Abstract
The standard for Clostridium difficile surface decontamination is bleach solution at a concentration of 10 % of sodium hypochlorite. Pulsed xenon UV light (PX-UV) is a means of quickly producing germicidal UV that has been shown to be effective in reducing environmental contamination by C. difficile spores. The purpose of this study was to investigate whether PX-UV was equivalent to bleach for decontamination of surfaces in C. difficile infection isolation rooms. High-touch surfaces in rooms previously occupied by C. difficile infected patients were sampled after discharge but before and after cleaning using either bleach or non-bleach cleaning followed by 15 min of PX-UV treatment. A total of 298 samples were collected by using a moistened wipe specifically designed for the removal of spores. Prior to disinfection, the mean contamination level was 2.39 c.f.u. for bleach rooms and 22.97 for UV rooms. After disinfection, the mean level of contamination for bleach was 0.71 c.f.u. (P = 0.1380), and 1.19 c.f.u. (P = 0.0017) for PX-UV disinfected rooms. The difference in final contamination levels between the two cleaning protocols was not significantly different (P = 0.9838). PX-UV disinfection appears to be at least equivalent to bleach in the ability to decrease environmental contamination with C. difficile spores. Larger studies are needed to validate this conclusion.
Background
Environmental contamination has been identified as an important factor for the transmission of Clostridium difficile (Cristina et al., 2013; Weber et al., 2013). According to current C. difficile control guidelines, rooms previously occupied by patients with C. difficile infection (CDI) should be cleaned with an Environmental Protection Agency-approved disinfectant that is registered as effective against C. difficile (Cohen et al., 2010; Dubberke, 2012; Carrico et al., 2013). Thorough cleaning of these rooms is essential, but is often difficult to achieve on multiple surfaces and complex equipment. C. difficile endospores have been found to survive up to 5 months in a hospital environment (Cohen et al., 2010; Hacek et al., 2010; Carrico et al., 2013). C. difficile has also been shown to be resistant to alcohol-based disinfectants and quaternary ammonium compounds, and some detergents may even encourage sporulation of this organism (Fraise, 2011).
The most common disinfectant used for C. difficile is a 1 : 10 dilution of sodium hypochlorite (bleach). Terminal cleaning with bleach of rooms once occupied by patients with CDI has shown a significant reduction in the rate of nosocomial CDI (Hacek et al., 2010). Although effective when applied for the correct dwell times, long-term use of bleach may cause corrosion, harmful effects on some metals and pitting of equipment and other surfaces (Carrico et al., 2013). Bleach also produces fumes that have led to respiratory complaints from environmental service workers and may also cause irritation to mucous membranes as well as emitting odour (Carrico et al., 2013).
Germicidal UV irradiation has been shown to be effective in deactivating C. difficile endospores in laboratory and clinical settings (Nerandzic et al., 2010; Rutala et al., 2010; Stibich et al., 2011). Specifically, the UV-C frequency, which ranges from 200 to 280 nm, is known to have germicidal properties through a variety of mechanisms, including photodimerization, photohydration, photo cross-linking, and photoseparation (Rutala et al., 2010; Stibich et al., 2011). Pulsed xenon UV (PX-UV) is a means of quickly producing germicidal UV that has been shown effective against C. difficile (Stibich et al., 2011). PX-UV light may have greater efficacy than other forms of UV, such as mercury UV, against C. difficile because of the broad spectrum produced within the UV-C range and a greater intensity (Levin et al., 2013; Qureshi & Yassin, 2013; Rutala & Weber, 2013). PX-UV technology has been associated with a reduction in facility-wide C. difficile rates (Levin et al., 2013).
The purpose of the current study was to assess whether PX-UV cleaning and bleach were equivalent in terms of reducing environmental C. difficile contamination and to determine whether PX-UV could be an alternative to corrosive chemicals for the disinfection of C. difficile infection isolation rooms in the clinical setting.
