Abstract
Background
Pulmonary hypertension (PH) is associated with increased morbidity across the cardiopulmonary disease spectrum. Based largely on expert consensus opinion, PH is defined by a mean pulmonary artery pressure (mPAP) ≥25 mmHg. Although mPAP levels below this threshold are common among populations at risk for PH, the relevance of mPAP <25 mmHg to clinical outcome is unknown.
Methods and Results
We analyzed retrospectively all US veterans undergoing right heart catheterization (RHC)(2007–2012) in the Veterans Affairs health care system (N=21,727; 908 day median follow-up). Cox proportional hazards models were used to evaluate the association between mPAP and outcomes of all-cause mortality and hospitalization, adjusted for clinical covariates. When treating mPAP as a continuous variable, the mortality hazard increased beginning at 19 mmHg (HR=1.183, 95% CI [1.004–1.393]) relative to 10 mmHg. Therefore, patients were stratified into three groups: referent (≤18 mmHg; N=4,207), borderline PH (19–24 mmHg; N=5,030), and PH (≥25 mmHg; N=12,490). The adjusted mortality hazard was increased for borderline PH (HR=1.23, 95% CI [1.12–1.36], P<0.0001) and PH (HR=2.16, 95% CI [1.96–2.38], P<0.0001) compared to the referent group. The adjusted hazard for hospitalization was also increased in borderline PH (HR=1.07, 95% CI [1.01–1.12], P=0.0149) and PH (HR=1.15, 95% CI [1.09–1.22], P<0.0001). The borderline PH cohort remained at increased risk for mortality after excluding the following high-risk subgroups: patients with pulmonary artery wedge pressure >15 mmHg, pulmonary vascular resistance ≥3.0 Wood units, or inpatient status at the time of RHC.
Conclusions
These data illustrate a continuum of risk according to mPAP level, and that borderline PH is associated with increased mortality and hospitalization. Future investigations are needed to test the generalizability of our findings to other populations and study the effect of treatment on outcome in borderline PH.
Keywords: pulmonary heart disease, outcomes research
INTRODUCTION
Elevated mean pulmonary artery pressure (mPAP) is the principal criterion to diagnose pulmonary hypertension (PH), which is highly prevalent in a wide variety of diseases encountered commonly in clinical practice including primary lung, cardiovascular, rheumatologic, hematologic, and sleep disorders.1,2 The traditional definition of PH stipulates that mPAP ≥25 mmHg measured by right heart catheterization (RHC) supine at rest is required for disease diagnosis.3 However, this threshold value is extrapolated largely from early era data acquired from normal subjects, and expert opinion published originally four decades ago.4 While numerous reports affirm that mPAP ≥25 mmHg independently predicts adverse outcome across multiple populations,5,6 the relevance of mPAP values near but below the current threshold, defined as “borderline”,7,8 to adverse clinical events is not known. Identifying elevated clinical risk in patients with mPAP <25 mmHg, in turn, has important implications for PH diagnosis and risk stratification.
Prior studies addressing the possibility that mPAP <25 mmHg is prognostic have been inconsistent and are limited. For example, studies in which PA systolic pressure (PASP) was measured non-invasively indicate that PASP 30–35 mmHg, which is near but below a range consistent with PH, is common and associated with increased clinical events.9–12 However, echocardiography-based measurement of PA pressure is relatively imprecise at discriminating lower values.13 Furthermore, studies performed in small patient cohorts at risk for PH demonstrate that mPAP ≥21 mmHg predicts impaired exercise tolerance,14 again suggesting that mPAP levels below the current criterion for PH may be meaningful, though these studies were not powered sufficiently to assess hard clinical endpoints. Hence, while these observations identify the borderline PH population as potentially sizeable and likely vulnerable clinically, definitive data regarding the association of borderline PH as assessed by RHC with outcomes is lacking.
In order to address this, we used the Veterans Affairs Clinical Assessment, Reporting, and Tracking (CART) program, which links cardiopulmonary hemodynamic and outcome data from all 76 VA catheterization centers nationally,15 to determine if there is an association between borderline PH and mortality or hospitalization among a national cohort of patients undergoing RHC.
