Nettleton P., Dukes J., Irvine R. J., Mougeot F. (2007). Use of real-time RT-PCR to determine the prevalence of louping ill virus in live red grouse chicks. Vet Rec 161, 660–661. 10.1136/vr.161.19.660 [DOI] [PubMed] [Google Scholar]
- Norberg P., Roth A., Bergström T. (2013). Genetic recombination of tick-borne flaviviruses among wild-type strains. Virology 440, 105–116. 10.1016/j.virol.2013.02.017 [DOI] [PubMed] [Google Scholar]
- Orlinger K. K., Hofmeister Y., Fritz R., Holzer G. W., Falkner F. G., Unger B., Loew-Baselli A., Poellabauer E. M., Ehrlich H. J. & other authors (2011). A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J Infect Dis 203, 1556–1564. 10.1093/infdis/jir122 [DOI] [PubMed] [Google Scholar]
- Papa A., Pavlidou V., Antoniadis A. (2008). Greek goat encephalitis virus strain isolated from Ixodes ricinus, Greece. Emerg Infect Dis 14, 330–332. 10.3201/eid1402.070889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Landt O., Kaiser M., Faye O., Koppe T., Lass U., Sall A. A., Niedrig M. (2013). Development of one-step quantitative reverse transcription PCR for the rapid detection of flaviviruses. Virol J 10, 58. 10.1186/1743-422X-10-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R., Norman R. A., Gilbert L. (2013). A model to test how ticks and louping ill virus can be controlled by treating red grouse with acaricide. Med Vet Entomol 27, 237–246. 10.1111/j.1365-2915.2012.01047.x [DOI] [PubMed] [Google Scholar]
- Randolph S. E., Green R. M., Hoodless A. N., Peacey M. F. (2002). An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int J Parasitol 32, 979–989. 10.1016/S0020-7519(02)00030-9 [DOI] [PubMed] [Google Scholar]
- Reid H. W. (1975). Experimental infection of red grouse with louping-ill virus (Flavivirus group): I. The viraemia and antibody response. J Comp Pathol 85, 223–229. 10.1016/0021-9975(75)90063-8 [DOI] [PubMed] [Google Scholar]
- Reid H. (1984). Epidemiology of louping-ill. In Vectors in Virus Biology, pp. 161–178. Edited by Mayo M. A., Harrap K. A. London: Academic Press. [Google Scholar]
- Reid H. W. (1991). Louping Ill. In Practice 13, 157–160. 10.1016/0021-9975(71)90103-4 [DOI] [PubMed] [Google Scholar]
- Reid H. W. (2011). Louping ill (ovine encephalomyelitis): etiology and transmission. The Merck Veterinary Manual. [Last full review/revision July 2011]. http://www.merckmanuals.com/vet/nervous_system/louping_ill_ovine_encephalomyelitis/overview_of_louping_ill.html Accessed 19 April 2013. [Google Scholar]
- Reid H. W., Boyce J. B. (1974). Louping-ill virus in red grouse in Scotland. Vet Rec 95, 150. 10.1136/vr.95.7.150-a [DOI] [PubMed] [Google Scholar]
- Reid H. W., Chianini F. (2007). Louping ill. In Diseases of Sheep, 4th edn, pp. 250–255. Edited by Aitken I. D. Oxford: Blackwell Publishing; 10.1002/9780470753316.ch36 [DOI] [Google Scholar]
- Reid H. W., Doherty P. C. (1971). Experimental louping-ill in sheep and lambs: I. Viraemia and the antibody response. J Comp Pathol 81, 291–298. 10.1016/0021-9975(71)90103-4 [DOI] [PubMed] [Google Scholar]
- Reid H. W., Gibbs C. A., Burrells C., Doherty P. C. (1972). Laboratory infections with louping-ill virus. Lancet 299, 592–593. 10.1016/S0140-6736(72)90385-6 [DOI] [PubMed] [Google Scholar]
- Reid H. W., Barlow R. M., Boyce J. B. (1976). Isolation of louping-ill virus from a roe deer (Capreolus capreolus). Vet Rec 98, 116. 10.1136/vr.98.6.116 [DOI] [PubMed] [Google Scholar]
- Reid H. W., Barlow R. M., Pow I. (1978a). Isolation of louping-ill virus from red deer (Cervus elaphus). Vet Rec 102, 463–464. 10.1136/vr.102.21.463 [DOI] [PubMed] [Google Scholar]
