Abstract
Insulin-like growth factor binding protein-1 (IGFBP-1), secreted by fetal liver, is a key regulator of IGF-I bioavailability and fetal growth. IGFBP-1 phosphorylation decreases IGF-I bioavailability and diminishes its growth-promoting effects. Growth-restricted fetuses have decreased levels of circulating essential amino acids. We recently showed that IGFBP-1 hyperphosphorylation (pSer101/119/169) in response to leucine deprivation is regulated via activation of the amino acid response (AAR) in HepG2 cells. Here we investigated nutrient-sensitive protein kinases CK2/PKC/PKA in mediating IGFBP-1 phosphorylation in leucine deprivation. We demonstrated that leucine deprivation stimulated CK2 activity (enzymatic assay) and induced IGFBP-1 phosphorylation (immunoblotting/MRM-MS). Inhibition (pharmacological/siRNA) of CK2/PKC, but not PKA, prevented IGFBP-1 hyperphosphorylation in leucine deprivation. PKC inhibition also prevented leucine deprivation-stimulated CK2 activity. Functionally, leucine deprivation decreased IGF-I-induced-IGF-1R autophosphorylation when CK2/PKC were not inhibited. Our data strongly support that PKC promotes leucine deprivation-induced IGFBP-1 hyperphosphorylation via CK2 activation, mechanistically linking decreased amino acid availability and reduced fetal growth.
Keywords: Amino acid restriction, fetal growth, HepG2 cells, Insulin-Like Growth Factor Binding Protein, Insulin-Like Growth Factor-1 receptor, protein kinases, phosphorylation sites, mass spectrometry
1. Introduction
Fetal growth restriction (FGR) is often a result of placental insufficiency, which is associated with impaired maternal-fetal amino acid transfer. In particular, a body of evidence suggests that placental amino acid transport capacity is reduced (Glazier et al 1997, Jansson et al 2002, Norberg et al 1998, Mahendran et al 1993) and fetal amino acid availability is diminished in FGR (Economides 1989). Decreased fetal circulating levels of essential amino acids, such as leucine, have been reported in FGR pregnancies (Paolini et al 2001, Cetin et al 1988, Cetin et al 1990, Cetin et al 1992, Economides et al 1989). Fetal growth is critically regulated by the insulin-like growth factor (IGF) system (Baker et al 1993). IGF-I, in particular, is a key regulator of overall growth in the human fetus (Gluckman and Pinal 2003), and its mitogenic activity is highly sensitive to nutrient availability (Kaaks 2004). The predominant IGF-I binding protein during fetal development is IGFBP-1 (Murphy et al 2006, Chard 1994), which is primarily secreted by the fetal liver (Han et al 1996). IGFBP-1 sequesters IGF-I from its receptor and prevents its ability to promote cell growth (Firth and Baxter 2002). Phosphorylated IGFBP-1, having significantly higher affinity for IGF-I (Jones et al 1991, Westwood et al 1997), further diminishes IGF-I bioavailability and subsequent cellular growth and proliferation.
Our earlier report indicates that IGFBP-1 is hyperphosphorylated (pSer101, pSer119 and pSer169) in umbilical cord plasma from human babies with FGR due to placental insufficiency as well as in the fetal liver in a baboon model of FGR induced by maternal nutrient restriction (MNR) (Abu Shehab et al 2014). Amino acid availability is a key regulator of IGFBP-1 phosphorylation with leucine deprivation causing pronounced IGFBP-1 hyperphosphorylation at Ser101, 119 and 168 residues and a concomitant increase in affinity for IGF-I (Seferovic et al 2009). We have recently identified the amino acid response (AAR) signaling pathway as the principle mediator of IGFBP-1 phosphorylation in leucine deprivation in vitro (Malkani et al 2015). The protein kinases responsible for IGFBP-1 hyperphosphorylation in leucine deprivation, however, have not been systematically studied.
Protein kinase CK2 is an evolutionarily conserved constitutively active kinase and CK2 activity is dynamically regulated in response to nutrient availability (Tripodi et al 2011, Sanchez-Casalongue et al 2015). We previously reported a potential link between MNR, elevated CK2 activity and IGFBP-1 hyperphosphorylation in baboon FGR fetal liver in vivo (Abu Shehab et al 2014). These findings nonetheless do not address whether CK2 or other nutrient-responsive kinases mediate IGFBP-1 hyperphosphorylation in response to amino acid deprivation. Conservation of optimal CK2 consensus sequence(s) in IGFBP-1 (pS/T-X-X-D/E), defined by the presence of acidic amino acids (Aspartic Acid, D and Glutamic Acid, E) surrounding the phospho-acceptor residues (Ser101, 119 and 169) (Litchfield 2003), is consistent with the possibility that acidophilic CK2 can directly phosphorylate IGFBP-1 (Coverley and Baxter 1997). As shown in Table 1, two of the three phosphorylated serine residues (Ser119 and 169) in IGFBP-1 conform precisely to this general recognition motif, providing strong basis to investigate CK2 as a key candidate in the regulation of IGFBP-1 phosphorylation in leucine deprivation.
Table 1.
IGFBP-1 peptide sequence (45-180) and possible phosphorylation sites for CK2, PKC and PKA.
|
IGFBP-1
sequence (45-180) |

|
||
|---|---|---|---|
|
Protein
Kinase |
Consensus phosphorylation site
motif sequence (X=any amino acid): |
IGFBP-1 peptides
with potential substrate sites |
Validated by in
vitro kinase assay |
| CK2 | pS/T-X-X-D/E |
*pS101TEITEEE pS119EED pS169GEE |
Validated
Validated Validated |
| PKC | pS/T-X-R/K |
pT50 AR pS58 CR |
-- |
| PKA | R/K-R/K-X-pS/T |
pT50AR pS58CR |
-- |
IGFBP-1 residues Ser101, Ser119 and Ser169 have been shown to be potential sites for phosphorylation by CK2 in HepG2 cells in response to leucine deprivation in vitro, in human FGR umbilical cord plasma from FGR pregnancies and in baboon FGR fetal liver from maternal nutrient restriction in vivo.
pSer101 is not a precise consensus sequence site for CK2. Conversely, PKC and PKA are not likely to directly phosphorylate IGFBP-1 at Ser 101, 119 and 169 sites.
Additionally, the protein kinase C (PKC) family is a group of at least 12 known members that have serine/threonine kinase activity. Several studies have shown that PKC is responsive to nutritional status (Lee et al 2008, Vary et al 2005, da Silva Lippo et al 2015). PKC exists in a variety of isotypes (i.e. the conventional (cPKCs), novel (nPKCs) and atypical (aPKCs) isoforms (Steinberg 2008)), all of which are dynamically regulated by a variety of intra- and extra-cellular stimuli (Steinberg 2008, Nishizuka 1992). Although the regulation of the specific PKC isoforms under nutrient restriction is currently unclear, it is known that PKC is involved in the regulation of hepatic IGFBP-1 production in HepG2 cells (Lee et al 1992). While PKC has the potential to phosphorylate multiple residues on the IGFBP-1 molecule (due to the presence of consensus sequence motifs (Table 1), IGFBP-1 phosphorylation by PKC has not been explicitly reported in normal or leucine deprivation conditions.
Furthermore, it is recognized that protein kinase A (PKA) is also sensitive to fluctuations in nutrient availability (O'Brien et al 1998, Milanski et al 2005, Stephen and Nagy 1996, Rozwadowski et al 1995, Goss et al 1994). Reduction in PKA activity has been linked to total IGFBP-1 mRNA and protein expression in HepG2 cells (Suwanichkul et al 1993). Although the consensus motif for IGFBP-1 phosphorylation at Ser101, 119 and 168 residues by PKA is not present within IGFBP-1 (Table 1), PKA-mediated phosphorylation of IGFBP-1 in vitro has been proposed previously (Ankrapp et al 1996, Frost and Tseng 1991).
In this study we tested the hypothesis that multiple nutrient sensitive kinases (CK2, PKA and PKC) contribute to regulation of IGFBP-1 phosphorylation in response to leucine deprivation in HepG2 cells. We employed pharmacological and RNAi approaches with and without leucine to inhibit respective kinases in HepG2 cells. We performed western immunoblot and enzymatic assays to study the mechanistic link between the kinases that mediate IGFBP-1 hyperphosphorylation under leucine deprivation. Furthermore, Multiple Reaction Monitoring Tandem Mass Spectrometry (MRM-MS) was used to quantitatively validate immunoblot data and IGF-IR autophosphorylation assay was employed to determine the functional relevance of our findings.
2. Methods
A schematic of the methodology used in major experiments is presented in supplemental figure 1 (Figure S1).
2.1 Cell culture
We utilized human hepatocellular carcinoma HepG2 cells (ATCC (Mananassas, VA)) which exhibit key characteristics of human fetal hepatocytes (Kelly and Darlington 1989, Wilkening et al 2003, Hart et al 2010, Pal et al 2012, Maruyama et al 2007). Cells were cultured in regular DMEM/F-12 media supplemented with 10% FBS (Invitrogen Corp., Carlsbad, CA) and maintained in 20% O2 and 5% CO2 at 37°C as we described previously (Seferovic et al 2009).