METHODS
Data sources
Data for this analysis were from the VA CART program, which is a national clinical quality program for all VA cardiac catheterization laboratories, as well as from VA administrative and fee-basis data sources to capture cardiac catheterization procedures performed at non-VA medical centers. The CART program uses a software application embedded in the VA electronic health record (EHR) for documentation of all cardiac catheterization procedures. Key patient characteristics and procedural data were collected from procedures conducted in the 76 VA cardiac catheterization laboratories nationwide and linked to the VA EHR to allow tracking of longitudinal outcomes. Regularly scheduled quality checks of the CART data are performed to ensure completeness and accuracy. Additional details on CART and the validity and timeliness of CART data have been described previously.15
Study Population
We evaluated all veterans with procedural data recorded in CART who underwent RHC as an outpatient or inpatient in the VA system between October 1, 2007 and September 30, 2012. In patients undergoing multiple RHCs, the first RHC was considered the index procedure and was the only one included in the analysis. A minimum of one year of follow-up data for outcomes was available for all subjects included in the cohort. The Colorado Multiple Institutional Review Board approved this study.
The RHC volume for participating centers grouped by U.S. Census boundaries is provided in Supplemental Table 1. Patients were included in the analyses if data from a complete RHC were available, defined as recorded values for mPAP, pulmonary artery wedge pressure (PAWP), cardiac output (CO) measured by either assumed Fick and/or thermodilution methods, and height and weight for calculation of body surface area (BSA). For subjects with CO measured by both assumed Fick and thermodilution methods, values acquired by thermodilution were used. BSA was calculated by using the Du Bois formula (BSA = weight [kg].425 × height [cm].725).
We identified 21,818 index RHC records that were complete and available for analysis. These variables were checked for physiologically implausible values,16,17 defined as: CO <0.5 or >15 L/min; mPAP <5 or >80 mmHg; or PAWP <0 or >60 mmHg. As a result, 0.8% (N=179) of subjects had a change either by assigning a corrected value based on ancillary data (i.e. values that could be confirmed in the medical record or could be calculated from other variables), or assigning a value of missing where correct value(s) could not be identified. A total of 91 subjects (0.4%) were excluded either due to a missing value in at least one of the key hemodynamic variables or because internal consistency within patients hemodynamic profile could not be validated by two cardiologists with expertise in hemodynamic analysis, resulting in a final cohort of 21,727 subjects. Of subjects in the cohort, 0.5% (N=109) had a value corrected as according to the methods described above.
Covariates
Clinical characteristics abstracted included age, sex, race (Black or African American, White, or Other which includes American Indian, Alaskan Native, Asian, Native Hawaiian, or other Pacific Islander), weight, height, body mass index, history of systemic hypertension, left heart failure (i.e., heart failure due to left ventricular dysfunction), congestive heart failure (i.e., heart failure that may include right ventricular or biventricular dysfunction), diabetes mellitus, coronary artery disease (considered present if history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, or presence of any obstructive disease on cardiac catheterization was present) chronic obstructive pulmonary disease (COPD), human immunodeficiency virus (HIV), liver cirrhosis (viral and non-viral hepatitis), chronic kidney disease (including patients receiving renal replacement therapy), portal hypertension, connective tissue disease, atrial fibrillation or flutter, interstitial lung disease, obstructive sleep apnea, pulmonary embolism, any valvular disease (regurgitation or stenosis), tobacco use, and sickle cell anemia. These variables represent a comprehensive group of risk factors for the development of pulmonary hypertension based on prior published reports1–3,8 and were included to decrease the probability that differences in outcome between patient groups were a consequence of patients’ PH-associated disease status rather than due to mPAP level. All clinical characteristics were captured via CART,15 except for left heart failure, pulmonary embolism, HIV, liver cirrhosis and dementia, which were obtained from VA administrative data.
We validated the values for pulmonary artery systolic pressure (PASP) by evaluating the concordance between measured right ventricular systolic pressure and PASP, and for mPAP and PVR by comparing the reported values with derived values using standard definitions. Derived mPAP was calculated as (PASP + [2*PADP])/3 where PADP is pulmonary artery diastolic pressure, and derived PVR as [(mPAP-PAWP)/CO] (Supplemental Table 2).
