Abstract
Genetic variation due to mutation and phase variation has a considerable impact on the commensal and pathogenic behaviours of Campylobacter jejuni. In this study, we provide an example of how second-site mutations can interfere with gene function analysis in C. jejuni. Deletion of the flagellin B gene (flaB) in C. jejuni M1 resulted in mutant clones with inconsistent motility phenotypes. From the flaB mutant clones picked for further analysis, two were motile, one showed intermediate motility and two displayed severely attenuated motility. To determine the molecular basis of this differential motility, a genome resequencing approach was used. Second-site mutations were identified in the severely attenuated and intermediate motility flaB mutant clones: a TA-dinucleotide deletion in fliW and an A deletion in flgD, respectively. Restoration of WT fliW, using a newly developed genetic complementation system, confirmed that the second-site fliW mutation caused the motility defect as opposed to the primary deletion of flaB. This study highlights the importance of (i) screening multiple defined gene deletion mutant clones, (ii) genetic complementation of the gene deletion and ideally (iii) screening for second-site mutations that might interfere with the pathways/mechanisms under study.
Introduction
Campylobacter jejuni is the leading bacterial cause of foodborne gastroenteritis worldwide. The impact of C. jejuni infections is significant due to their high incidence, duration of infection and possible post-infection sequelae (Ruiz-Palacios, 2007). C. jejuni has a broad range of environmental reservoirs including water, birds and other domestic animals, with chickens representing the largest source of human infection (Young et al., 2007). Although C. jejuni is considered as commensal in chickens, it has a significant impact on animal welfare in certain breeds of bird (Humphrey et al., 2014). The genome of C. jejuni is subject to considerable genetic variation, which is thought to play an important role in the survival of the species within host organisms and the environment, especially by altering surface exposed structures such as flagella, lipooligosaccharide and capsular polysaccharide (Balaban et al., 2009; Jerome et al., 2011; Joslin & Hendrixson, 2009; Mohawk et al., 2014; Thomas et al., 2014). Genetic heterogeneity arises in the C. jejuni population as a consequence of mutations due to replication errors, phase variation of homopolymeric or repetitive heteropolymeric tracts either in coding sequences or in promoter regions and to a lesser extent, recombination (Thomas et al., 2014; Wassenaar, 2011; Wilson et al., 2009). It has been suggested that the lack of a functional mismatch repair system enhances the frequency of phase variation and random mutations, and thus the overall heterogeneity of the C. jejuni population (Gaasbeek et al., 2009).
Flagellar-mediated motility plays a central role in commensal and pathogenic behaviours of C. jejuni (Guerry, 2007; Lertsethtakarn et al., 2011). The flagellar system is subject to genetic variation at multiple levels, which often leads to alteration of motility (Gao et al., 2014; Guerry, 2007; Hendrixson & DiRita, 2004). C. jejuni possesses polar flagella at one or both ends of the bacterial cell which consist of a membrane-embedded basal body, a hook structure and a protruding polymeric filament that is composed of the major flagellin subunit FlaA and the minor subunit FlaB (Gao et al., 2014; Guerry et al., 1991). In C. jejuni strain 81116, inactivation of flaA led to a non-flagellated and non-motile phenotype, whereas flagella and motility were retained after the inactivation of flaB (Nuijten et al., 1990; Wassenaar et al., 1991). The motor subunits at the flagellar base, encoded by motA and motB, are responsible for flagellar rotation. Mohawk et al. (2014) reported that non-motile variants of C. jejuni 81-176 carried distinct mutations [i.e. single nucleotide polymorphisms (SNPs) and insertions and deletions (INDELs)] in the motA gene. Flagellar gene expression is tightly controlled and requires the alternative δ54 and δ28 factors, the FlgSR two-component system, the FlhF GTPase and the flagellar secretion system (Balaban et al., 2009; Hendrixson & DiRita, 2003; Joslin & Hendrixson, 2009). Phase variation mechanisms acting on the FlgSR system that affect motility in C. jejuni 81-176 include reversible phase variation in (i) homopolymeric poly-A and poly-T tracts within the flgR response regulator (Hendrixson, 2006) and (ii) poly-A tracts and heteropolymeric repeats located in the flgS sensor histidine kinase (Hendrixson, 2008). It is noteworthy that the C. jejuni flagellar filament is extensively glycosylated, predominantly with pseudaminic acid and legionaminic acid and derivatives thereof. Recombination within the flagellar glycosylation gene locus and homopolymeric poly-G tract phase variation are mechanisms of variation (Howard et al., 2009; Karlyshev et al., 2002; van Alphen et al., 2008).
Here we show that the propensity for stochastic mutation events in C. jejuni has clear implications for gene function analysis in an experimental setting. Analysis of C. jejuni M1 flaB mutant clones revealed inconsistent motility phenotypes. Using genome resequencing and in-depth genotypic and phenotypic characterization, we identified second-site mutations and confirmed that these were responsible for the observed inconsistent motility amongst flaB mutant clones. We also describe a new versatile and user-friendly system for genetic complementation in C. jejuni.
Methods
Bacterial strains and growth conditions
All WT strains, defined mutants, genetically complemented mutants and plasmids are summarized in Table 1. C. jejuni strain M1 was originally isolated from a researcher after visiting a poultry processing plant (Friis et al., 2010) and is fully motile and spiral-shaped. C. jejuni M1 was routinely cultured on brain heart infusion (BHI, Oxoid) agar plates supplemented with 5 % defibrinated horse blood (Oxoid) and 5 μg trimethoprim ml− 1 (TrM). Gene deletion mutants were cultured in the presence of 10 μg chloramphenicol ml− 1 and genetically complemented mutant strains were also supplemented with 50 μg kanamycin ml− 1. C. jejuni was cultured under microaerophilic conditions (5 % O2, 10 % CO2 and 85 % N2) in a MACS VA500 Variable Atmosphere Work Station (Don Whitley). Escherichia coli NEB 5α or 10-β (New England Biolabs) was used for cloning and was cultured in LB medium at 37 °C, supplemented as appropriate with antibiotics.