2.2 MRM-MS-based assessment of IGFBP-1 residues phosphorylated by CK2 using synthetic peptides
To validate that CK2 phosphorylates IGFBP-1 at the predicted residues (Table 1), we performed enzymatic assays using synthetic peptides (100 μM) containing respective IGFBP-1 phosphorylation sites (Ser101, 119 and 169). We performed CK2 activity assay according to established protocol (Turowec et al 2010) using pure CK2 (2.0 ng) and CK2 substrate peptide DSD (RRRDDDSDDD) (100 μM) (positive control) (a kind gift from Dr. D Litchfield). The relative abundance of each phosphorylated peptide was assessed using MRM-MS as detailed in section 2.10.
2.3 Leucine deprivation
Leucine deprivation treatment of HepG2 cells was performed using cells cultured in FBS-free DMEM/F-12 media. Based on dose dependency treatments performed by us previously (Seferovic et al 2009), 450 µM leucine was used in controls (leucine plus) and 0 μM leucine for culturing cells in leucine minus media.
2.4 Treatments of HepG2 cells with kinase inhibitors
HepG2 cells were cultured with leucine plus or leucine minus media and treated with CK2 inhibitor TBB (Litchfield 2003) using 1 μM concentration as per our previous dose-dependency optimization (Abu Shehab et al 2014). We performed titrations (1-20 µM) with a selective PKC inhibitor bisindolylmaleimide (BIS) (Brehmer et al 2004) (Figure S2 A-E) and a widely employed PKA specific inhibitor (cAMP-dependent protein kinase inhibitor (5-24) (PKI) (Hearst et al 2014) (5-200 nM) to select final concentrations for BIS (7.5 µM) and PKI (100 nM), respectively. HepG2 cells were incubated with inhibitors for 24 hours. Following treatments cell media and lysates (Abu Shehab et al 2014, Malkani et al 2015) were collected (Figure S1) and stored at −80 °C.
2.5 RNA interference (RNAi)-mediated silencing
Silencing of CK2 holoenzyme in HepG2 cells was achieved using SMART pool (Thermo Scientific, Rockford, IL, USA) siRNA (100nM) targeting all three subunits of human CK2 (α, α’ and β) as described previously (Abu Shehab et al 2014). PKC was silenced using pan-PKC (100 nM siRNA) (targeting PKC isoforms α, β, βII, γ, δ, ε, η, θ, ζ, and ν) (Santa Cruz Biotechnology, Dallas, TX, USA). Transfection was performed using Dharmacon transfection reagent 4 (Dharmacon,Thermo Fisher Scientific, Waltham, MA). Transfection media was removed after 24 hours. HepG2 cells were then incubated with leucine plus or leucine minus media for an additional 72 hours (Figure S1). Western immunoblot analysis was performed using CK2α, CK2α’, CK2β and PKCδ and PKCε antibodies to validate silencing efficiency.
2.6 Cell viability assays
To ensure that cell viability was not compromised by leucine deprivation and/or pharmacological inhibitors, we employed a trypan blue exclusion assay. Cells were counted using the Countess Automated Cell Counter (Life Technologies, Carlsbad, CA). Cell survival was determined as a ratio of live/total cells (Figure S3 A, B).
2.7 SDS-PAGE and Western Blotting
Equal volume of cell media (40-50 μL) from HepG2 cells plated in equal numbers was used to determine total and phosphorylated IGFBP-1 (Ser101, Ser119 and Ser169) by western immunoblot analysis as previously reported (Abu Shehab et al 2013, Abu Shehab et al 2014). Total IGFBP-1 was determined using mAb 6303 (Medix Biochemica, Kauniainen, Finland) and IGFBP-1 phosphorylation using our custom phosphosite specific IGFBP-1 antibodies (YenZyme Antibodies LLC, San Francisco, CA, USA). Expression of CK2α, CK2α’, CK2β, PKCδ, PKCε, CREB, IGF-1Rβ, β-actin, and phosphorylation of CREB (pSer133) and IGF-1Rβ (pTyr1135) was determined with equal amounts of total cell lysate protein (40-50 μg) from HepG2 cells using immunoblot analysis with respective antibodies. β-actin primary antibody was used for normalization of band intensities. CK2 antibodies were a kind gift from Dr. David Litchfield, UWO, Canada. Remaining primary antibodies were obtained from Cell Signaling Technologies (Beverly, MA, USA).
2.8 CK2 Activity Assay
CK2 activity was measured by incubating the synthetic CK2 substrate peptide DSD with HepG2 cell lysate and ATP32 (PerkinElmer, Waltham, MA, USA) (Vilk et al 1999) as we previously reported (Abu Shehab et al 2014).
2.9 PKC Activity Assay
We employed an established (D’Apolito et al 2015, Jia et al 2014) non radioactive ELISA-based PKC enzymatic assay (Enzo Life Science, Farmingdale, NY, USA) with HepG2 cells (0.5 µg total protein) in a 30 µL reaction volume following the manufacturer’s recommendations.
2.10 MRM-MS analyses of IGFBP-1 phosphorylation
2.10.1. Enrichment of IGFBP-1 phosphopeptides using immunoprecipitation
For MS analysis, samples were prepared by enrichment of IGFBP-1 using immunoprecipitation (IP) (Abu Shehab et al 2009). Equal volumes (3 mL) of conditioned HepG2 cell media were utilized from leucine minus or leucine plus treatments with and without TBB or BIS. MRM/MS analyses was performed as described in section 2.10.2.
2.10.2. MRM/MS analyses of IGFBP-1 phosphopeptides
We performed in-solution digestions using the IP samples of IGFBP-1 from HepG2 cell media as described earlier in section 2.10.1. IGFBP-1 was first digested using endoproteinase Asp-N (in 50 mM sodium phosphate buffer, pH 8.0) incubated overnight at 37ºC (Roche Diagnostics, Laval, QC, Canada). Half of each sample was then subjected to a subsequent digestion using trypsin (Roche Diagnostics) overnight at 37°C. IGFBP-1 digests were subsequently analyzed by LC-MS/MS with a triple quadruple mass spectrometer (4000 QTRAP AB Sciex, Concord, ON, Canada). A NanoAcquity UPLC system (Waters, Milford, MA, USA) equipped with a C18 analytical column (1.7 µm, 75 µm×200 mm) was used to separate the peptides at a flow rate of 300 nl/min and operating pressure of 8000 psi. Peptides were eluted using a 62 min gradient from 95% solvent A (H2O, 0.1% formic acid) and 5% solvent B (acetonitrile, 0.1% formic acid) to 50% solvent B for 40 min, 90 % solvent B for 6 min, and back to 5% solvent B for 10 min. Eluted peptides were electrosprayed (Nanosource, ESI voltage +2000V) into the mass spectrometer, which monitored 98 transitions per sample with a dwelling time of 50 msec/transition. Relative changes in IGFBP-1 phosphorylation were determined by the total peak height of combined transitions. An internal IGFBP-1 peptide (NH2-ALPGEQQPLHALTR-COOH) was used to normalize all pIGFBP-1 data.
2.11 Immunodepletion of IGFBP-1 for IGF-1 receptor (IGF-1R) activation assay
To confirm that changes in IGF-1Rβ autophosphorylation in P6 cells are not influenced by non-specific components of the treatment media other than IGFBP-1, we depleted IGFBP-1 derived from HepG2 cell media. IP was performed following our previously established protocol (Abu Shehab et al 2009) and supernatants collected were utilized as immunodepleted IGFBP-1 for the functional assay described in section 2.12.
2.12 IGF-1 receptor (IGF-1R) activation assay
The details of the bioassay using mouse embryo fibroblast P6 cells that over-express human IGF-1R (a kind gift from Dr. R. Baserga, Thomas Jefferson University, Philadelphia, PA) and a schematic representation have been described previously (Abu Shehab et al 2013). In brief, HepG2 conditioned media containing equal amounts of total IGFBP-1 from leucine plus and leucine minus treatments with/without TBB or BIS were incubated with rhIGF-I (25 ng/mL) for two hours at room temperature. Samples were then used to treat P6 cells. Equal amounts of total protein (45 µg) from post-treatment P6 cell lysates were used for western immunoblot analyses to assess changes in IGF-1R autophosphorylation using IGF-1Rβ (pTyr1135) antibody.
2.13 Data presentation and statistics
GraphPad Prism 5 (Graph Pad Software Inc., CA) was used for all data analyses. The mean of three biological replicates (n=3) of the band densitometry was normalized to the control of each experiment and assigned an arbitrary value of 1. For the PKC activity assay, each replicate was assessed in duplicate (n=6). The data was represented as mean ± SEM and considered significant where indicated (at *p<0.05, **p=0.01-0.05, ***p<0.01) as per one-way analysis of variance (ANOVA) with Dunnet’s Multiple Comparison Post-Test. For MRM-MS analysis, peak areas of the combined transitions for each peptide were determined using Skyline software and compared between samples and normalized to an internal, non-phosphorylated IGFBP-1 peptide.