Exposure
The main exposure was mPAP as reported in the CART dataset. A cubic spline model (B-spline with 3 knots) was created to provide a detailed description of the relationship between mPAP and hazard of all-cause mortality using 10 mmHg as the reference value. Optimization of the spline fit (e.g. number and placement of knots) was based on the Akaike Information Criterion (AIC). From this analysis, we identified elevation in risk for the hazard for mortality beginning between 19 – 20 mmHg, which was consistent with findings from a complementary analysis characterizing the relationship between mortality and mPAP sextile. Therefore, further analyses to calculate the mPAP range associated with increased clinical risk used the following categories: referent (mPAP ≤18 mmHg), borderline PH (19–24 mmHg), and the traditional definition of PH (mPAP ≥25 mmHg).3
Outcomes
The primary outcome measure was time to all-cause mortality assessed via the VA vital status file. The file has 98.3% sensitivity and 97.6% exact agreement with the National Death Index.18 The secondary outcome measures were time to hospitalization post index-procedure and time to all-cause mortality or hospitalization.
Statistical Analyses
Differences in baseline clinical and hemodynamic characteristics between the referent, borderline PH and PH groups were analyzed using the Kruskal-Wallis test for continuous variables, or a chi-squared test for categorical variables. Kaplan-Meier event-free curves were plotted for the categorical exposure variables using death, or hospitalization and death as events. Unadjusted group comparisons for time to event outcomes were made using the log-rank test.
Time to event data for the outcome measures of all-cause mortality and all-cause hospitalization were modeled using a Cox proportional hazards model that accounts for clustering by RHC site (i.e., a frailty model19). In these analyses, for the outcome of mortality time 0 is the date of the RHC. For the outcome of (re)hospitalization, it is either the date of discharge (for inpatients) or the date of the procedure (for outpatients). Covariates in this model included those baseline characteristics listed above and PAWP >15 mm Hg, as well as the addition of cancer, psychiatric disease including dementia, stroke, and inpatient hospital status based on their association with mortality or hospitalization in patients within the age range and demographic profile of the study population. The model included age (45 y, 45–55 y, 55–65 y, 65–75 y, 75–85 y and ≥85 y) and BMI (<18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2, ≥30 kg/m2) as categorical variables. All Cox models were assessed for proportional hazards violation. In the case of mortality as an outcome, inpatient status appeared to marginally violate this assumption, so interaction terms (inpatient status * time and inpatient status *time squared) were added to the model to address this issue. Estimates of interest were generated using estimate statements in the SAS PHREG procedure.
Data preparation and analyses were conducted using SAS version 9.4 (SAS Institute, Cary North Carolina) and R version 3.3.1. P<0.05 was considered statistically significant.
RESULTS
Study Population Characteristics
A total of 21,727 individual patients met study entry criteria and were included in the analysis. The cohort was predominately male (N=20,991; 96.6%) with a median age of 64.8 yrs (IQR: 60.1 – 73.4 yrs). The median mPAP was 26 mmHg (IQR: 20–35 mmHg; Supplemental Figure 1); the source for calculating cardiac index was thermodilution and the assumed Fick equation in 64.5% (N=14,010) and 35.5% (N=7717) of patients, respectively.
A total of 12,306 (56.6%) patients underwent RHC as an outpatient. Overall, a clinical indication for RHC was available for 16,557 (76.2%) patients. Among patients undergoing RHC as an outpatient, the test indication was valvular heart disease, cardiomyopathy, and heart failure in 28.9%, 11.4%, and 9.9% of patients, respectively. By comparison, heart failure was a more common test indication for patients undergoing RHC as an inpatient (22.9%), while cardiomyopathy and valvular heart disease were indications accounting for RHC in 17.0% and 13.1% of patients, respectively.