- Reid H. W., Duncan J. S., Phillips J. D. P., Moss R., Watson A. (1978b). Studies of louping-ill virus (Flavivirus group) in wild red grouse (Lagopus lagopus scoticus). J Hyg (Lond) 81, 321–329. 10.1017/S002217240002516X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid H. W., Buxton D., Pow I., Brodie T. A., Holmes P. H., Urquhart G. M. (1986). Response of sheep to experimental concurrent infection with tick-borne fever (Cytoecetes phagocytophila) and louping-ill virus. Res Vet Sci 41, 56–62. [PubMed] [Google Scholar]
- Rivers T. M., Schwentker F. F. (1934). Louping ill in man. J Exp Med 59, 669–685. 10.1084/jem.59.5.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross H. M., Evans C. C., Spence J. A., Reid H. W., Krueger N. (1994). Louping ill in free-ranging pigs. Vet Rec 134, 99–100. 10.1136/vr.134.4.99 [DOI] [PubMed] [Google Scholar]
- Sargison N. (2008). Sheep Flock Health: A Planned Approach. Oxford: Blackwell Publishing; 10.1002/9781444302592 [DOI] [Google Scholar]
- Shaw B., Reid H. W. (1981). Immune responses of sheep to louping-ill virus vaccine. Vet Rec 109, 529–531. [PubMed] [Google Scholar]
- Sheahan B. J., Moore M., Atkins G. J. (2002). The pathogenicity of louping ill virus for mice and lambs. J Comp Pathol 126, 137–146. 10.1053/jcpa.2001.0533 [DOI] [PubMed] [Google Scholar]
- Simpson E., Schock A., Nettleton P. F., Holliman A., Watson P. J., Scholes S. F. (2003). Ovine louping ill encephalitis: Lesion mapping and antigen distribution. In European Society of Veterinary Pathology, 21st Annual Meeting, Dublin, 2003. [Google Scholar]
- Smith C. E., McMahon D. A., O’Reilly K. J., Wilson A. L., Robertson J. M. (1964a). The epidemiology of louping ill in Ayrshire: the first year of studies in sheep. J Hyg (Lond) 62, 53–68. 10.1017/S0022172400039772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. E., Varma M. G., McMahon D. (1964b). Isolation of louping ill from small mammals in Ayrshire, Scotland. Nature 203, 992–993. 10.1038/203992a0 [DOI] [PubMed] [Google Scholar]
- Stockman S. (1918). Louping-ill. J. Comp. Pathol. Therap. 31, 137–193. 10.1016/S0368-1742(18)80019-4 [DOI] [Google Scholar]
- Timoney P. J., Donnelly W. J., Clements L. O., Fenlon M. (1976). Encephalitis caused by louping ill virus in a group of horses in Ireland. Equine Vet J 8, 113–117. 10.1111/j.2042-3306.1976.tb03311.x [DOI] [PubMed] [Google Scholar]
- Twomey D. F., Cranwell M. P., Reid H. W., Tan J. F. V. (2001). Louping ill on Dartmoor. Vet Rec 149, 687. [PubMed] [Google Scholar]
- Walkington J., Hulgur M., Yates D. (2013). A case of refractory seizures caused by an unusual zoonosis. J Intensive Care Soc 14, 65–67. http://journal.ics.ac.uk/pdf/1401065.pdf [Google Scholar]
- Whitby J. E., Whitby S. N., Jennings A. D., Stephenson J. R., Barrett A. D. T. (1993). Nucleotide sequence of the envelope protein of a Turkish isolate of tick-borne encephalitis (TBE) virus is distinct from other viruses of the TBE virus complex. J Gen Virol 74, 921–924. 10.1099/0022-1317-74-5-921 [DOI] [PubMed] [Google Scholar]
- Williams H., Thorburn H. (1962). Serum antibodies to louping-ill virus. Scott Med J 7, 353–355. [DOI] [PubMed] [Google Scholar]
- Williams H., Thorburn H., Ziffo G. S. (1963). Isolation of louping ill from the red grouse. Nature 200, 193–194. 10.1038/200193a0 [DOI] [PubMed] [Google Scholar]
- Zanotto P. M. de A., Gao G. F., Gritsun T., Marin M. S., Jiang W. R., Venugopal K., Reid H. W., Gould E. A. (1995). An arbovirus cline across the northern hemisphere. Virology 210, 152–159. 10.1006/viro.1995.1326 [DOI] [PubMed] [Google Scholar]