3. Results
3.1 MRM-MS-based assessment of IGFBP-1 residues phosphorylated by CK2 using synthetic IGFBP-1 peptides
Based on the presence of consensus sequence motif (Table 1) as well as consistent with our previous studies with HepG2 cells (Abu Shehab et al 2014, Malkani et al 2015), we expected CK2 to mediate IGFBP-1 phosphorylation at Ser101, 119 and 169. In order to confirm the contribution of CK2, we used three individual IGFBP-1 synthetic substrate peptides, which contained the residues Ser101, 119 and 169 in their non-phosphorylated state, respectively (Figure S4). Following CK2 activity assay, we monitored the status of site-specific phosphorylation of IGFBP-1 with MRM-MS. As expected, CK2 was able to phosphorylate IGFBP-1 peptides that contained the Ser 119, or Ser 169 but also Ser101 residue to detectable levels by MRM-MS transitions specific to the phosphorylated peptide state (Figure 1). We anticipate that Ser101, albeit not exactly conforming to the consensus sequence, is phosphorylated by CK2 because of its proximity to multiple acidic residues (D/E). In addition, MRM-MS analysis identified phosphorylation of Ser101 paired with Ser98 and residue Ser 169 paired with Ser174 on respective peptides. The presence of doubly phosphorylated residue Ser101/98 is consistent with our previous in vitro findings with HepG2 cells (Seferovic et al 2009) and with FGR samples (Abu Shehab et al 2010). However, doubly phosphorylated Ser169/174 has not been reported previously. Together these data provided the basis to investigate the role of CK2 as well as other potential kinases (PKA and PKC) in mediating IGFBP-1 hyperphosphorylation under leucine deprivation using cultured HepG2 cells.
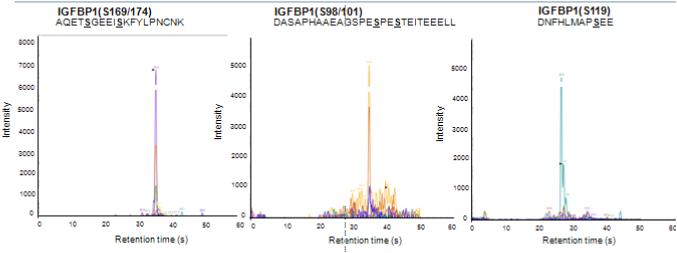
Figure 1. MRM spectra of synthetic IGFBP-1 peptides used for CK2 in vitro kinase assay.
MRM/MS analysis of phosphorylation of Ser residues (S) on IGFBP-1 peptides, non-phosphorylated synthetic peptides were used as substrates for CK2 enzyme. Following incubation, CK2-induced phosphorylation of each peptide was then monitored by targeting the phosphorylation-specific version of each IGFBP-1 peptide substrate by MRM-MS using a QTRAP 4000 mass spectrometer. CK2 phosphorylates IGFBP-1 at Ser101, Ser119 and Ser169. Ser101 was found to be doubly phosphorylated with Ser98, and Ser169 was indicated to be doubly phosphorylated with Ser174.
3.2 Pharmacological inhibition of PKC supports that PKC mediates leucine deprivation-induced IGFBP-1 phosphorylation
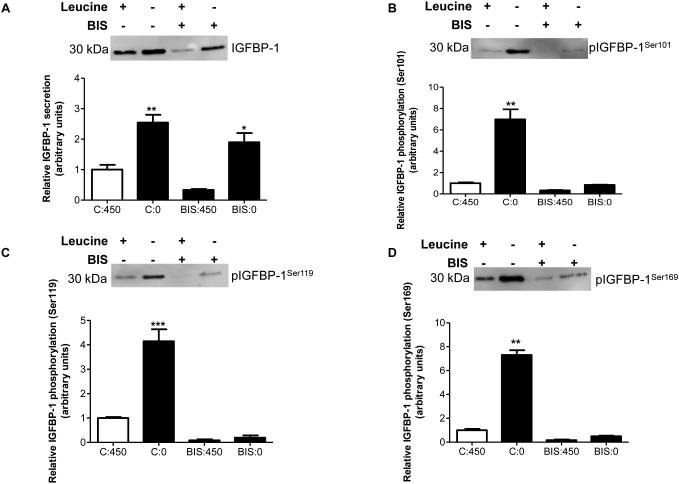
Using HepG2 cells, we first sought to determine whether inhibition of PKC using BIS in leucine plus or leucine deprived conditions affected IGFBP-1 phosphorylation at the sites of our interest (Ser101, 119, and 169). Although the exact consensus motif for PKC substrates is not present within these three serine residues, we demonstrated that under leucine deprivation there was an increase in total IGFBP-1 (+250%) independent of the presence of BIS (Figure 2A). Cell viability assay confirmed that HepG2 cells were not affected by BIS treatments (Figure S3A). In the presence of normal concentrations of leucine (450 μM), BIS-mediated inhibition of PKC decreased IGFBP-1 phosphorylation at all three IGFBP-1 phosphosites (Ser101 −30%, Ser119 −40%, Ser169 −50%) (Figure 2B-D). As expected, leucine deprivation also markedly induced IGFBP-1 phosphorylation (Ser101 +800%, 119 +300%, 169 +600%), however, this effect was completely mitigated by PKC inhibition (Figure 2B-D), demonstrating that an intact/active PKC is required for leucine deprivation to induce IGFBP-1 phosphorylation. These data provide strong evidence that PKC is involved in mediating IGFBP-1 phosphorylation under conditions of leucine deprivation in HepG2 cells. However, considering the lack of consensus sequence motifs at Ser101, 119 and 169 residues (Table 1), we speculated that PKC may be altering IGFBP-1 phosphorylation indirectly by potentially interacting with CK2.
Figure 2. Effect of pharmacological pan-PKC inhibitor Bisindolylmaleimide (BIS) on IGFBP-1 phosphorylation.
HepG2 cells were treated with BIS (7.5 μM) and cultured in leucine plus (450 μM) or in leucine deprived (0 μM) media for 24 hours (n=3 each). A representative western immunoblot of HepG2 cell media indicating A. total IGFBP-1 and B. IGFBP-1 phosphorylation at Ser101, Ser119 and Ser169 in leucine plus (450 μM), leucine deprivation, BIS, and leucine deprivation+BIS treatments. Inhibition of PKC in leucine deprivation attenuates IGFBP-1 hyperphosphorylation at Ser101, Ser119 and Ser169. Values are displayed as mean + SEM. *p< 0.05, **p= 0.001-0.05, ***p < 0.0001 versus control; One-way analysis of variance; Dunnet’s Multiple Comparison Test; n=3. C:450 Control, 450 μM leucine. C:0: Leucine deprivation, 0 μM leucine. BIS:450: Bisindolylmaleimide (7.5 μM), 450 μM leucine. BIS:0: Bisindolylmaleimide (7.5 μM), 0 μM leucine.
3.3 Inhibition of PKA does not affect IGFBP-1 phosphorylation in response to leucine deprivation
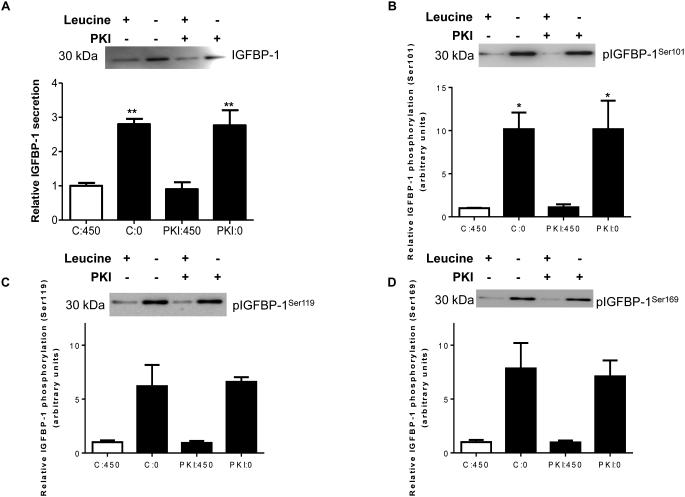
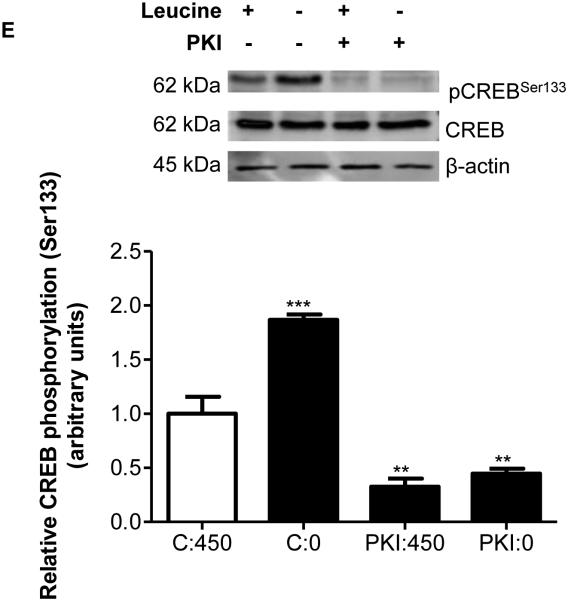
Previous studies have suggested that purified PKA can phosphorylate IGFBP-1 isolated from human decidual cells (Frost and Tseng 1991). However, based on consensus sequence motifs (Table 1), PKA mediated IGFBP-1 phosphorylation may not be possible at Ser101, Ser119 and Ser169 residues, which we have shown to be hyperphosphorylated due to leucine deprivation (Seferovic et al 2009). To examine whether PKA signaling is involved, we used a selective PKA inhibitor PKI, in leucine plus or leucine minus conditions in cultured HepG2 cells. Figures 3A-D show that leucine deprivation increased total IGFBP-1 (+300%) and IGFBP-1 phosphorylation (Ser101 +1000%, Ser119 +500%, Ser169 +750%) in the presence of the PKA inhibitor confirming that IGFBP-1 phosphorylation was not affected by PKA inhibition. In order to further prove that PKA is not involved, we tested the effects of leucine deprivation on PKA signaling using Creb (Ser133) phosphorylation as a functional readout for PKA activity (Gonzalez and Montminy 1989). As expected, our data show an increase in Creb (Ser133) phosphorylation (+200%) due to leucine deprivation. We further demonstrated that Creb phosphorylation (pSer133) was reduced (−75%) in the presence of PKI (Figure 3E) independent of leucine deprivation status. These data demonstrate that PKA does not mediate IGFBP-1 hyperphosphorylation in response to leucine deprivation.