Table 1. Bacterial strains and plasmids used in this study.
| Strain or plasmid * | Relevant genotype† or description | Source/reference |
|---|---|---|
| C. jejuni | ||
| M1 | WT | Friis et al. (2010) |
| M1 coupled to ΔflaB | WT | This study |
| M1 coupled to ΔflaB*, ΔflaA, ΔflaAB | WT | This study |
| M1 coupled to ΔflaB+empty GC | WT; Kmr | This study |
| M1 coupled to ΔflaD, ΔpflA | WT | This study |
| M1 coupled to ΔfliW | WT | This study |
| M1 coupled to ΔfliW+empty GC | WT; Kmr | This study |
| M1 ΔflaB clones A, B, C, D, E | ΔCJM1_1294; Cmr | This study |
| M1 ΔflaB* clones A, B, C | ΔCJM1_1295-1294; Cmr | This study |
| M1 ΔflaA clones A, B, C | ΔCJM1_1296; Cmr | This study |
| M1 ΔflaAB clones A, B, C | ΔCJM1_1295-1294/CJM1_1296; Cmr | This study |
| M1 ΔflaD clones A, B, C | ΔCJM1_0851; Cmr | This study |
| M1 ΔpflA clones A, B, C | ΔCJM1_1501; Cmr | This study |
| M1 ΔfliW clone A, B, C | ΔCJM1_1052; Cmr | This study |
| M1 ΔfliW clone A+empty GC | ΔCJM1_1052; Cmr Kmr | This study |
| M1 ΔfliW clone A+fliW GC | ΔCJM1_1052+CJM1_1052; Cmr Kmr | This study |
| M1 ΔflaB clone D+empty GC | ΔCJM1_1294; Cmr Kmr | This study |
| M1 ΔflaB clone D+fliW GC | ΔCJM1_1294+CJM1_1052; Cmr Kmr | This study |
| M1 ΔflaB clone E+empty GC | ΔCJM1_1294; Cmr Kmr | This study |
| M1 ΔflaB clone E+fliW GC | ΔCJM1_1294+CJM1_1052; Cmr Kmr | This study |
| E. coli strains | ||
| NEB 10-β or 5-α | Host strains for pSV009 and derivatives thereof | New England Biolabs |
| Plasmids | ||
| pCC027 | Cmr donor plasmid | Coward et al. (2008) |
| pMiniT | E. coli PCR cloning plasmid | New England Biolabs |
| pUC19 | E. coli cloning plasmid | New England Biolabs; Yanisch-Perron et al. (1985) |
| pSV009 | C. jejuni genetic complementation plasmid, pUC19 backbone; donor for empty GC region | This study |
| pSV012 | pSV009 containing fliW; donor for fliW GC region | This study |
empty GC, Empty genetic complementation region amplified from pSV009; fliW GC, genetic complementation region containing the fliW promoter and coding sequence amplified from pSV012.
Cmr, chloramphenicol resistance; Kmr, kanamycin resistance.
Electroporation and natural transformation of C. jejuni
Electroporation of C. jejuni was performed as described previously (Holt et al., 2012) with minor adjustments. C. jejuni M1 was cultured on BHI-TrM blood agar plates for ∼48 h then replated onto fresh BHI-TrM blood agar plates and grown for ∼16 h. Bacteria were harvested from plates in BHI broth, washed four times in ice-cold wash buffer [272 mM sucrose and 15 % (v/v) glycerol] and then resuspended in wash buffer. Of this suspension, 100 μl was added per 0.2 cm pre-chilled electroporation MicroPulser cuvette (Bio-Rad) and mixed with DNA (∼3 μg). Electroporation was performed using a GenePulser Xcell system (Bio-Rad) using the following settings: 2.5 kV, 200 Ω and 25 μF. After a single pulse, 200 μl of pre-warmed SOC medium was added and the suspension was transferred onto 2 ml BHI-TrM blood agar in a Universal tube (Greiner). After 5 h incubation under microaerophilic conditions, 1 ml of pre-warmed BHI medium was added to resuspend the bacteria, which were plated onto selective agar plates and grown under microaerophilic conditions for 2 to 3 days.
Natural transformation of C. jejuni was performed using an adapted biphasic method (Holt et al., 2012; van Vliet et al., 1998). C. jejuni was grown on BHI-TrM blood agar plates for ∼48 h and replated onto fresh BHI-TrM blood agar plates and grown for ∼16 h. Bacteria were harvested from plates in BHI broth and the OD600 was adjusted to ∼0.5. Of this suspension, 0.5 ml was pipetted onto 2 ml BHI-TrM blood agar in a Universal tube (Greiner) and incubated for 3 h under microaerophilic conditions. Thereafter, DNA was added and incubated for 3–5 h before bacteria were harvested and then plated on selective agar plates and grown for 2 to 3 days under microaerophilic conditions.
Construction of defined gene deletion mutants
C. jejuni defined gene deletion mutants were generated by allelic replacement of the gene with a chloramphenicol resistance cassette (cat). The cat cassette was amplified by PCR from the plasmid pCC027 (Coward et al., 2008) and the 5′ (L1 and L2 primers) and 3′ (R1 and R2 primers) flanking regions of the target gene were amplified by PCR from C. jejuni M1 genomic DNA. PCRs were performed using Q5 DNA polymerase (New England Biolabs) and purified using the QIAquick PCR purification kit (Qiagen). The gene flanking region PCR primers L2 (reverse primer 5′ flank) and R1 (forward primer 3′ flank) contain extensions that are complementary to the primers used for amplification of the cat cassette. The cat cassette (640 ng) and gene flanking regions (200 ng each) were joined in an overlap PCR without primers, resulting in the incorporation of the cat cassette in between the 5′ and 3′ flanking regions. Two microlitres of the product of this overlap PCR were then used as a template in a second round of amplification, this time using the primers L1 (forward primer 5′ flank) and R2 (reverse primer 3′ flank), which flank the original gene sequence and thus amplify the whole fragment; a total of four 50 μl PCRs were performed for each mutant, and subsequently pooled and purified. Of this, ∼3 μg of the overlap PCR product was used for electroporation of C. jejuni M1 to generate first generation defined gene deletion mutants, which were selected on BHI-TrM-Cm blood agar. Next, first generation mutant DNA was isolated using the DNeasy Blood and Tissue kit (Qiagen) and checked by PCR for recombination at the desired genomic location using the L1 forward primer (5′ gene flanking region) and a control reverse primer located in the gene (‘A’ primer, which should not generate a product) and a cat cassette primer (CC069, which should generate a product of predicted size). First generation gene deletion mutant DNA (50 ng) was used for natural transformation of C. jejuni M1 WT; the gene replacement event was selected and confirmed by PCR. For flaB deletion mutant clones, correct integration of the cat cassette was also analysed by DNA sequencing. In addition, the WT was processed in parallel through the transformation procedure without any added mutagenic DNA to obtain a ‘coupled’ WT strain. This was done to reduce the genetic variation between the WT and defined mutant strains. Primer sequences are given in Table S1.
Motility assay
C. jejuni was grown on BHI blood agar plates for ∼48 h then replated onto fresh BHI blood agar plates and grown for ∼16 h. BHI suspensions of OD600 ∼0.5 were made and stabbed, using a 20 μl pipette tip, into the middle of a 9 cm petri dish containing 25 ml of 0.4 % select agar (Sigma Aldrich) BHI medium and grown overnight under microaerophilic conditions. The diameter of the ring of bacterial growth was measured using a ruler (n = 3).