Association of mPAP with adverse outcomes
When mPAP was modeled as a continuous variable, the adjusted hazard for mortality increased progressively over a wide mPAP range beginning at levels between 19 mmHg (HR 1.183, 95% CI [1.004–1.393]) and 20 mmHg (HR 1.255, 95% CI [1.061–1.486]) and extending through values >50 mmHg (Figure 1). Furthermore, we also observed that the impact of a 1-mmHg incremental increase in mPAP on mortality hazard was highest between 19–24 mmHg, beyond which a decline is observed (Supplemental Figure 2). This trend indicates that the relative change in adjusted mortality risk per 1-mmHg increment is higher at mPAP levels between ~19–24 mmHg as compared to changes in risk per 1-mmHg increment observed at mPAP levels indicative of moderate or severe PH.
Figure 1.
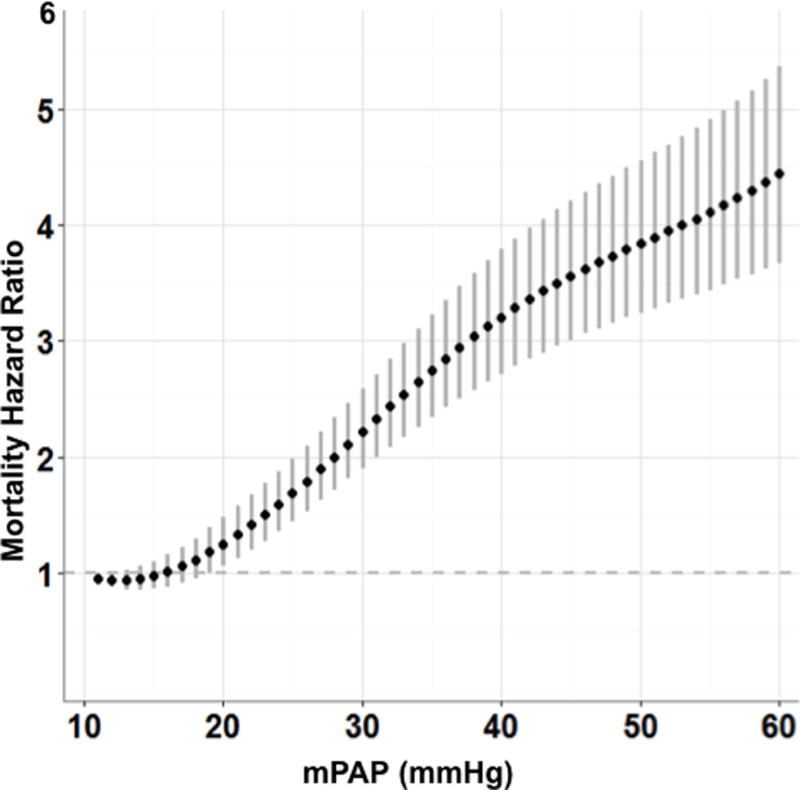
The adjusted hazard ratio for mortality according to mean pulmonary artery pressure (mPAP). The hazard ratio (95% CI) for all-cause mortality is plotted for mPAP between 11–60 mmHg relative to a reference value of 10 mmHg.
These patterns were consistent when the outcome was analyzed according to mPAP sextiles. We observed a statistically significant increase in risk of mortality relative to the referent sextile (mPAP values 5–18 mmHg) beginning with the second sextile (mPAP of 19–22 mmHg) and increasing through all subsequent sextiles (Supplemental Figures 3). The time to mortality and hospitalization-free survival according to mPAP sextile is presented in Supplemental Figure 4 and Supplemental Figure 5, respectively.
Association between borderline PH and adverse outcomes
From these analyses, 19 mmHg emerged as a lower mPAP level associated with increased risk in the study population. Thus, to determine the risk of adverse outcome associated with mPAP levels below the current definition of PH, we stratified the total cohort according to referent (mPAP ≤18 mmHg), borderline PH (19–24 mmHg), and PH (mPAP ≥25 mmHg) status. The referent group comprised 19.3% (N=4207) of the study cohort, whereas the prevalence of borderline PH (N=5030) and PH (N=12490) was 23.2% and 57.5%, respectively.