Figure 3. Effects of PKI (5-24) inhibition of PKA on leucine deprivation-induced IGFBP-1 phosphorylation.
HepG2 cells were incubated with PKI (5-24) (100 nM) in leucine plus (450 μM) or leucine deprived (0 μM) media for 24 hours (n=3 each). A representative western immunoblot of HepG2 cell media indicating A. total IGFBP-1 and B. IGFBP-1 phosphorylation at Ser101, Ser119 and Ser169 in control, leucine deprivation, PKI, and leucine deprivation+PKI treatments. PKA inhibition did not affect leucine deprivation-induced IGFBP-1 phosphorylation. Values are displayed as mean + SEM. *p< 0.05, **p= 0.001-0.05, ***p < 0.0001 versus control; One-way analysis of variance; Dunnet’s Multiple Comparison Test; n=3. C:450: Control, 450 μM leucine. C:0: Leucine deprivation, 0 μM leucine. PKI:450: PKI (5-24) (100 nM), 450 μM leucine. PKI:0:
3.4 siRNA targeting of the CK2 holoenzyme confirms the role of CK2 in mediating IGFBP-1 phosphorylation in response to leucine deprivation
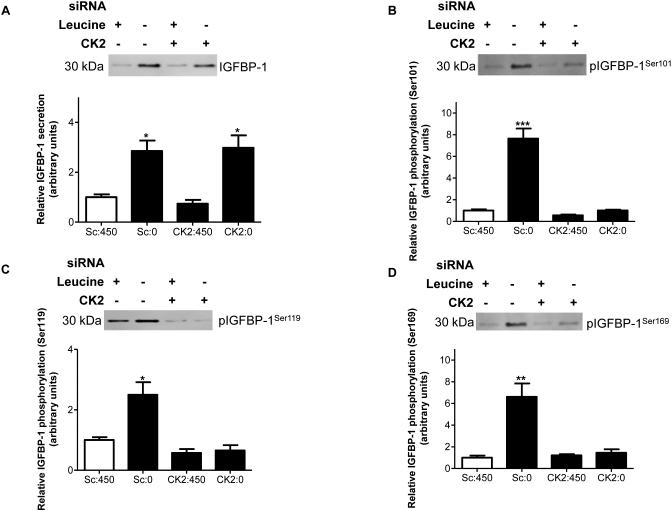
We have recently obtained preliminary support suggesting that TBB, a pharmacological inhibitor of CK2, prevents leucine deprivation-induced IGFBP-1 phosphorylation in HepG2 cells (Malkani et al 2015). To confirm these findings in the current study, we used a targeted RNAi approach. We inhibited the CK2 holoenzyme (Turowec et al 2010), by siRNA targeting all three CK2 (α+α’+β) subunits. The expression of CK2α and CK2α’ and CK2β were reduced by up to 55% (Figure S5). We then assessed whether CK2 silencing affected leucine deprivation-induced total IGFBP-1 secretion and/or IGFBP-1 phosphorylation. While leucine deprivation increased total IGFBP-1 (+350%) (Figure 4A) and phosphorylation (Ser101 +800%, Ser119 +300%, Ser169 +600%), importantly, CK2 silencing prevented IGFBP-1 phosphorylation at the three sites (Ser 101, 119 and 169) in leucine deprivation (Figures 4B-D). This data confirms that CK2 mediates IGFBP-1 hyperphosphorylation in response to leucine deprivation.
Figure 4. The effect of siRNA silencing targeting CK2 holoenzyme (CK2α+α’+β) on leucine deprivation-induced IGFBP-1 phosphorylation.
HepG2 cells were treated with scrambled or CK2α+α’+β siRNA for 24 hours and incubated in leucine plus (450 μM) or leucine deprived (0 μM) media for 72 hours (n=3 each). Representative immunoblots of conditioned HepG2 cell media (50 μL per well) treated with scrambled, or CK2α+α’+β siRNA in media that was leucine plus or leucine deprived assessed for A. total IGFBP-1 and B. IGFBP-1 phosphorylation at Ser101, Ser119 and Ser169. Knockdown of the CK2 holoenzyme prevents IGFBP-1 hyperphosphorylation in leucine deprivation. Values are displayed as mean + SEM. *p< 0.05, **p= 0.001-0.05, ***p < 0.0001 versus control; One-way analysis of variance; Dunnet’s Multiple Comparison Test; n=3. Sc: Scrambled, 450 μM leucine. Sc:0: Scrambled, 0 μM leucine. CK2:450: CK2α+α’+β siRNA, 450 μM leucine. CK2:0: CK2α+α’+β siRNA, 0 μM leucine.
3.5 siRNA targeting confirms a role for PKC in modulating leucine deprivation induced IGFBP-1 phosphorylation
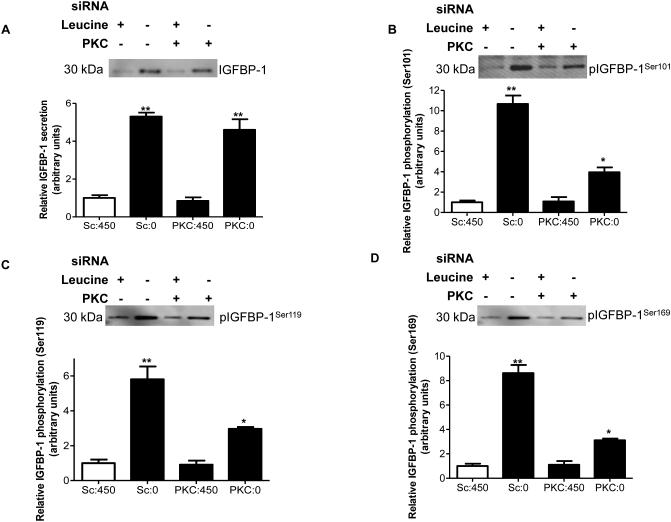
Next we used RNAi to verify the involvement of PKC in mediating IGFBP-1 phosphorylation in response to leucine deprivation. We validated efficient PKC silencing by western immunoblot analysis of two representative PKC isoforms, nPKCδ and nPKCε, with known expression in HepG2 cells (Rypka et al 2005). These isoforms are down-regulated in nutrient deprivation (Vary et al 2005). The expression of both PKCδ and PKCε was decreased 50% due to PKC silencing regardless of leucine status (Figure S6). In response to leucine deprivation, total IGFBP-1 (+450%) (Figure 5A) and IGFBP-1 phosphorylation (Ser101 +1000%, Ser119 +500%, Ser169 +800%) were significantly induced (Figures 5B-D). PKC silencing strongly attenuated the increase in IGFBP-1 phosphorylation (Ser101-60%, 119-50% and 169-70%) in response to leucine deprivation (Figures 5B-D) consistent with our findings with pharmacological PKC inhibition (Figure 2B-D). These data demonstrate a specific role of PKC in modulating IGFBP-1 phosphorylation in leucine deprivation. However, because IGFBP-1 lacks the consensus sequences for PKC phosphorylation at Ser101, 119 and 169, to validate the above results, we employed MRM/MS analysis.
Figure 5. The effect of pan-PKC siRNA on IGFBP-1 phosphorylation in leucine deprivation.