Genetic complementation
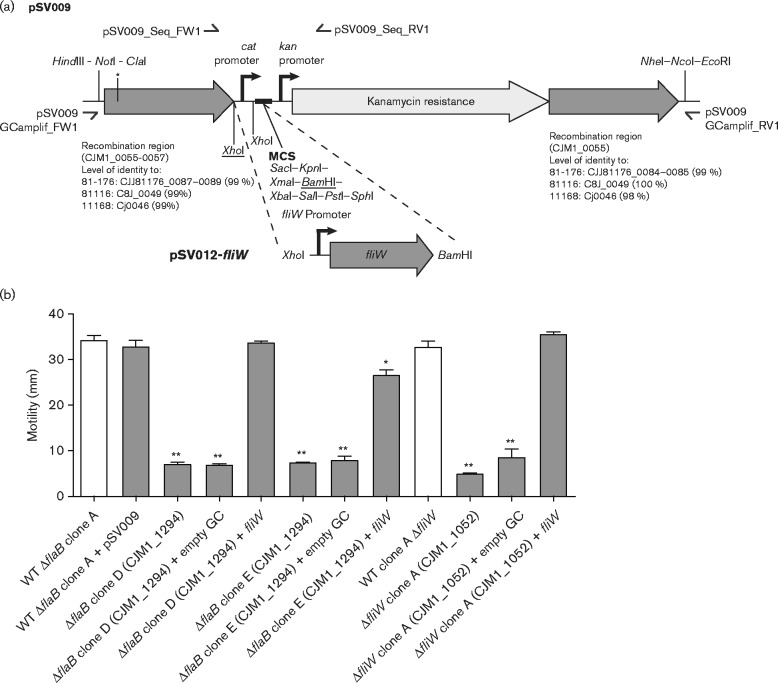
For genetic complementation of defined gene deletion mutants, the target gene was inserted into a pseudogene region between CJM1_0055 and CJM1_0057 (Coward et al., 2008). For this, a new genetic complementation system was designed in silico and synthesized de novo from oligonucleotides (GeneArt Strings, Life Technologies). The genetic complementation system consists of the cat promoter region from pRY011 (Coward et al., 2008; Yao et al., 1993), a partial pUC19 multiple cloning site and the kanamycin resistance cassette including its promoter region from pRY107 (Yao et al., 1993), all of which is flanked by 400 bp of 5′ and 3′ sequence of the CJM1_0055–0057 pseudogene region (98–100 % identity with sequences in C. jejuni 11168, 81116 and 81-176), see Fig. 5(a) for a schematic overview. The de novo synthesized genetic complementation region was first cloned into pMiniT using the NEB PCR cloning kit (New England Biolabs) and subsequently subcloned into pUC19 using the HindIII and EcoRI restriction enzyme sites, yielding the plasmid pSV009. Of note, the 5′ pseudogene region (CJM1_0055–0057) sequenced originally harboured a HindIII site, which was eliminated by introducing a synonymous mutation during the in silico design of the genetic complementation insert. The fliW coding sequence including its upstream sequence was amplified by PCR and subcloned into pSV009 using the XhoI and BamHI sites, yielding pSV012-fliW. Sequences of pSV009 and pSV012-fliW were confirmed using DNA sequencing. The fliW complementation region and the empty genetic complementation region were amplified by PCR from pSV012 or pSV009, respectively, using the primers pSV009_GCampl_FW1/RV1, which amplify the complete genetic complementation region. The fliW complementation or the empty genetic complementation region was subsequently introduced into C. jejuni by electroporation. For a schematic overview of the genetic complementation strategy, see Fig. 5(a). Primer sequences are given in Table S1.
Fig. 5.
Genetic complementation of fliW in severely attenuated motility flaB (CJM1_1294) mutant clones and a fliW (CJM1_1052) mutant restores motility. (a) Overview of the genetic complementation strategy using plasmid pSV009. The fliW gene including its upstream sequence was cloned into the BamHI site located in the multiple cloning site (MCS) and XhoI site of pSV009, yielding pSV012-fliW. This replaced the pSV009 cat promoter and enabled fliW expression from its native promoter. The genetic complementation region is flanked by regions for homologous recombination to facilitate insertion into the CJM1_0055–0057 pseudogene region, which is predicted to be compatible with other commonly used C. jejuni isolates (e.g. 11168, 81116 and 81-176). For insertion of fliW (CJM1_1052) into the pseudogene region, the complementation region was amplified by PCR using the pSV009_GCamplif primers and subsequently introduced by electroporation. (b) As expected, insertion of fliW (CJM1_1052) in the CJM1_0055–0057 pseudogene region in a fliW (CJM1_1052) mutant restored motility. Insertion of fliW (CJM1_1052) in the pseudogene region also restored motility in the severely attenuated motility flaB (CJM1_1294) mutant clones D and E, whereas insertion of the empty genetic complementation (‘empty GC’) region did not restore motility. The white bars indicate the coupled WT strains for flaB (CJM1_1294) and fliW (CJM1_1052) gene deletion mutants. Differential motility of mutant clones was tested against coupled WT isolates using a Mann–Whitney test (n = 3). Data shown are the mean and sem, with *P < 0.05 and **P < 0.01.
Scanning and transmission electron microscopy (EM) analysis
C. jejuni M1 WT and defined gene deletion mutants were cultured for ∼48 h, harvested and washed four times in water. For scanning EM, bacteria were fixed in 2 % glutaraldehyde in 0.05 M sodium cacodylate buffer at pH 7.4 for 48 h, allowed to settle onto 10 mm poly-l-lysine-coated glass coverslips, rinsed twice in deionized water and quench frozen in propane cooled in liquid nitrogen. Samples were freeze-dried overnight from − 95 to +30 °C in a K775X freeze dryer (Quorum Emitech). The coverslips were mounted on Cambridge stubs with silver DAG and coated with 4 nm of gold in a K575X sputter coater (Quorum Emitech), then imaged using a Verios 460 (FEI) scanning electron microscope operated at 2 or 5 kV. For transmission EM, bacteria were fixed in 2 % glutaraldehyde in 0.05 M sodium cacodylate buffer at pH 7.4 for 6–12 h, and adsorbed onto glow discharged 400 mesh copper grids with a carbon film attached for 30 s. They were rinsed twice with deionized water and stained for 30 s with 1 % aqueous uranyl acetate. Grids were allowed to dry for 20 min and viewed in a Tecnai G2 (FEI) transmission electron microscope operated at 200 keV. Images were captured with an AMT XR60B camera running Deben software.
Illumina sequencing
Genomic DNA was isolated using Genomic-tip 20/G columns (Qiagen) or the DNeasy Blood and Tissue kit (Qiagen) according to manufacturer's instructions. Libraries for Illumina sequencing were prepared using the NEBNext Ultra DNA library prep kit (New England Biolabs) or the Nextera XT DNA kit (Illumina). For the NEBNext Ultra DNA kit, the DNA was first sheared to ∼300 or 400 bp fragments in microTUBE screw-cap tubes in an M220 focused-ultrasonicator (Covaris). Following NEBNext Ultra DNA library preparation, the library size was determined with a Bioanalyser 2100 (Agilent) and the DNA concentration was measured with the Qubit dsDNA BR kit (Life Technologies) and pooled at equimolar amounts. Nextera XT library preparation was performed according to the manufacturer's instructions. The libraries for pflA (CJM1_1501), flaD (CJM1_0851), flaB (CJM1_1294) gene deletion mutant clones and their coupled WT strains were sequenced using 76 bp paired-end sequencing, whereas the fliW gene deletion mutant clone A and its coupled WT were sequenced on the Illumina MiSeq platform using 301 bp paired-end sequencing (v3 chemistry).