Although there were no meaningful differences between referent, borderline PH, and PH groups in age or sex, a gradient was observed for body mass index (BMI) (28.0, IQR: 24.6–31.5 vs. 29.6, IQR: 26.0–34.0 vs. 30.6, IQR: 26.3–35.8, P<0.0001) and rates of other key clinical PH risk factors including systemic hypertension (82.3% vs. 86.0% vs. 89.8%, P<0.0001), congestive heart failure (34.1% vs. 42.7% vs. 67.9%, P<0.0001), COPD (23.0% vs. 30.8% vs. 37.9%, P<0.0001), obstructive sleep apnea (7.3% vs. 10.2% vs. 14.9%, P<0.0001), and chronic kidney disease (17.6% vs. 21.2% vs. 32.6%, P<0.0001)(Table 1).
Table 1.
Clinical characteristics of patients stratified by referent, borderline pulmonary hypertension (PH), and PH status.
| Clinical Variable | mPAP (mmHg) | P Value | ||
|---|---|---|---|---|
| ≤18 (N=4207) | 19–24 (N=5030) | ≥25 (N=12490) | ||
| Age (yr) | 64.3 [59.6–73.0] | 65.2 [60.3–73.5] | 64.9 [60.2–73.4] | <0.0001 |
| Sex (M) | 96.5 (4059) | 96.6 (4861) | 96.6 (12071) | 0.8730 |
| Race | <0.0001 | |||
| White | 79.1 (3328) | 79.3 (3987) | 73.4 (9166) | |
| Black or African American | 12.0 (506) | 13.1 (659) | 19.3 (2405) | |
| Other | 8.9 (373) | 7.6 (384) | 7.3 (919) | |
| Body Mass Index (BMI) | 28.0 [24.6–31.5] | 29.6 [26.0–34.0] | 30.6 [26.3–35.8] | <0.0001 |
| BMI Categorized | <0.0001 | |||
| Normal (18.5–24.9kg/m2) | 26.2 (1103) | 18.0 (906) | 17.1 (2138) | |
| Underweight (<18.5 kg/m2) | 1.4 (57) | 1.1 (53) | 1.0 (125) | |
| Overweight (25–29.9 kg/m2) | 38.7 (1628) | 33.6 (1692) | 28.8 (3603) | |
| Obese (≥30 kg/m2) | 33.7 (1419) | 47.3 (2379) | 53.0 (6624) | |
| Systemic hypertension | 82.3 (3462) | 86.0 (4327) | 89.8 (11221) | <0.0001 |
| Left heart failure | 4.8 (201) | 6.6 (333) | 15.5 (1939) | <0.0001 |
| Congestive heart failure | 34.1 (1434) | 42.7 (2146) | 67.9 (8478) | <0.0001 |
| Diabetes | 33.4 (1405) | 40.6 (2043) | 52.6 (6568) | <0.0001 |
| Coronary Summary | <0.0001 | |||
| Normal | 21.4 (779) | 17.6 (754) | 15.9 (1477) | |
| Non-Obstructive | 33.0 (1200) | 34.5 (1480) | 30.9 (2872) | |
| 1-vessel obstructive | 16.9 (616) | 17.7 (760) | 17.7 (1645) | |
| 2-vessel obstructive | 11.8 (428) | 12.5 (535) | 13.5 (1255) | |
| 3-vessel/LMCA obstructive | 16.3 (593) | 17.2 (739) | 21.6 (2007) | |
| Other | 0.7 (25) | 0.6 (28) | 0.6 (53) | |
| COPD | 23.0 (968) | 30.8 (1548) | 37.9 (4731) | <0.0001 |
| Interstitial lung disease | 0.4 (18) | 0.7 (37) | 0.7 (93) | 0.0840 |
| Obstructive sleep apnea | 7.3 (307) | 10.2 (513) | 14.9 (1858) | <0.0001 |
| HIV | 0.5 (23) | 0.3 (16) | 0.5 (57) | 0.2390 |
| Sickle cell anemia* | 0 (1) | 0 (1) | 0.1 (8) | |
| Liver cirrhosis | 9.7 (406) | 8.1 (405) | 8.7 (1083) | 0.0242 |
| Chronic kidney disease | 17.6 (742) | 21.2 (1065) | 32.6 (4068) | <0.0001 |
| Connective tissue disease | 4.9 (205) | 4.3 (217) | 3.7 (465) | 0.0031 |
Clinical and hemodynamic data for patients undergoing right heart catheterization between 2007–2012 were accessed from the VA Clinical Assessment Reporting and Tracking Program (N=21,717) and patients were stratified into three groups according to mean pulmonary artery pressure (mPAP) level: referent (≤18 mmHg), borderline PH (19–24 mmHg), and PH (≥25 mmHg). COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; LMCA, left main coronary artery. Data are expressed as % (N) for categorical variables and median [interquartile range] for continuous variables.