HepG2 cells were treated with scrambled or pan-PKC siRNA for 24 hours and cultured in leucine plus (450 μM) or leucine deprived (0 μM) media for an additional 72 hours (n=3 each). A. A representative western immunoblot of total IGFBP-1 in equal amounts (50 μL) of cell media treated with scrambled or ERK siRNA with and without leucine deprivation. B-D. Representative western immunoblots of HepG2 cell media (50 μL) treated with scrambled or pan-PKC siRNA in leucine plus or leucine deprived conditions and assayed for IGFBP-1 phosphorylation at Ser101, Ser119 and Ser169. PKC knockdown attenuates IGFBP-1 hyperphosphorylation in response to leucine deprivation. Values are displayed as mean + SEM. *p< 0.05, **p= 0.001-0.05, ***p < 0.0001 versus control; One-way analysis of variance; Dunnet’s Multiple Comparison Test; n=3. Sc: Scrambled, 450 μM leucine. Sc:0: Leucine deprivation, 0 μM leucine. PKC:450: PKC siRNA, 450 μM leucine. PKC:0: PKC siRNA, 0 μM leucine.
3.6 MRM-MS based validation of IGFBP-1 phosphorylation sites following pharmacological inhibition of CK2 and PKC activity in leucine deprivation
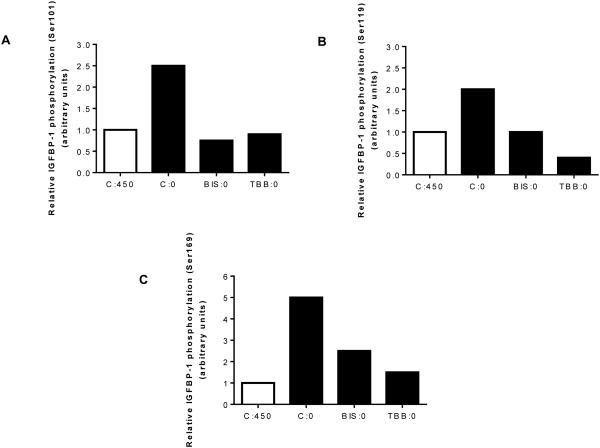
As an alternate independent strategy to assess the involvement of CK2 or PKC in mediating IGFBP-1 hyperphosphorylation, we used MRM-MS to ensure that inactivation of either CK2 or PKC (TBB or BIS) prevents leucine deprivation-induced site-specific IGFBP-1 phosphorylation. As detected by MRM-MS, leucine deprivation increased IGFBP-1 phosphorylation at Ser101, 119, and 169 by +280%, +200%, and +540%, respectively (Figures 6A-C), consistent with our immunoblot analyses. Treatment of cells with TBB decreased largely prevented leucine deprivation-induced IGFBP-1 phosphorylation (Figures 6A-C). Similarly, leucine deprivation did not induce IGFBP-1 phosphorylation at Ser101 and Ser119 in the presence of BIS, whereas BIS only partially prevented phosphorylation at Ser169 in response to leucine deprivation (Figures 6A-C). Thus, MRM/MS analyses confirmed that IGFBP-1 phosphorylation at Ser 101, Ser119 and Ser169 in response to leucine deprivation involves both CK2 and PKC.
Figure 6. MRM/MS analysis for assessment of IGFBP-1 phosphorylation.
Mass spectrometry analysis of the relative IGFBP-1 phosphorylation induced by leucine deprivation in the presence or absence of TBB or BIS. TBB or BIS attenuate IGFBP-1 phosphorylation at A. Ser101, B. Ser119 and C. Ser169 in the presence of either inhibitor (TBB samples (set to a value of 1) and normalized to a non-phosphorylated peptide within the IGFBP1 protein. C:450 Control, 450 μM leucine. C:0: Leucine deprivation, 0 μM leucine. BIS:0: Bisindolylmaleimide (7.5 μM), 0 μM leucine. TBB:0: TBB (1 μM), 0 μM leucine.
3.7 Inhibition of CK2 and/or PKC in leucine deprivation of HepG2 cells eliminates the inhibitory effects of IGFBP-1 phosphorylation on IGF-I bioactivity
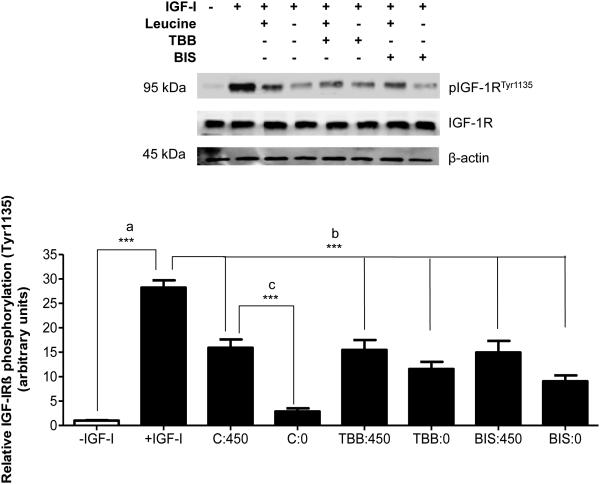
Next, to understand the functional relevance of our findings we determined the effects of pharmacological inhibition of CK2 or PKC-mediated IGFBP-1 phosphorylation in leucine deprivation using IGF-1 receptor (IGF-1Rβ) autophosphorylation assay (Figure 7). Addition of 25 ng/mL IGF-I to P6 cell media (Figure 7, lane 2 (positive control) stimulated the phosphorylation of IGF-IRβ (Tyr1135) (+2700%) compared to P6 cells incubated in media lacking IGF-I (Figure 7, lane 1, negative control), confirming that IGF-I stimulates IGF-1R activity in P6 cells.
Figure 7. The effects of CK2 and PKC inhibition on IGF-1R autophosphorylation.
HepG2 cells were treated in leucine plus (450 μM) or leucine deprived (0 μM) media with BIS or with TBB, a pharmacological CK2 inhibitor, for 24 hours (n=3). HepG2 cell media samples were aliquoted to contain equal concentrations of IGFBP-1 and buffer-exchanged to serum-free P6 media (DMEM/F12 with pyruvate). Aliquots were then incubated with human recombinant IGFI (25 ng/mL) for 2 hours to allow IGFBP-1 binding to IGF-I, followed by a 10 minute exposure to P6 cells to allow induction of IGF-I-mediated IGF-1Rβ autophosphorylation (Tyr1135). A representative western immunoblot of post-treatment P6 cell lysates (50 μg per lane) assessed for IGF-IR autophosphorylation (Tyr1135). Leucine deprivation-stimulated IGFBP-1 phosphorylation reduced IGF-1R activation; leucine deprivation was unable to elicit this effect in the presence of BIS or TBB. Values are displayed as mean + SEM. *p< 0.05, **p= 0.001-0.05, ***p < 0.0001 versus control; One-way analysis of variance; Dunnet’s Multiple Comparison Test; n=3. – IGF-I: Negative control, no IGF-I, no IGFBP-1. +IGF-I: Positive control, 25 ng/mL IGF-I, no IGFBP-1. C:450: Control, 450 μM leucine. C:0: Leucine deprivation, 0 μM leucine. TBB:450: TBB (1 μM), 450 μM leucine. TBB:0: TBB (1 μM), 0 μM leucine. BIS:450: Bisindolylmaleimide (7.5 μM), 450 μM leucine. BIS:0: Bisindolylmaleimide (7.5 μM), 0 μM leucine.
HepG2 cell media from control (450 µM leucine) was used to determine the effect of basal IGFBP-1 phosphorylation on IGF-I activity (Figure 7, lane 3) compared to media lacking leucine in the presence and absence of CK2 and PKC inhibitors (TBB and BIS). Equal concentration of total IGFBP-1 from each treatment was used in the assay to ensure that changes in IGF-1Rβ autophosphorylation are caused specifically by precise changes in the phosphorylation status of IGFBP-1 and not due to total IGFBP-1. Additionally, to ensure that changes in IGF-1Rβ autophosphorylation are mainly due to IGFBP-1 in HepG2 cell media and not any other factors, we treated P6 cells with HepG2 cell media immunodepleted of IGFBP-1. Autophosphorylation of IGF-1Rβ in P6 cells was stimulated to similar levels to IGF-I alone (positive control), confirming that decreases in receptor autophosphorylation are specific to IGFBP-1 only (Figure S7).
As expected, HepG2 cell media from (450 µM leucine, basal IGFBP-1 phosphorylation) (Figure 7, lane 3) reduced IGF-1Rβ autophosphorylation (Tyr1135) (−45%), compared to positive control (Figure 7, lane 2). Lane 3 subsequently served as a control for the basis of comparison of IGF-I bioactivity from the various treatments in this experiment. Furthermore, the elevated IGFBP-1 phosphorylation in leucine-deprived HepG2 cell media inhibited IGF-1Rβ autophosphorylation (−90%) (Figure 7, lane 4). When IGFBP-1 phosphorylation had been prevented by CK2 inhibition (TBB) or PKC inhibition (BIS) in HepG2 cells and the respective cell media were used to treat P6 cells, IGF-1R activity in P6 cells was restored to basal levels.
Therefore, these data indicate that when either CK2 or PKC activity was inhibited, leucine deprivation failed to inhibit IGF-I bioactivity (Figure 7, lanes 6 and 8). Together, these data demonstrate the functional role of both CK2 and PKC in modulating IGFBP-1 phosphorylation, and as a consequence, IGF-I bioactivity in leucine deprivation.