Illumina sequence data analysis
Raw sequence reads for pflA (CJM1_1501), flaD (CJM1_0851), flaB (CJM1_1294) mutant clones and their coupled WTs were mapped to the M1 reference genome sequence [GenBank accession no. CP001900 (Friis et al., 2010)] using the CLC Genomics Workbench version 7.5.1. Variants were called only if covered by >20 reads, a variant count of >5 reads and detected in both forward and reverse sequence reads. Hierarchical clustering based on SNPs and INDELs presence/absence was performed using CLC Main Workbench (v7); variants present in all sequenced mutants and WTs were excluded from the clustering analysis.
Variant analysis of fliW mutant clone A and its coupled WT was performed as follows: (1) Illumina sequence reads were mapped to the M1cam reference genome (accession no. CP012149, see details below) using Stampy (Lunter & Goodson, 2011), (2) Samtools was used to identify variants (Li et al., 2009); the minimum quality for variant calling was set to 100. The variant effect at the protein level was predicted using SnpEff (Cingolani et al., 2012).
Genome resequencing and annotation of C. jejuni M1 ‘Cambridge’
The M1 WT strain used in our laboratory and ‘coupled’ WT strains (i.e. the M1 WT processed through the natural transformation procedure) obtained during the generation of flaB (one WT), pflA and flaD (two WTs) gene deletion mutants were Illumina sequenced and their sequence reads mapped to the M1 reference sequence [GenBank accession no. CP001900 (Friis et al., 2010)]. SNPs and INDELs detected in all four sequenced M1 WTs were collected and incorporated into the C. jejuni M1 reference genome sequence using a custom Python script, yielding an in-house C. jejuni M1 genome sequence (designated CJM1cam). The effect of SNPs and INDELs at the protein level was predicted using SnpEff (Cingolani et al., 2012). Sequence variants detected in this study by Sanger DNA sequencing were also incorporated into the CJM1cam genome sequence. An overview of the SNPs and INDELs relative to the published M1 reference genome can be found in Table S2. The obtained CJM1cam genome sequence was annotated with Prokka (Seemann, 2014) and assigned locus tags starting with CJM1cam; where possible the locus tag numbering of the original M1 reference genome was retained. The annotation of the flagellin locus CJM1_1295-1294 (flaB) and CJM1_1296 (flaA) was manually corrected. Automated ORF calling using Prokka resulted in the convergence of 33 partial coding sequences (CDSs) into 16 CDSs relative to the published M1 genome (Friis et al., 2010). The CJM1cam proteins were assigned Cluster of Orthologous Genes (Tatusov et al., 1997) functional categories using a custom R script. The obtained CJM1cam genome sequence and annotation are available through GenBank (accession no. CP012149).
Statistical analyses
Statistical analyses were performed in GraphPad Prism 6.0 (GraphPad software) with P < 0.05 considered to be significant.
Sequencing data
The CJM1cam genome sequence is deposited at GenBank (CP012149) and the sequence of the pSV009 genetic complementation plasmid is deposited KT373982. The Illumina sequence data are deposited at the European Nucleotide Archive under study accession number PRJEB10223 (http://www.ebi.ac.uk/ena).
Results and discussion
Differential motility of defined gene deletion mutant clones
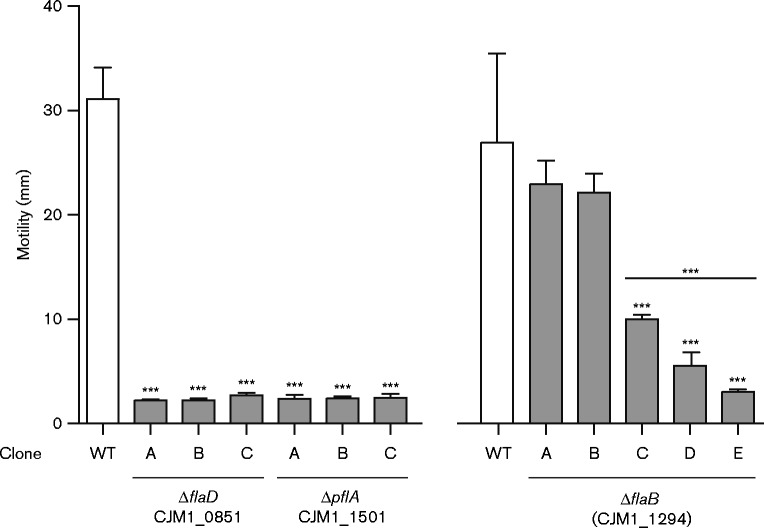
For our studies into the molecular mechanisms of C. jejuni pathogenesis, e.g. invasion of gut epithelial cells (to be reported elsewhere), three defined deletion mutants were generated in C. jejuni M1 flagella genes using an overlap PCR method. Gene deletions were constructed in flaD, also referred to as flgL (CJM1_0851; hook filament junction protein) (Neal-McKinney & Konkel, 2012), pflA (CJM1_1501; paralysed flagellum protein) (Yao et al., 1994) and CJM1_1294, predicted to encode the major flagellin subunit FlaA (CJM1_1294) in the published C. jejuni M1 reference genome (Friis et al., 2010). We anticipated that deletion of these flagella genes would result in non-motile phenotypes (Neal-McKinney & Konkel, 2012; Wassenaar et al., 1991; Yao et al., 1994). As expected (Neal-McKinney & Konkel, 2012; Yao et al., 1994) three independent mutant clones for each of flaD (CJM1_0851) and pflA (CJM1_1501) were found to be non-motile (Fig. 1). Scanning EM confirmed the previously reported non-flagellated and flagellated phenotype of flaD and pflA gene deletion mutants, respectively (data not shown) (Neal-McKinney & Konkel, 2012; Yao et al., 1994). In contrast, five independent flaA (CJM1_1294) mutant clones were tested for motility with inconsistent results: mutant clones A and B displayed WT motility, clone C showed intermediate motility and clones D and E had severely attenuated motility (Fig. 1).
Fig. 1.
Motility of C. jejuni M1 flagella defined gene deletion mutants. Motility was analysed on BHI plates with 0.4 % (w/v) agar after overnight incubation. Three flaD (CJM1_0851) and plfA (CJM1_1501) deletion mutant clones were assayed along with their coupled WT strain and five flaB [CJM1_1294, previously annotated to encode FlaA (Friis et al., 2010)] mutant clones were tested with two coupled WT strains (A and B), with the average motility shown for the latter. All three flaD (CJM1_0851) and pflA (CJM1_1501) mutant clones were non-motile; however, motility of flaB (CJM1_1294) mutant clones was inconsistent: clones A and B showed WT motility, clone C displayed intermediate motility, whereas the motility of clones D and E was severely attenuated. Differential motility of mutant clones was tested against the coupled WT strain(s) with a Mann–Whitney test (n = 3). Data shown are the mean and sem, with ***P < 0.001.