A Chi-squared test was not performed in this case due to very low counts of individuals with this diagnosis.
The event-free curves for mortality and the combined end-point of mortality and hospitalization are presented in Figure 2A and Figure 2B, respectively. Patients were followed for a median of 908 days. The estimated one-year mortality rates from the Kaplan-Meier analysis were 6.7%, 8.2%, and 19.1% for the referent, borderline PH, and PH groups, respectively. At 5 years, mortality rates were 23.3%, 29.9%, and 48.0%, respectively. The estimated one-year hospitalization rates from the Kaplan-Meier analysis for the referent, borderline PH, and PH groups were 51.5%, 53.6%, and 60.9%, respectively. At 5 years, hospitalization rates were 74.9%, 78.7%, and 84.1%, respectively.
Figure 2.
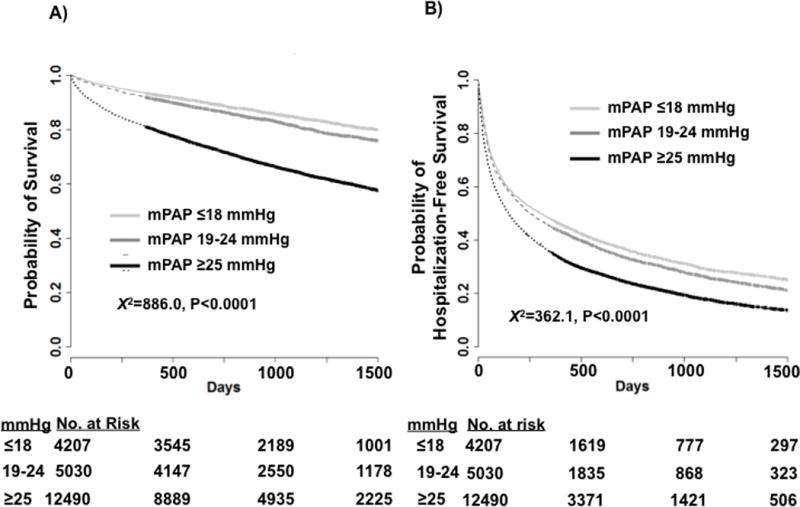
Time to event plot for unadjusted mortality and hospitalization-free survival for referent, borderline pulmonary hypertension (PH), and PH patients. The study cohort was stratified according to mean pulmonary artery pressure (mPAP) ≤18 mmHg (referent), mPAP=19–24 mmHg (borderline PH), and mPAP ≥25 mmHg (PH), and Kaplan–Meier analysis of the probability of (A) all-cause mortality (log-rank test X2=886.0, P<0.0001) and (B) combined all-cause mortality or hospitalization (log-rank test X2=362.1, P<0.0001) was performed. Censoring begins at and beyond 1 year, and is indicated by thickening of the curve.
Compared to the referent group, classically defined PH patients had the highest hazard for mortality (HR 2.16, 95% CI [1.96–2.38], P<0.0001) and hospitalization (1.15, 95% CI [1.09–1.22], P<0.0001) after adjusting for clinical variables. However, we also observed that borderline PH patients had a 23% (95% CI [12–36%], P<0.0001) increase in the adjusted hazard for mortality and a 7% (95% CI [1–12%], P=0.0149) increase in adjusted hazard for hospitalization compared to the referent group (Table 2).
Table 2.