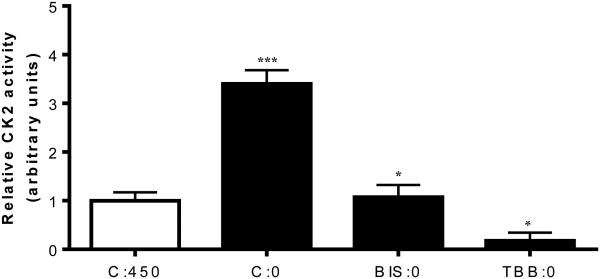
3.8 Pharmacological inhibition of CK2 or PKC signaling attenuates leucine deprivation-induced CK2 activity
Considering the significance of PKC in IGFBP-1 phosphorylation demonstrated with functional effects on IGF-1 bioactivity, we tested whether CK2 activity in leucine deprivation is affected by PKC. We assessed CK2 activity in cell lysates from HepG2 cells treated with either TBB or BIS in leucine deprivation. We first confirmed that TBB reduced CK2 activity without affecting PKC activity (Figure S8A). We also confirmed in parallel that BIS specifically reduced PKC activity without affecting CK2 activity (Figure S8B).
Leucine deprivation stimulated CK2 activity (+300%) (Figure 8, lane 2) but this stimulation was effectively prevented in the presence of BIS (Figure 8, lane 3) or TBB (Figure 8, lane 4). These results, suggest that CK2 activation, and the subsequent induction of IGFBP-1 phosphorylation due to leucine deprivation, is dependent on PKC. Together these data strongly support the conclusion that activation of CK2 following leucine deprivation is PKC-dependent thus implicating the involvement of the two kinases in a common mechanism in regulating IGFBP-1 phosphorylation in leucine deprivation.
Figure 8. Effects of various inhibitor treatments on CK2 activity.
A. CK2 activity assay demonstrates that leucine deprivation induces CK2 activity, an effect that is attenuated by BIS. Expectedly, TBB decreases CK2 activity in leucine deprivation. Values are displayed as mean + SEM. *p< 0.05, **p= 0.001-0.05, ***p < 0.0001 versus control; One-way analysis of variance; Dunnet’s Multiple Comparison Test; n=3. C:450: Control, 450 μM leucine. C:0: Leucine deprivation, 0 μM leucine. BIS:0: Bisindolylmaleimide (7.5 μM), 0 μM leucine. TBB:0: TBB (1 μM), 0 μM leucine.
4. Discussion
We report here for the first time that increased IGFBP-1 phosphorylation in response to leucine deprivation is mediated by CK2 and PKC. Using pharmacological and/or RNAi approaches in situ we provide highly convincing evidence that the inhibition of CK2 or PKC, but not PKA, decreases IGFBP-1 phosphorylation and markedly attenuates or prevents IGFBP-1 phosphorylation caused by leucine deprivation. We show that PKC promotes IGFBP-1 phosphorylation via activation of CK2 in response to leucine deprivation. Our data are consistent with our premise that amino acid deprivation leads to increased IGFBP-1 phosphorylation in the fetal liver via CK2, which is dependent on PKC.
As the predominant circulating fetal IGFBP, the ability of IGFBP-1 to regulate IGF-I bioavailability is critical to fetal growth. Phosphorylation of human IGFBP-1 markedly increases its affinity for IGF-I (Jones et al 1991), thereby significantly reducing its bioavailability further (Westwood et al 1997). Preliminary support for a role of site-specific IGFBP-1 phosphorylation in FGR comes from our previous studies. We reported a marked increase in IGFBP-1 phosphorylation and higher affinity for IGF-I in amniotic fluid derived from human FGR pregnancies (Abu Shehab et al 2010). We also demonstrated that human FGR fetuses display elevated phosphorylated IGFBP-1 (Ser101, 119 and 169) in cord serum. Enhanced IGFBP-1 phosphorylation at the same serine residues was also observed in the liver of baboon fetuses with FGR induced by maternal nutrient restriction (MNR), which was linked with substantial increases in CK2 activity (Abu Shehab et al 2014). However, whether CK2 or any additional protein kinases modulate IGFBP-1 phosphorylation and constitute an underlying molecular mechanism of regulation of IGF-I bioavailability in response to amino acid deprivation in FGR remains unclear.
The consensus sequence for CK2 phosphorylation requires an acidic residue in position +3 downstream from the phospho-acceptor site and is generally accompanied by additional acidic residues (Litchfield 2003, Coverley and Baxter 1997). Two of the three IGFBP-1 phosphorylation sites (Ser119 and Ser169) in IGFBP-1 conform precisely to this general recognition motif. Although the region surrounding Ser101 is not an exact match, sequence adjacent to S101 is highly similar to the precise CK2 consensus sequence and rich in surrounding acidic residues (Table 1). Using three synthetic non-phosphorylated IGFBP-1 peptides and MRM-MS strategy in this study we demonstrate that CK2 can directly phosphorylate IGFBP-1 at all three serine residues. Furthermore, we provided experimental evidence to also show that IGFBP-1 was hyperphosphorylated (Ser101, 119 and 169) by CK2 in HepG2 cells in response to leucine deprivation. These results are consistent between two independent approaches: immunoblotting and MRM-MS analyses. Together these findings provide strong supporting evidence to show that CK2 is mediating the effects of leucine deprivation on IGF-I bioactivity and suggest that CK2 may be a key protein kinase involved in FGR.
Previous studies have proposed that in addition to CK2, PKA and PKC can also phosphorylate IGFBP-1 (Ankrapp et al 1996, Frost and Tseng 1991). In vitro kinase assays employing purified/isolated preparations may or may not be reflective of the actual functionality of the kinases within live cells. Although the consensus motif for PKC and PKA substrate is present within IGFBP-1, these are distinct than that of CK2 (Table 1). Furthermore, a direct phosphorylation by PKC requires the proximity of basic amino acids to the phospho-acceptor site such as at T50AR and S58CR residues (Coverley and Baxter 1997). Considering that we have shown that Ser 101, 119 and 169 residues were hyperphosphorylated in response to leucine deprivation (Seferovic et al 2009, Malkani et al 2015) and/or in FGR (Abu Shehab et al 2010), it is less plausible that IGFBP-1 is directly phosphorylated by PKC or PKA at these serine sites.
Interestingly, despite lack of precise consensus sequence for PKC at Ser 101, 119 and 169, our current data showed that BIS, a PKC-specific inhibitor, prevents CK2 induction by leucine deprivation, placing PKC upstream of CK2. These data suggest that PKC regulates IGFBP-1 phosphorylation by modulating CK2 activity. Alternatively, PKC may phosphorylate IGFBP-1 at discrete sites such as Thr50 or Ser58 (Table 1). It is possible that IGFBP-1 phosphorylation at these new sites may elicit functional effects on IGF-I affinity directly or through synergistic interactions with Ser101, 119 or 169 or other alternate sites. For example, phosphorylation of IGFBP-1 at Ser95 and Ser98 has been previously identified by us (Abu Shehab et al 2010, Nissum et al 2009) as well as others (Dolcini et al 2009) and might be functionally relevant in modulating IGFBP-1 phosphorylation via CK2/PKC in FGR.
The functional implications of phosphorylation of IGFBP-1 at single sites on IGF-I-bioactivity have been demonstrated by us previously (Abu Shehab et al 2013) but similar effects of dual/multiple site phosphorylation on IGFBP-1 have not yet been tested. We have previously provided indirect evidence for a role of IGFBP-1 phosphorylation at dually phosphorylated residues in FGR. Employing LC-MS and LC-MS/MS of amniotic fluid we earlier showed up to 7-fold increase in IGFBP-1 phosphorylation at Ser98/101 along with a 23-fold increase on Ser169 (Abu Shehab et al, 2010) in FGR together with an increased IGF-I affinity. Additionally, hypoxic treatment of HepG2 cells enhanced IGFBP-1 phosphorylation up to 4-fold at Ser98/101 together with Ser 169, which was accompanied by a pronounced increase (300-fold) in IGFBP-1 affinity to IGF-I (Seferovic et al 2009) and reduced IGF-I dependent cell proliferation. In the current study using synthetic peptides and highly sensitive MRM-MS analysis, we found IGFBP-1 phosphorylation at Ser98 paired with Ser101, but also at a novel site Ser174 combined with Ser169. Whether Ser 174/169 phosphorylation singly or in combination with new Ser/Thr residues (Table 1) is specific to enhanced CK2/PKC activity in leucine deprivation is yet to be determined. Considering dual phosphorylation of IGFBP-1 could remarkably enhance affinity and reduce IGF-I bioavailability than due to a single site (Seferovic et al 2009), we speculate that multi-site interactions can synergistically alter IGF-I affinity and bioactivity in FGR and may have potential implications in the etiology of FGR.