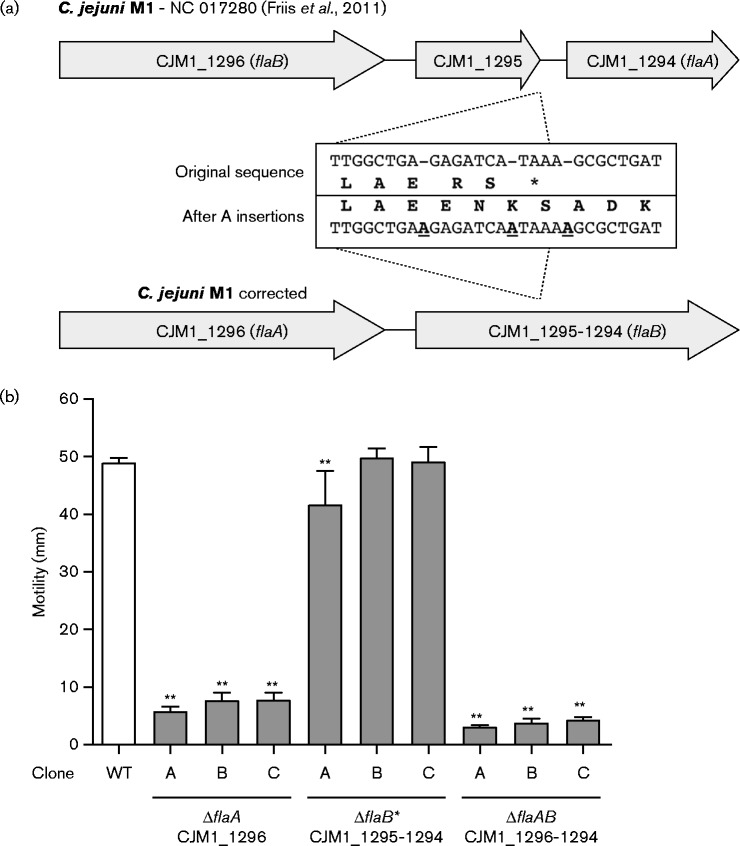
To unravel the basis of the inconsistent motility phenotypes of flaA (CJM1_1294) mutant clones, we first confirmed the correct insertion of the chloramphenicol (cat) resistance cassette in flaA (CJM1_1294) by Sanger sequencing. Importantly, this revealed the insertion of three A nucleotides in an 11 bp stretch at the 3′ end of CJM1_1295 [annotated as ‘hypothetical protein’ (Friis et al., 2010)], which was also independently confirmed by Sanger sequencing of the complete CJM1_1295–1294 region. Incorporation of the A insertions into the original M1 sequence (Friis et al., 2010) led to CJM1_1295 and CJM1_1294 being merged into a larger ORF of 1731 bp (Fig. 2a). The difference between the published C. jejuni M1 reference genome (Friis et al., 2010) and the sequence of our M1 strain may reflect genotypic variation or sequencing or assembly errors in the original M1 genome sequence.
Fig. 2.
Sequence corrections in the C. jejuni M1 flagellin locus and motility analysis of flagellin locus gene deletion mutants. (a) Insertion of three A nucleotides at the 3′ of CJM1_1295 led to elimination of the TAA stop codon and resulted in CJM1_1295 and CJM1_1294 being merged into one large ORF. (b) Motility was assessed on 0.4 % (w/v) agar BHI plates after overnight incubation. Deletion of the full flaB CDS (flaB* mutant; CJM1_1295–1294) did not affect motility in clones B and C; however, we observed a very marginal decrease in motility in clone A. In contrast, mutant clones in which flaA (CJM1_1296) or both the flaA and flaB gene (flaAB; CJM1_1296 and CJM1_1295–1294) were deleted all showed severely attenuated motility. Differential motility of mutant clones was tested against the coupled WT strain with a Mann–Whitney test (n = 3). Data shown are the mean and sem, with **P < 0.01.
In-depth analysis of the altered flagellin locus in C. jejuni M1 revealed that: (1) the merged CJM1_1295–1294 gene actually represents the full-length flaB gene as it was identical to the published flaB sequence of C. jejuni 81116 (Nuijten et al., 1990; Pearson et al., 2007) and (2) CJM1_1296 was incorrectly annotated as flaB; it is identical to flaA of C. jejuni 81116 (Friis et al., 2010), see Fig. 2(a). Therefore, our CJM_1294 gene deletion mutant, described above, is not a flaA mutant but the cat cassette is replacing a region ranging from position 822 to 1671 bp of the flaB gene. Consequently, the motile phenotype of flaB (CJM1_1294) mutant clones (Fig. 1) confirmed the findings by Wassenaar et al., (1991), reporting a motile phenotype after the inactivation of flaB in C. jejuni 81116. Deletion of the full-length flaB gene (CJM1_1295–1294, referred to as the flaB* mutant) also resulted in WT motility levels for mutant clones B and C, although the motility of flaB* (CJM1_1295–1294) mutant clone A was slightly lower compared to the WT (Fig. 2b). In line with previous findings of Wassenaar et al. (1991) in C. jejuni 81116, deletion of flaA (CJM1_1296) and the complete flagellin locus (flaAB; CJM1_1296 and CJM1_1295–1294) led to abolished motility (Fig. 2b). Analysis of the combined motility data for the flaA (CJM1_1296) and flaAB mutant clones showed that deletion of the complete flagellin locus resulted in more severely attenuated motility compared to deletion of flaA alone (P < 0.001), suggesting that flaB plays a minor role in motility, at least in C. jejuni strain M1.
Based on our findings, we hypothesized that the attenuated motility in flaB (CJM1_1294) mutant clones C, D and E might be caused by loss-of-function mutations in other motility-associated genes or as previously reported, by a compensatory mechanism, for example, by increased flaB expression or by genome rearrangements, which have been shown to (partially) restore motility (Nuijten et al., 2000; Wassenaar et al., 1994, 1995).