Methods
The study was conducted at a major comprehensive cancer centre in the United States. The environmental surfaces in 30 C. difficile infection isolation rooms were sampled immediately after patients with a CDI were discharged. Five surfaces in each room were sampled before being cleaned, and the same five surfaces were sampled after being cleaned via one of two protocols.
In the first arm of the study, 15 rooms were cleaned according to the standard protocol, which included bleach disinfection. In the second arm, 15 rooms were visually cleaned without bleach and then disinfected using a PX-UV device (Xenex Disinfection Services). After the samples were collected post PX-UV clean, these 15 rooms were again cleaned with bleach disinfection to be in accordance with the standard cleaning protocol. The samples were taken from the following five surfaces: (1) the bathroom handrail, horizontal/vertical surface facing into the room; (2) the bed control panel, on the side facing the door; (3) the bedrail, at the midpoint upper surface; (4) the top of the bedside table, near the centre; and (5) an IV pump control panel or other equipment control panel, when available.
Disinfection arm 1: bleach.
Rooms were visually cleaned using an activated hydrogen peroxide disinfectant and then with bleach at a concentration of 10 % of sodium hypochlorite according to guidelines published by the Society for Healthcare Epidemiology of America (Cohen et al., 2010).
Disinfection arm 2: PX-UV light.
After a visual cleaning using an activated hydrogen peroxide disinfectant and without bleach, a PX-UV device was placed in the bathroom and on both sides of the bed and then run for 5 min in each position (Fig. 1).
Fig. 1.
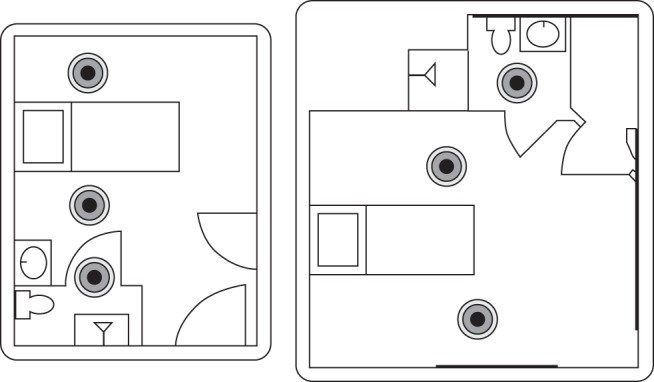
PX-UV device positions.
Device description.
The PX-UV device contains a xenon flash lamp that emits a broad spectrum of light covering the germicidal, or UV-C, spectrum of 200–280 nm as well as the visible light spectrum (Nerandzic et al., 2010). The device weighs approximately 150 lb (68 kg) and has dimensions of 20 in (51 cm) wide by 30 in (76 cm) long by 38 in (97 cm) high. The PX-UV system produces a pulsed flash at a frequency of 1.5 Hz with an approximate output of 505 J per pulse and duration of less than 360 µs. The device is typically operated by housekeeping personnel and includes safety features such as motion sensors. The operating time for the device for C. difficile deactivation is 5 min per position for a total of three positions based on the average size of each room at our institution.
Laboratory analysis.
Environmental samples were taken from five high-touch surfaces in rooms in which the previous occupant had been diagnosed with CDI. Briefly, sponges were moistened with 1 ml of sterile normal saline (0.9 %, w/v, NaCl) and used (with sterile gloves) to scrub the surfaces. The sponge was then placed in a 50 ml conical tube containing 10 ml sterile 10 % (v/v) Dey–Engley neutralizing broth (Difco, BD) and refrigerated. The contents of each tube were passed through a 0.45 µm membrane filter (EMD Millipore). Each tube was washed two additional times with 30 ml sterile PBS, and each wash volume was passed through the same membrane filter. The membrane filter was placed directly onto C. difficile agar supplemented with 7 % horse blood, 0.5 g l−1 cycloserine, 0.016 g l−1 cefoxitin, 1 g l−1 taurocholate and 10 mg l−1 lysozyme. All enumeration plates were incubated anaerobically for 48±4 h at 36±1 °C. Samples demonstrating growth were morphologically compared with C. difficile (ATCC 43598) grown on the same membrane filter type. Alternatively, samples demonstrating growth were subcultured onto supplemented C. difficile agar, incubated anaerobically, compared with C. difficile (ATCC 43598), and subjected to Gram staining for confirmation. The c.f.u. were determined by directly counting the colonies on each filter. Each filter represented one submitted sample (e.g. sponge), and more than 100 colonies were determined to be too numerous to count.