A previous report has suggested a role of PKC in FGR; specifically, the expression of some PKC isoforms was decreased in placentas of rats with glucocorticoid-induced FGR (Sugden and Langdown 2001). Specific isoforms of PKC (nPKCδ and nPKCε), which are known to be down-regulated in nutrient deprivation (Vary et al 2005) and to have a role in placental remodeling, are expressed in HepG2 cells (Rypka et al 2005). These observations are consistent with the possibility that altered PKC signaling is involved in the pathophysiology of FGR although the exact role of PKC function in FGR pregnancies has not been established. In this study, siRNA silencing of PKC resulted in reduction in the expression of these two specific isoforms (nPKCδ and nPKCε) in HepG2 cells, which accompanied by a reduced IGFBP-1 phosphorylation. The identification of the specific isoform(s) of PKC that underlie(s) leucine deprivation induced IGFBP-1 phosphorylation is critical but is beyond the scope of this study. However, our data show that BIS, a PKC-specific inhibitor, prevents CK2 induction by leucine deprivation, which suggest that PKC regulates IGFBP-1 phosphorylation via upstream modulation of CK2 activity. The data from this work are consistent with the possibility that altered PKC signaling along with CK2 is involved in the pathophysiology of FGR.
PKA on the other hand, has been previously been suggested to phosphorylate IGFBP-1 isolated from stromal cells (Frost et al 2000). Because Ser101, 119 and 169 sites are not compatible with PKA consensus sequence (Table 1), it is possible that PKA phosphorylates stromal IGFBP-1 at additional discrete residues where the surrounding sequence is similar to the exact PKA compatible sequence. It is also possible that PKA-mediated IGFBP-1 phosphorylation is cell-type specific. However, given the inability of PKA to phosphorylate IGFBP-1 in HepG2 cells at Ser 101, 119 and 169, which we have shown to be hyperphosphorylated due to leucine deprivation (Seferovic et al 2009), we suggest that PKA is not a kinase linking leucine deprivation to hepatic IGFBP-1 phosphorylation.
Taken together, from the above results we propose a model in which leucine deprivation activates CK2, which directly phosphorylates IGFBP-1 at Ser101, 119 and 169, while PKC regulates IGFBP-1 phosphorylation via modulating CK2 activity under leucine deprivation. This model is consistent with the established role of PKC as a regulator of the function of other proteins (Geraldes and King 2010), however future studies are required to identify the specific PKC isoforms involved in linking leucine deprivation to IGFBP-1 phosphorylation (Meier et al 2007; Sossin, W. S. (2007). Nonetheless, our demonstration that PKC regulates both CK2 activity and IGFBP-1 phosphorylation in leucine deprivation contends, for the first time, that PKC functions in a combined signaling mechanism with CK2 to modulate IGF-I bioavailability. We speculate that activation of CK2 via PKC in the fetal liver constitutes a mechanistic link between reduced amino acid availability, increased IGFBP-1 phosphorylation, decreased IGF-I bioavailability and restricted fetal growth in FGR.
Supplementary Material
Highlights.
A novel mechanistic link between reduced amino acid availability and restricted fetal growth in FGR.
PKC promotes both CK2 activity and IGFBP-1 phosphorylation in leucine deprivation
CK2/PKC regulate IGF-I-bioactivity in a common mechanism in leucine deprivation by reducing IGF-I action
Acknowledgements
This work was supported in part by the National Institutes of Health (HD 078313 to MBG and TJ), Natural Science and Engineering Council of Canada (NSERC) Discovery grant and a grant from the Lawson Health Research Institute, UWO, Canada to MBG. We gratefully acknowledge Dr. D W Litchfield, Chair, Department of Biochemistry, UWO, Canada for providing the specialized reagents for CK2. NM received Department of Pediatrics (UWO) Summer Student Scholarship. KKB is the recipient of an NSERC Banting fellowship. SSCL holds a Canada Research Chair in Functional Genomics and Cellular Proteomics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Abu Shehab M, Damerill I, Shen T, Rosario FJ, Nijland M, Nathanielsz PW, Kamat A, Jansson T, Gupta MB. Liver mTOR controls IGF-I bioavailability by regulation of protein kinase CK2 and IGFBP-1 phosphorylation in fetal growth restriction. Endocrinology. 2014;155:1327–1339. doi: 10.1210/en.2013-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Shehab M, Iosef C, Wildgruber R, Sardana G, Gupta MB. Phosphorylation of IGFBP-1 at discrete sites elicits variable effects on IGF-I receptor autophosphorylation. Endocrinology. 2013;154:1130–1143. doi: 10.1210/en.2012-1962. [DOI] [PubMed] [Google Scholar]
- Abu Shehab M, Khosravi J, Han VK, Shilton BH, Gupta MB. Site-specific IGFBP-1 hyper-phosphorylation in fetal growth restriction: clinical and functional relevance. J. Proteome Res. 2010;9:1873–1881. doi: 10.1021/pr900987n. [DOI] [PubMed] [Google Scholar]
- Ankrapp DP, Jones JI, Clemmons DR. Characterization of insulin-like growth factor binding protein-1 kinases from human hepatoma cells. J. Cell. Biochem. 1996;60:387–399. doi: 10.1002/(sici)1097-4644(19960301)60:3<387::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Barker DJ. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Brehmer D, Godl K, Zech B, Wissing J, Daub H. Proteome-wide identification of cellular targets affected by bisindolylmaleimide-type protein kinase C inhibitors. Mol. Cell. Proteomics. 2004;3:490–500. doi: 10.1074/mcp.M300139-MCP200. [DOI] [PubMed] [Google Scholar]
- Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol. 1988;158:120–126. doi: 10.1016/0002-9378(88)90792-2. [DOI] [PubMed] [Google Scholar]
- Cetin I, Marconi AM, Corbetta C, Lanfranchi A, Baggiani AM, Battaglia FC, Pardi G. Fetal amino acids in normal pregnancies and in pregnancies complicated by intrauterine growth retardation. Early Hum Dev. 1992;29:183–186. doi: 10.1016/0378-3782(92)90136-5. [DOI] [PubMed] [Google Scholar]
- Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol. 1990;162:253–261. doi: 10.1016/0002-9378(90)90860-a. [DOI] [PubMed] [Google Scholar]
- Chard T. Insulin-like growth factors and their binding proteins in normal and abnormal fetal growth. Growth Regul. 1994;4:91–100. [PubMed] [Google Scholar]
- Coverley JA, Baxter RC. Phosphorylation of insulin-like growth factor binding proteins. Mol. Cell. Endocrinol. 1997;128:1–5. doi: 10.1016/s0303-7207(97)04032-x. [DOI] [PubMed] [Google Scholar]
- D’Apolito M, Du X, Pisanelli D, Pettoello-Mantovani M, Campanozzi A, Giacco F, Maffione AB, Colia AL, Brownlee M, Giardino I. Urea-induced ROS cause endothelial dysfunction in chronic renal failure. Atherosclerosis. 2015;239:393–400. doi: 10.1016/j.atherosclerosis.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Lippo BR, Batista TM, de Rezende LF, Cappelli AP, Camargo RL, Branco RC, Barbosa Sampaio HC, Protzek AO, Wanderley MI, Arantes VC, Corat MA, Carneiro EM, Udrisar DP, Wanderley AG, Ferreira F. Low-protein diet disrupts the crosstalk between the PKA and PKC signaling pathways in isolated pancreatic islets. J. Nutr. Biochem. 2015;26(5):556–62. doi: 10.1016/j.jnutbio.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell. Signal. 2005;17:1343–1351. doi: 10.1016/j.cellsig.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Dolcini L, Sala A, Campagnoli M, Labo S, Valli M, Visai L, Minchiotti L, Monaco HL, Galliano M. Identification of the amniotic fluid insulin-like growth factor binding protein-1 phosphorylation sites and propensity to proteolysis of the isoforms. Febs j. 2009;276:6033–6046. doi: 10.1111/j.1742-4658.2009.07318.x. [DOI] [PubMed] [Google Scholar]
- Economides DL, Nicolaides KH, Gahl WA, Bernadini I, Evans MI. Plasma amino acids in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;161:1219–1227. doi: 10.1016/0002-9378(89)90670-4. [DOI] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Stimulation of insulin-like growth factor binding protein-1 synthesis by interleukin-1beta: requirement of the mitogen-activated protein kinase pathway. Endocrinology. 2000;141:3156–3164. doi: 10.1210/endo.141.9.7641. [DOI] [PubMed] [Google Scholar]
- Frost RA, Tseng L. Insulin-like growth factor-binding protein-1 is phosphorylated by cultured human endometrial stromal cells and multiple protein kinases in vitro. J. Biol. Chem. 1991;266:18082–18088. [PubMed] [Google Scholar]
- Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Pinal CS. Regulation of fetal growth by the somatotrophic axis. J. Nutr. 2003;133:1741S–1746S. doi: 10.1093/jn/133.5.1741S. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–80. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gupta MB. The role and regulation of IGFBP-1 phosphorylation in fetal growth restriction. J. Cell Commun Signal. 2015;9:111–123. doi: 10.1007/s12079-015-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han VK, Matsell DG, Delhanty PJ, Hill DJ, Shimasaki S, Nygard K. IGF-binding protein mRNAs in the human fetus: tissue and cellular distribution of developmental expression. Horm. Res. 1996;45:160–166. doi: 10.1159/000184780. [DOI] [PubMed] [Google Scholar]