Genotypic and phenotypic analysis of motility defects
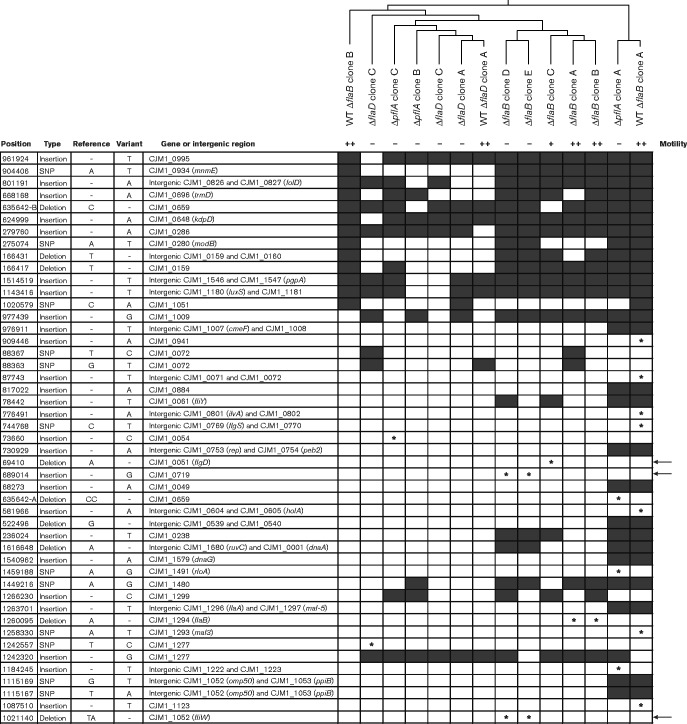
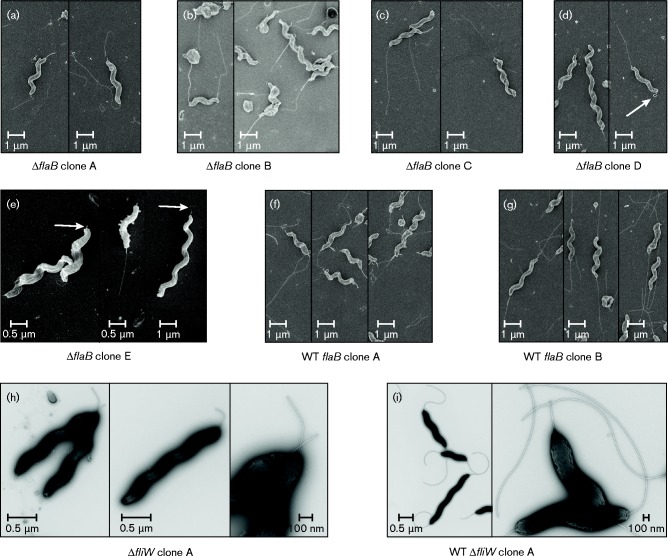
A genome resequencing approach was used to assess whether second-site mutations might be responsible for the attenuated motility of flaB (CJM1_1294) mutant clones C, D and E. Three pflA, three flaD and five flaB mutant clones and their ‘coupled’ M1 WT strains were genome sequenced. Eighty-one variants, i.e. SNPs and INDELs, were detected relative to the C. jejuni M1 reference genome [GenBank CP001900 (Friis et al., 2010)]. Of these, 34 SNPs and INDELs were present in all sequenced gene deletion mutant clones and their ‘coupled’ WTs, whereas 47 SNPs and INDELs were only detected in a subselection of them (Fig. 3). Although the flaB mutant clones subclustered, limited mutant-specific hierarchical clustering was detected, indicating that variation may be introduced throughout all growth steps during the construction of the gene deletion mutants. The SNPs and INDELs nucleotide position numbers correspond to the position in the original C. jejuni M1 reference genome sequence (Friis et al., 2010). Interestingly, the genome resequencing approach revealed the presence of SNPs and INDELs unique to the severely attenuated motility flaB (CJM1_1294) mutant clones D and E. These were an insertion of a G at position 689 014 within CJM1_0719 (encoding a putative periplasmic protein) and deletion of a TA-dinucleotide at position 1 021 140 within CJM1_1052 (fliW). The TA-deletion in fliW is predicted to result in a three amino acid C-terminal truncation of the FliW protein. FliW encodes a predicted flagellin filament-stabilizing chaperone, although it has not yet been demonstrated whether FliW interacts with FlaA and/or FlaB flagellin subunits (Barrero-Tobon & Hendrixson, 2014; Titz et al., 2006). Previously it was shown that deletion of fliW in C. jejuni 81-176 resulted in a phenotype consisting of severely attenuated motility with the presence of a probable flagellar hook structure but without flagellin filaments (Barrero-Tobon & Hendrixson, 2014). Scanning EM analysis revealed that the majority of the flaB (CJM1_1294) mutant clones D and E also lacked flagellar filaments and instead possessed hook-like structures, but with very few bacteria displaying a possible filament structure (Fig. 4d, e). In contrast, the majority of the bacteria imaged for the flaB (CJM1_1294) coupled WTs (Fig. 4f, g) and flaB (CJM1_1294) mutant clones A, B and C harboured a flagellar filament at one or both poles (Fig. 4a–c). Notably, the flaB (CJM1_1294) mutant clones D and E differed at two variant positions (Fig. 3), which suggests that they did not originate from a single transformation event. Further, the intermediate motile flaB (CJM1_1294) mutant clone C possessed a unique deletion of an A at position 69 410 in CJM1_0051 (flgD), encoding a flagellar hook assembly protein. This deletion was predicted to result in a severely truncated flgD ORF, encoding a protein of 55 aa instead of 294 aa. In C. jejuni 81-176 a flgD transposon mutant was previously reported to be non-motile (Hendrixson et al., 2001).
Fig. 3.
Genome sequencing revealed second-site mutations associated with motility defects. Genetic variation, i.e. SNPs and INDELs, in C. jejuni M1 defined gene deletion mutant clones and corresponding WT strains (Table 1) were analysed by Illumina sequencing; their presence is indicated with black boxes or with an asterisk when either found to be unique to individual mutant clones, WT strains, or associated with differential motility. Motility phenotypes are indicated as severely attenuated motility ( − ), intermediate motility (+) or WT motility (++) (see Fig. 1). SNPs or INDELs that are associated with attenuated motility in flaB (CJM1_1294) mutant clones C, D and E are indicated with an arrow on the right side of the panel. SNPs/INDELs that were detected in all sequenced M1 WTs and mutant strains compared to the M1 reference (Friis et al., 2010) were excluded from the analysis (Table S2). Hierarchical clustering was performed based on the presence/absence of SNPs and INDELs.
Fig. 4.
EM analysis of the flagellar structure in flaB (CJM1_1294) and fliW (CJM1_1052) gene deletion mutants. Scanning EM was used to assess the flagellar structure in flaB (CJM1_1294) mutant clones A–E (a–e) and coupled WT clones A and B (f, g) and transmission EM was used for analysis of the fliW deletion mutant clone A (h) and its parental WT (i). (a–c) The motile (clones A and B) and intermediate motility (clone C) flaB (CJM1_1294) mutants harboured bipolar flagella. (d, e) Non-motile flaB clones D and E lack flagellar structures at both poles; a protruding structure (flagellar filament or hook-like structure) was observed (indicated with white arrows), or rarely bacteria were observed that harboured a flagellar structure at a single pole as shown in the right panels of (d) and (e). (f, g) Motile WT isolates (clones A and B) coupled to flaB mutants displayed flagellar structures at both poles. (h) No flagellar filaments were observed for the non-motile fliW deletion mutant clone A; however, short hook-like structures were observed. (i) The WT isolate coupled to the fliW mutant clone A possessed flagellar structures at both poles.
The genome sequencing data obtained in this study for the M1 WTs were used to generate a reference genome sequence (designated CJM1cam) for the C. jejuni M1 strain used in our laboratory. The CJM1cam genome sequence and annotation are available via GenBank accession no. CP012149; for more information see Methods.