Ethics.
Ethical approval was not sought because this was a study evaluating the performance of two cleaning protocols.
Statistical analysis.
Because a non-parametric distribution of the data was likely, the Wilcoxon rank-sum test was used to compare the mean C. difficile colony counts for the bleach and PX-UV disinfected rooms.
Results
In the pre-disinfection period, 26 of 74 samples were positive for C. difficile (mean 2.39 c.f.u.) in the bleach arm. After disinfection with bleach, 18 of 74 samples were positive (mean 0.71 c.f.u.), a decrease of 70 % (P = 0.1380). In the PX-UV disinfection arm, 34 of 75 samples were positive (mean 22.97 c.f.u.). This decreased to 18 samples (mean 1.19 c.f.u.) after PX-UV disinfection, a significant reduction of 95 % in the level of environmental contamination as measured by bacterial load (P = 0.0017).
Because five of the samples in the pre-group were outliers and created high leverage in favour of the UV device, an analysis was done with these outliers removed, and the results are shown in Table 1. In the pre-disinfection period, 29 of 70 samples were positive for C. difficile (mean 4.61 c.f.u.) in the PX-UV (Table 1). After disinfection with PX-UV, 16 of 70 samples were positive (mean 0.80 c.f.u.), a decrease of 83 % (P = 0.007).
Table 1. Impact of standard cleaning and PX-UV disinfection on Clostridium difficile counts in patient rooms.
| Room status | Samples taken (n) | Samples positive for C. difficile [n (%)] | c.f.u. | Reduction (%) | P-value | ||||
| Min. | Mean | Median | Max. | IQR | |||||
| Pre-bleach cleaning | 74 | 26 (35) | 0 | 2.39 | 0 | 81 | 11 | 70 | 0.13 |
| Post-bleach cleaning | 74 | 18 (24) | 0 | 0.71 | 0 | 18 | 0 | ||
| Pre-PX-UV | 70 | 29 (41) | 0 | 4.61 | 0 | 71 | 2 | 83 | 0.007 |
| Cleaning | |||||||||
| Post-PX-UV | 70 | 16 (23) | 0 | 0.80 | 0 | 13 | 0 | ||
IQR, Interquartile range.
Outcomes of the two methods of environmental cleaning were compared using the Wilcoxon–Mann–Whitney two-sample rank-sum test (12 observations in 2012 for bleach alone and 12 in 2013 for PX-UV alone), which confirmed the equivalence of these two methods of cleaning (z = 0.058).
Discussion
The current study shows that PX-UV disinfection appears to be equivalent to bleach in decreasing environmental contamination with C. difficile spores. The pre-disinfection C. difficile contamination level in the second arm was approximately 8.6 times higher than that in the bleach arm, although the reason for this difference in c.f.u. is unclear.
The study is limited by the small sample size and the targeted nature of the surface sampling. An additional limitation is the use of a customized laboratory protocol that, although validated in the laboratory, could have introduced additional variables.