- Hanif IM, Shazib MA, Ahmad KA, Pervaiz S. Casein Kinase II: an attractive target for anti-cancer drug design. Int J Biochem Cell Biol. 2010;42:1602–1605. doi: 10.1016/j.biocel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Hart SN, Li Y, Nakamoto K, Subileau EA, Steen D, Zhong XB. A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab. Dispos. 2010;38:988–994. doi: 10.1124/dmd.109.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst SM, Shao Q, Lopez M, Raucher D, Vig PJ. The design and delivery of a PKA inhibitory polypeptide to treat SCA1. J. Neurochem. 2014;131:101–114. doi: 10.1111/jnc.12782. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ylvén K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- Jia X, Cong B, Zhang J, Li H, Liu W, Chang H, Dong M, Ma C. CCK8 negatively regulates the TLR9-induced activation of human peripheral blood pDCs by targeting TRAF6 signaling. Eur.J.Immunol. 2014;2:489–499. doi: 10.1002/eji.201343725. [DOI] [PubMed] [Google Scholar]
- Jones JI, D'Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc. Natl. Acad. Sci. U. S. A. 1991;88:7481–7485. doi: 10.1073/pnas.88.17.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: A summary of epidemiological evidence. Novartis Found Symp.; 2004. pp. 247–60. [PubMed] [Google Scholar]
- Kelly JH, Darlington GJ. Modulation of the liver specific phenotype in the human hepatoblastoma line Hep G2. In Vitro Cell. Dev. Biol. 1989;25:217–222. doi: 10.1007/BF02626182. [DOI] [PubMed] [Google Scholar]
- Lee MY, Jo SD, Lee JH, Han HJ. L-leucine increases [3H]-thymidine incorporation in chicken hepatocytes: involvement of the PKC, PI3K/Akt, ERK1/2, and mTOR signaling pathways. J. Cell. Biochem. 2008;105:1410–1419. doi: 10.1002/jcb.21959. [DOI] [PubMed] [Google Scholar]
- Lee PD, Abdel-Maguid LS, Snuggs MB. Role of protein kinase-C in regulation of insulin-like growth factor-binding protein-1 production by HepG2 cells. J. Clin. Endocrinol. Metab. 1992;75:459–464. doi: 10.1210/jcem.75.2.1379255. [DOI] [PubMed] [Google Scholar]
- Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am. J. Physiol. 1999;277:C580–8. doi: 10.1152/ajpcell.1999.277.3.C580. [DOI] [PubMed] [Google Scholar]
- Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RDH, Sibley CP. Amino acid (System A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Malkani N, Jansson T, Gupta MB. IGFBP-1 hyperphosphorylation in response to leucine deprivation is mediated by the AAR pathway. Mol. Cell. Endocrinol. 2015;412:182–95. doi: 10.1016/j.mce.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M, Matsunaga T, Harada E, Ohmori S. Comparison of basal gene expression and induction of CYP3As in HepG2 and human fetal liver cells. Biol. Pharm. Bull. 2007;30:2091–2097. doi: 10.1248/bpb.30.2091. [DOI] [PubMed] [Google Scholar]
- Meier M, Menne J, Park J-K, Haller H. Nailing down PKC isoform specificity in diabetic nephropathy—two's company, three's a crowd. Nephrology Dialysis Transplantation. 2007;22:2421–2425. doi: 10.1093/ndt/gfm320. [DOI] [PubMed] [Google Scholar]
- Milanski M, Arantes VC, Ferreira F, de Barros Reis MA, Carneiro EM, Boschero AC, Collares-Buzato CB, Latorraca MQ. Low-protein diets reduce PKAalpha expression in islets from pregnant rats. J. Nutr. 2005;135:1873–1878. doi: 10.1093/jn/135.8.1873. [DOI] [PubMed] [Google Scholar]
- Montenarh M. Cellular regulators of protein kinase CK2. Cell Tissue Res. 2010;342:139–146. doi: 10.1007/s00441-010-1068-3. [DOI] [PubMed] [Google Scholar]
- Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27:141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nissum M, Abu Shehab M, Sukop U, Khosravi JM, Wildgruber R, Eckerskorn C, Han VK, Gupta MB. Functional and complementary phosphorylation state attributes of human insulin-like growth factor-binding protein-1 (IGFBP-1) isoforms resolved by free flow electrophoresis. Mol. Cell. Proteomics. 2009;8:1424–1435. doi: 10.1074/mcp.M800571-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res. 1998;44:233–238. doi: 10.1203/00006450-199808000-00016. [DOI] [PubMed] [Google Scholar]
- O'Brien LJ, Levac KD, Nagy LE. Moderate dietary protein and energy restriction modulate cAMP-dependent protein kinase activity in rat liver. J. Nutr. 1998;128:927–933. doi: 10.1093/jn/128.6.927. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, Hartil K, Petry CJ, Buckley AJ, Mosthaf-Seedorf L. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J. Endocrinol. 2003;177:235–241. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- Pal R, Mamidi MK, Das AK, Gupta PK, Bhonde R. A simple and economical route to generate functional hepatocyte-like cells from hESCs and their application in evaluating alcohol induced liver damage. J. Cell. Biochem. 2012;113:19–30. doi: 10.1002/jcb.23391. [DOI] [PubMed] [Google Scholar]
- Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin. Obstet. Gynecol. 2006;49:257–269. doi: 10.1097/00003081-200606000-00008. [DOI] [PubMed] [Google Scholar]
- Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z, Sell C, Lammers R, Ullrich A, Baserga R. Roles of insulinlike growth factor 1 (IGF-1) and the IGF-1 receptor in epidermal growth factor-stimulated growth of 3T3 cells. Mol. Cell. Biol. 1992;12:3883–3889. doi: 10.1128/mcb.12.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Umbilical uptakes and transplacental concentration ratios of amino acids in severe fetal growth restriction. Pediatr Res. 2013;73(5):602–611. doi: 10.1038/pr.2013.30. doi: 10.1038/pr.2013.30; 10.1038/pr.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypka M, Cervenkova K, Uherkova L, Poczatkova H, Bogdanova K, Vesely J. Changes in mRNA levels of intracellular fatty acid metabolism regulators in human hepatoma HepG2 cells following their treatment with non-esterified fatty acids and dehydroepiandrosterone. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2005;149:251–256. [PubMed] [Google Scholar]
- Sanchez-Casalongue ME, Lee J, Diamond A, Shuldiner S, Moir RD, Willis IM. Differential phosphorylation of a regulatory subunit of protein kinase CK2 by target of rapamycin complex 1 signaling and the cdc-like kinase Kns1. J Biol Chem. 2015;290(11):7221–7233. doi: 10.1074/jbc.M114.626523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seferovic MD, Ali R, Kamei H, Liu S, Khosravi JM, Nazarian S, Han VK, Duan C, Gupta MB. Hypoxia and leucine deprivation induce human insulin-like growth factor binding protein-1 hyperphosphorylation and increase its biological activity. Endocrinology. 2009;150:220–231. doi: 10.1210/en.2008-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin WS. Isoform specificity of protein kinase Cs in synaptic plasticity. Learn Mem. 2007;14:236–46. doi: 10.1101/lm.469707. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen LL, Nagy LE. Very low protein diets induce a rapid decrease in hepatic cAMP-dependent protein kinase followed by a lower increase in adenylyl cyclase activity in rats. J. Nutr. 1996;126:1799–1807. doi: 10.1093/jn/126.7.1799. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Langdown ML. Possible involvement of PKC isoforms in signalling placental apoptosis in intrauterine growth retardation. Mol. Cell. Endocrinol. 2001;185:119–126. doi: 10.1016/s0303-7207(01)00630-x. [DOI] [PubMed] [Google Scholar]
- Suwanichkul A, DePaolis LA, Lee PD, Powell DR. Identification of a promoter element which participates in cAMP-stimulated expression of human insulin-like growth factor-binding protein-1. J. Biol. Chem. 1993;268:9730–9736. [PubMed] [Google Scholar]
- Tripodi F, Cirulli C, Reghellin V, Brambilla L, Marin O, Coccetti P. Nutritional modulation of CK2 in saccharomyces cerevisiae: Regulating the activity of a constitutive enzyme. Mol Cell Biochem. 2011;356(1-2):269–275. doi: 10.1007/s11010-011-0958-3. [DOI] [PubMed] [Google Scholar]
- Vary TC, Goodman S, Kilpatrick LE, Lynch CJ. Nutrient regulation of PKCepsilon is mediated by leucine, not insulin, in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005;289:E684–94. doi: 10.1152/ajpendo.00613.2004. [DOI] [PubMed] [Google Scholar]
- Vilk G, Saulnier RB, St Pierre R, Litchfield DW. Inducible expression of protein kinase CK2 in mammalian cells. Evidence for functional specialization of CK2 isoforms. J. Biol. Chem. 1999;274:14406–14414. doi: 10.1074/jbc.274.20.14406. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, White A. Purification and characterization of the insulin-like growth factor-binding protein-1 phosphoform found in normal plasma. Endocrinology. 1997;138:1130–1136. doi: 10.1210/endo.138.3.5020. [DOI] [PubMed] [Google Scholar]
- Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line HepG2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.