Second-site mutations in fliW responsible for motility defects in flaB mutants
We hypothesized that the predicted C-terminal truncation of the FliW protein by 3 aa may be responsible for the observed attenuated motility of flaB (CJM1_1294) mutant clones D and E. To confirm that fliW is also required for motility in C. jejuni M1, a fliW gene deletion mutant was generated, which showed abolished motility (Fig. 5b). To exclude that this was a clone-specific phenotype, we assayed two other fliW gene deletion mutant clones; both clones were found to be non-motile (Fig. S1). Genome sequencing of fliW mutant clone A and its coupled WT showed that 14 SNPs and INDELs were shared between them, five variants were unique to the WT and two were only found in the fliW mutant clone A (Table S3). Unique variants to the fliW mutant clone A were an intergenic A insertion between CJM1cam_0120 (putative metalloprotease) and CJM1cam_0121 (hypothetical protein) and a synonymous SNP (A to G) in CJM1cam_0791 (hypothetical protein), indicating that the non-motile phenotype is not caused by second-site mutations. Electron micrographs of the C. jejuni M1 fliW mutant were similar to those reported previously of a C. jejuni 81-176 fliW mutant, i.e. lack of flagellar filaments and presence of a hook-like structure (Barrero-Tobon & Hendrixson, 2014) (Fig. 4h). The motility of the fliW mutant was slightly less than that of the flaB (CJM1_1294) mutant clones D and E, although this difference was not statistically significant. It is possible that the predicted 3 aa truncated FliW protein in flaB (CJM1_1294) mutant clones D and E could still possess limited functional activity.
To confirm that the TA-deletion in fliW was responsible for the severely attenuated motility of flaB (CJM1_1294) mutant clones D and E, the WT fliW gene including its native promoter was inserted into the CJM1_0055–0057 pseudogene region. For this, the new genetic complementation plasmid pSV009 was developed, which enables: (1) flexible cloning options through a whole range of restriction sites, (2) the possibility to drive the expression from the cat promoter or replace this with the native promoter of the gene of interest and (3) pseudogene region insertion in well-studied C. jejuni strains, e.g. M1, 81-176, 81116 and 11168, via homologous recombination regions with >98 % identity (Fig. 5a). Using the newly developed complementation system, insertion of fliW into the CJM1_0055–0057 pseudogene region in the fliW mutant as well as in flaB (CJM1_1294) mutant clones D and E was shown to restore motility to WT levels, suggesting that the flagella are restored, although this was not confirmed by EM. Insertion of the empty genetic complementation backbone did not restore motility (Fig. 5b). These results confirm that the TA-deletion in fliW was responsible for the attenuated motility phenotype of flaB (CJM1_1294) mutant clones D and E.
Concluding remarks
Many studies aimed at investigating the role of genes in C. jejuni make use of targeted deletion of genes by replacing them with an antibiotic resistance cassette. Here we have demonstrated that it is of critical importance to assess the phenotype of multiple mutant clones and/or genetically complement the gene deletion. Ideally, genome sequencing may be used to screen for second-site mutations that may interfere with the pathways/mechanism under study, especially when mutant clones show inconsistent behaviour or when genetic complementation fails. As shown in this study, genome sequencing can be used as a troubleshooting tool and may also yield novel genes associated with the phenotypes under study.
Acknowledgements
This work was funded by BBSRC grant RG66581. We thank Gemma Murray from the Department of Genetics, University of Cambridge for providing the R script used for Cluster of Orthologous Genes assignment and Diane Newell for providing the C. jejuni M1 strain. Jeremy Skepper at the Cambridge Advanced Imaging Centre, Department of Physiology, Development and Neuroscience, University of Cambridge kindly performed the scanning electron microscopy.
Supplementary Data
Supplementary Data
Abbreviations:
- cat
chloramphenicol resistance cassette
- CDS
coding sequence
- EM
electron microscopy
- flaB
flagellin B
- INDEL
insertion and deletion
- TrM
trimethoprim
References
- Balaban M., Joslin S. N., Hendrixson D. R. (2009). FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni J Bacteriol 191 6602–6611 10.1128/JB.00884-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero-Tobon A. M., Hendrixson D. R. (2014). Flagellar biosynthesis exerts temporal regulation of secretion of specific Campylobacter jejuni colonization and virulence determinants Mol Microbiol 93 957–974 10.1111/mmi.12711 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P., Platts A., Wang L., Coon M., Nguyen T., Wang L., Land S. J., Lu X., Ruden D. M. (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3 Fly (Austin) 6 80–92 10.4161/fly.19695 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward C., van Diemen P. M., Conlan A. J., Gog J. R., Stevens M. P., Jones M. A., Maskell D. J. (2008). Competing isogenic Campylobacter strains exhibit variable population structures in vivo Appl Environ Microbiol 74 3857–3867 10.1128/AEM.02835-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis C., Wassenaar T. M., Javed M. A., Snipen L., Lagesen K., Hallin P. F., Newell D. G., Toszeghy M., Ridley A., other authors (2010). Genomic characterization of Campylobacter jejuni strain M1 PLoS One 5 e12253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaasbeek E. J., van der Wal F. J., van Putten J. P., de Boer P., van der Graaf-van Bloois L., de Boer A. G., Vermaning B. J., Wagenaar J. A. (2009). Functional characterization of excision repair and RecA-dependent recombinational DNA repair in Campylobacter jejuni J Bacteriol 191 3785–3793 10.1128/JB.01817-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Lara-Tejero M., Lefebre M., Goodman A. L., Galán J. E. (2014). Novel components of the flagellar system in epsilonproteobacteria MBio 5 e01349–e14 10.1128/mBio.01349-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P. (2007). Campylobacter flagella: not just for motility Trends Microbiol 15 456–461 10.1016/j.tim.2007.09.006 . [DOI] [PubMed] [Google Scholar]
- Guerry P., Alm R. A., Power M. E., Logan S. M., Trust T. J. (1991). Role of two flagellin genes in Campylobacter motility J Bacteriol 173 4757–4764 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrixson D. R. (2006). A phase-variable mechanism controlling the Campylobacter jejuni FlgR response regulator influences commensalism Mol Microbiol 61 1646–1659 10.1111/j.1365-2958.2006.05336.x . [DOI] [PubMed] [Google Scholar]
- Hendrixson D. R. (2008). Restoration of flagellar biosynthesis by varied mutational events in Campylobacter jejuni Mol Microbiol 70 519–536 10.1111/j.1365-2958.2008.06428.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrixson D. R., DiRita V. J. (2003). Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus Mol Microbiol 50 687–702 10.1046/j.1365-2958.2003.03731.x . [DOI] [PubMed] [Google Scholar]
- Hendrixson D. R., DiRita V. J. (2004). Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract Mol Microbiol 52 471–484 10.1111/j.1365-2958.2004.03988.x . [DOI] [PubMed] [Google Scholar]