In summary, both methods of disinfection, bleach and PX-UV light, produced equivalent results in reducing the level of C. difficile contamination in patient rooms. PX-UV technology may be an attractive alternative to bleach for the disinfection of C. difficile environmental surfaces. Other technologies, such as the use of hydrogen peroxide vapour (HPV) for decontamination, have also been investigated for decontaminating rooms occupied by patients with CDI (Boyce et al., 2008; Zoutman et al., 2011; Passaretti et al., 2013). In spite of showing some promise in eliminating C. difficile from the room surfaces (Boyce et al., 2008), the HPV method has some drawbacks. The time taken for disinfection is between 2 and 4.5 h, which may present a real challenge for routine use, especially when a rapid bed turnaround time is necessary (Otter et al., 2009). Environmental cleaning with HPV is also an intensive procedure requiring specialized personnel and equipment, increasing the operational costs of using this method (Blazejewski et al., 2011). On the other hand, one of the limitations of the UV technology when compared with HPV is that the former does not disinfect evenly throughout a room, with areas in shadow receiving a smaller dose than directly exposed areas. This could be lessened by manually moving the PX-UV device to two or three locations within each room.
PX-UV technology can be easily incorporated in routine environmental decontamination and has a potentially faster turnaround time than either HPV or bleach. In staff time, HPV costs approximately $175 per room (Doan et al., 2012). It takes approximately 45 min to clean a room with bleach and 15 min with PX-UV, resulting in staff savings (Boschert, 2012). The PX-UV device costs approximately $3000 per month and can disinfect more than 30 rooms per day at a per-room cost of approximately $3 excluding labour costs. Five-year materials damage testing has not indicated that PX-UV causes any kind of damage to materials in hospital settings.
Additional studies on a larger scale are needed to verify these findings and to determine their impact on CDI rates.
Acknowledgements
This research was supported in part by the National Institutes of Health through MD Anderson Cancer Center Support Grant CA016672. Xenex Disinfection Services, LLC, provided funding for laboratory analysis. Presented in part at the Fifty-second Interscience Conference on Antimicrobial Agents & Chemotherapy (ICAAC) on 10 September 2012 in San Francisco, CA (Poster K-934). Editorial support for this manuscript was provided by Innovative Strategic Communications, LLC, and was funded by Xenex Disinfection Services, LLC. Conflicts of Interest: F. C. has received Research Grants and acts as a consultant to Xenex Disinfection Services, LLC. M. S. and J. S. are employed by Xenex Disinfection Services, LLC. All other authors have no conflict of interest to declare.
Abbreviations:
- CDI
C. difficile infection
- PX
pulsed xenon
References
- Blazejewski C., Guerry M. J., Preau S., Durocher A., Nseir S. (2011). New methods to clean ICU rooms. Infect Disord Drug Targets 11, 365–375. 10.2174/187152611796504818 [DOI] [PubMed] [Google Scholar]
- Boschert S. (2012). UV light beat bleach for C. difficile decontamination. Family Practice News. http://www.familypracticenews.com/news/infectious-diseases/single-article/uv-light-beat-bleach-for-ic-difficile-idecontamination/d75849f049f47fcfed3ed70bcc7bf6ab.html
- Boyce J. M., Havill N. L., Otter J. A., McDonald L. C., Adams N. M., Cooper T., Thompson A., Wiggs L., Killgore G., et al. (2008). Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect Control Hosp Epidemiol 29, 723–729. 10.1086/589906 [DOI] [PubMed] [Google Scholar]
- Carrico R. M., Bryant K., Lessa F., Limbago B., Fauerbach L. L., Marx J. F., Sands F., Stephens D., Westhusing K., Wiemken T. (2013). Guide to Preventing Clostridium Difficile Infections. Washington, DC: Association for Professionals in Infection Control and Epidemiology (APIC). [Google Scholar]