- Hendrixson D. R., Akerley B. J., DiRita V. J. (2001). Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility Mol Microbiol 40 214–224 10.1046/j.1365-2958.2001.02376.x . [DOI] [PubMed] [Google Scholar]
- Holt J. P., Grant A. J., Coward C., Maskell D. J., Quinlan J. J. (2012). Identification of Cj1051c as a major determinant for the restriction barrier of Campylobacter jejuni strain NCTC11168 Appl Environ Microbiol 78 7841–7848 10.1128/AEM.01799-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. L., Jagannathan A., Soo E. C., Hui J. P., Aubry A. J., Ahmed I., Karlyshev A., Kelly J. F., Jones M. A., other authors (2009). Campylobacter jejuni glycosylation island important in cell charge, legionaminic acid biosynthesis, and colonization of chickens Infect Immun 77 2544–2556 10.1128/IAI.01425-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., Humphrey T., Wigley P. (2014). Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare MBio 5 e01364–e14 10.1128/mBio.01364-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome J. P., Bell J. A., Plovanich-Jones A. E., Barrick J. E., Brown C. T., Mansfield L. S. (2011). Standing genetic variation in contingency loci drives the rapid adaptation of Campylobacter jejuni to a novel host PLoS One 6 e16399 10.1371/journal.pone.0016399 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslin S. N., Hendrixson D. R. (2009). Activation of the Campylobacter jejuni FlgSR two-component system is linked to the flagellar export apparatus J Bacteriol 191 2656–2667 10.1128/JB.01689-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlyshev A. V., Linton D., Gregson N. A., Wren B. W. (2002). A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni Microbiology 148 473–480 10.1099/00221287-148-2-473 . [DOI] [PubMed] [Google Scholar]
- Lertsethtakarn P., Ottemann K. M., Hendrixson D. R. (2011). Motility and chemotaxis in Campylobacter and Helicobacter Annu Rev Microbiol 65 389–410 10.1146/annurev-micro-090110-102908 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup (2009). The Sequence Alignment/Map format and SAMtools Bioinformatics 25 2078–2079 10.1093/bioinformatics/btp352 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunter G., Goodson M. (2011). Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads Genome Res 21 936–939 10.1101/gr.111120.110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk K. L., Poly F., Sahl J. W., Rasko D. A., Guerry P. (2014). High frequency, spontaneous motA mutations in Campylobacter jejuni strain 81-176 PLoS One 9 e88043 10.1371/journal.pone.0088043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal-McKinney J. M., Konkel M. E. (2012). The Campylobacter jejuni CiaC virulence protein is secreted from the flagellum and delivered to the cytosol of host cells Front Cell Infect Microbiol 2 31 10.3389/fcimb.2012.00031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten P. J., van Asten F. J., Gaastra W., van der Zeijst B. A. (1990). Structural and functional analysis of two Campylobacter jejuni flagellin genes J Biol Chem 265 17798–17804 . [PubMed] [Google Scholar]
- Nuijten P. J., van den Berg A. J., Formentini I., van der Zeijst B. A., Jacobs A. A. (2000). DNA rearrangements in the flagellin locus of an flaA mutant of Campylobacter jejuni during colonization of chicken ceca Infect Immun 68 7137–7140 10.1128/IAI.68.12.7137-7140.2000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson B. M., Gaskin D. J., Segers R. P., Wells J. M., Nuijten P. J., van Vliet A. H. (2007). The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828) J Bacteriol 189 8402–8403 10.1128/JB.01404-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M. (2007). The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken Clin Infect Dis 44 701–703 10.1086/509936 . [DOI] [PubMed] [Google Scholar]
- Seemann T. (2014). Prokka: rapid prokaryotic genome annotation Bioinformatics 30 2068–2069 10.1093/bioinformatics/btu153 . [DOI] [PubMed] [Google Scholar]
- Tatusov R. L., Koonin E. V., Lipman D. J. (1997). A genomic perspective on protein families Science 278 631–637 10.1126/science.278.5338.631 . [DOI] [PubMed] [Google Scholar]
- Thomas D. K., Lone A. G., Selinger L. B., Taboada E. N., Uwiera R. R., Abbott D. W., Inglis G. D. (2014). Comparative variation within the genome of Campylobacter jejuni NCTC 11168 in human and murine hosts PLoS One 9 e88229 10.1371/journal.pone.0088229 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titz B., Rajagopala S. V., Ester C., Häuser R., Uetz P. (2006). Novel conserved assembly factor of the bacterial flagellum J Bacteriol 188 7700–7706 10.1128/JB.00820-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L. B., Wuhrer M., Bleumink-Pluym N. M., Hensbergen P. J., Deelder A. M., van Putten J. P. (2008). A functional Campylobacter jejuni maf4 gene results in novel glycoforms on flagellin and altered autoagglutination behaviour Microbiology 154 3385–3397 10.1099/mic.0.2008/019919-0 . [DOI] [PubMed] [Google Scholar]
- van Vliet A. H., Wood A. C., Henderson J., Wooldridge K. G., Ketley J. M. (1998). Genetic manipulation of enteric Campylobacter species. In Methods in Microbiology, pp. 405–419. Edited by Williams P., Ketley J., Salmond G. London, UK: Academic Press. [Google Scholar]
- Wassenaar T. M. (2011). Following an imaginary Campylobacter population from farm to fork and beyond: a bacterial perspective Lett Appl Microbiol 53 253–263 10.1111/j.1472-765X.2011.03121.x . [DOI] [PubMed] [Google Scholar]
- Wassenaar T. M., Bleumink-Pluym N. M., van der Zeijst B. A. (1991). Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion EMBO J 10 2055–2061 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar T. M., Bleumink-Pluym N. M., Newell D. G., Nuijten P. J., van der Zeijst B. A. (1994). Differential flagellin expression in a flaA flaB+ mutant of Campylobacter jejuni Infect Immun 62 3901–3906 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar T. M., Fry B. N., van der Zeijst B. A. (1995). Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer Microbiology 141 95–101 10.1099/00221287-141-1-95 . [DOI] [PubMed] [Google Scholar]
- Wilson D. J., Gabriel E., Leatherbarrow A. J., Cheesbrough J., Gee S., Bolton E., Fox A., Hart C. A., Diggle P. J., Fearnhead P. (2009). Rapid evolution and the importance of recombination to the gastroenteric pathogen Campylobacter jejuni Mol Biol Evol 26 385–397 10.1093/molbev/msn264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. (1985). Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors Gene 33 103–119 10.1016/0378-1119(85)90120-9 . [DOI] [PubMed] [Google Scholar]
- Yao R., Alm R. A., Trust T. J., Guerry P. (1993). Construction of new Campylobacter cloning vectors and a new mutational cat cassette Gene 130 127–130 10.1016/0378-1119(93)90355-7 . [DOI] [PubMed] [Google Scholar]
- Yao R., Burr D. H., Doig P., Trust T. J., Niu H., Guerry P. (1994). Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells Mol Microbiol 14 883–893 10.1111/j.1365-2958.1994.tb01324.x . [DOI] [PubMed] [Google Scholar]
- Young K. T., Davis L. M., Dirita V. J. (2007). Campylobacter jejuni: molecular biology and pathogenesis Nat Rev Microbiol 5 665–679 10.1038/nrmicro1718 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data