- Cohen S. H., Gerding D. N., Johnson S., Kelly C. P., Loo V. G., McDonald L. C., Pepin J., Wilcox M. H., Society for Healthcare Epidemiology of America. Infectious Diseases Society of America (2010). Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31, 431–455. 10.1086/651706 [DOI] [PubMed] [Google Scholar]
- Cristina M. L., Spagnolo A. M., Orlando P., Perdelli F. (2013). The role of the environment in the spread of emerging pathogens in at-risk hospital wards. Rev Med Microbiol 24, 104–112. 10.1097/MRM.0b013e328365c506 [DOI] [Google Scholar]
- Doan L., Forrest H., Fakis A., Craig J., Claxton L., Khare M. (2012). Clinical and cost effectiveness of eight disinfection methods for terminal disinfection of hospital isolation rooms contaminated with Clostridium difficile 027. J Hosp Infect 82, 114–121. 10.1016/j.jhin.2012.06.014 [DOI] [PubMed] [Google Scholar]
- Dubberke E. (2012). Strategies for prevention of Clostridium difficile infection. J Hosp Med 7 (Suppl. 3), S14–S17. 10.1002/jhm.1908 [DOI] [PubMed] [Google Scholar]
- Fraise A. (2011). Currently available sporicides for use in healthcare, and their limitations. J Hosp Infect 77, 210–212. 10.1016/j.jhin.2010.06.029 [DOI] [PubMed] [Google Scholar]
- Hacek D. M., Ogle A. M., Fisher A., Robicsek A., Peterson L. R. (2010). Significant impact of terminal room cleaning with bleach on reducing nosocomial Clostridium difficile. Am J Infect Control 38, 350–353. 10.1016/j.ajic.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Levin J., Riley L. S., Parrish C., English D., Ahn S. (2013). The effect of portable pulsed xenon ultraviolet light after terminal cleaning on hospital-associated Clostridium difficile infection in a community hospital. Am J Infect Control 41, 746–748. 10.1016/j.ajic.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Nerandzic M. M., Cadnum J. L., Pultz M. J., Donskey C. J. (2010). Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis 10, 197. 10.1186/1471-2334-10-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J. A., Puchowicz M., Ryan D., Salkeld J. A., Cooper T. A., Havill N. L., Tuozzo K., Boyce J. M. (2009). Feasibility of routinely using hydrogen peroxide vapor to decontaminate rooms in a busy United States hospital. Infect Control Hosp Epidemiol 30, 574–577. 10.1086/597544 [DOI] [PubMed] [Google Scholar]
- Passaretti C. L., Otter J. A., Reich N. G., Myers J., Shepard J., Ross T., Carroll K. C., Lipsett P., Perl T. M. (2013). An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin Infect Dis 56, 27–35. 10.1093/cid/cis839 [DOI] [PubMed] [Google Scholar]
- Qureshi Z., Yassin M. H. (2013). Role of ultraviolet (UV) disinfection in infection control and environmental cleaning. Infect Disord Drug Targets 13, 191–195. 10.2174/1871526511313030007 [DOI] [PubMed] [Google Scholar]
- Rutala W. A., Weber D. J. (2013). Disinfectants used for environmental disinfection and new room decontamination technology. Am J Infect Control 41 (Suppl.), S36–S41. 10.1016/j.ajic.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Rutala W. A., Gergen M. F., Weber D. J. (2010). Room decontamination with UV radiation. Infect Control Hosp Epidemiol 31, 1025–1029. 10.1086/656244 [DOI] [PubMed] [Google Scholar]
- Stibich M., Stachowiak J., Tanner B., Berkheiser M., Moore L., Raad I., Chemaly R. F. (2011). Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on hospital operations and microbial reduction. Infect Control Hosp Epidemiol 32, 286–288. 10.1086/658329 [DOI] [PubMed] [Google Scholar]
- Weber D. J., Anderson D. J., Sexton D. J., Rutala W. A. (2013). Role of the environment in the transmission of Clostridium difficile in health care facilities. Am J Infect Control 41 (Suppl.), S105–S110. 10.1016/j.ajic.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Zoutman D., Shannon M., Mandel A. (2011). Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces. Am J Infect Control 39, 873–879. 10.1016/j.ajic.2011.01.012 [DOI] [PubMed] [Google Scholar]