Hazard ratio for mortality and hospitalization among patients assigned to the borderline PH and PH groups compared to the referent group.
| mPAP (mmHg) | Unadjusted Mortality | P Value | Adjusted Mortality* | P Value | Unadjusted Hospitalization | P Value | Adjusted Hospitalization* | P Value |
|---|---|---|---|---|---|---|---|---|
| ≤18 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | ||||
| 19–24 | 1.26 (1.15–1.39) | <0.0001 | 1.23 (1.12–1.36) | <0.0001 | 1.08 (1.03–1.13) | <0.0001 | 1.07 (1.01–1.12) | 0.0149 |
| ≥25 | 2.57 (2.37–2.78) | <0.0001 | 2.16 (1.96–2.38) | <0.0001 | 1.30 (1.25–1.36) | <0.0001 | 1.15 (1.09–1.22) | <0.0001 |
mPAP, mean pulmonary artery pressure; PH, pulmonary hypertension; PAWP, pulmonary artery wedge pressure.
Adjustment model included the following clinical variables: categorical age, sex, race, categorical body mass index, and history of systemic hypertension, congestive heart failure, left heart failure, diabetes mellitus, coronary artery disease, COPD, HIV, liver cirrhosis (viral and non-viral hepatitis), chronic kidney disease that included patient receiving renal replacement therapy, portal hypertension, connective tissue disease, atrial fibrillation or flutter, interstitial lung disease, pulmonary embolism, valvular disease, tobacco use, sickle cell anemia, psychiatric disease including dementia, stroke, PAWP >15 mmHg, and inpatient hospital status. For the mortality model, an additional interaction term of time * inpatient status was included to address a moderate proportional hazards violation observed for the inpatient status variable. Data are presented as hazard ratio (95% CI) relative to the referent group.
Association between adverse clinical events and borderline PH after excluding high-risk patient subgroups
In the setting of decreased cardiac output due to right heart failure, mPAP may be only mildly elevated despite severe pulmonary vascular disease.1,2 However, we observed no clinically meaningful differences in cardiac index between the referent and borderline PH groups (Table 3). By contrast, meaningful differences were observed between these groups for the number of patients with PAWP >15 ([referent] 2.5% vs. [borderline PH] 22.0% vs. [PH] 78.2%, P<0.0001) and PVR ≥3.0 Wood Units ([referent] 2.1% vs. [borderline PH] 7.7% vs. [PH] 38.2%, P<0.0001).
Table 3.
Hemodynamic characteristics of patients stratified by referent, borderline pulmonary hypertension (PH), and PH status.
| Hemodynamic Variable | mPAP (mmHg) | P Value | ||
|---|---|---|---|---|
| ≤18 (N=4207) | 19–24 (N=5030) | ≥25 (N=12490) | ||
| mPAP (mmHg) | 15 [13–17] | 22 [20–23] | 34 [29–41] | |
| mPAP (Min, Max)(mmHg) | (5–18) | (19–24) | (25–79) | |
| PASP (mmHg)* | 25 [22–28] | 33 [30–36] | 50 [42–61] | <0.0001 |
| PADP (mmHg)** | 9 [7–11] | 14 [12–15) | 23 [19–28] | <0.0001 |
| PAWP (mmHg) | 8 [6–11] | 13 [10–15) | 21 [16–26] | <0.0001 |
| PAWP >15 mmHg | 2.5 (106) | 22.0 (1108) | 78.2 (9769) | <0.0001 |
| Cardiac index (l/min/m2) | 2.5 [2.2–3.0] | 2.5 [2.1–2.9) | 2.3 [1.9–2.8] | <0.0001 |
| PVR (Wood units)§ | 1.2 [0.8–1.7] | 1.6 [1.2–2.2) | 2.5 [1.7–3.8] | <0.0001 |
| PVR >3.0 Wood units | 2.1 (88) | 7.7 (385) | 38.2 (4752) | <0.0001 |
| SVR (dyne*s*cm−5)§§ | 1295 [1060–1585] | 1286 [1040–1583) | 1306 [1023–1662] | 0.0291 |
Hemodynamic data for patients undergoing right heart catheterization between 2007–2012 were accessed from the VA Clinical Assessment Reporting and Tracking Program (N=21,717) and patients were stratified into three groups according to mean pulmonary artery pressure (mPAP) level: referent (≤18 mmHg), borderline PH (19–24 mmHg), and PH (≥25 mmHg). mPAP, mean pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PADP, pulmonary artery diastolic pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance. Data are expressed as % (N) for categorical variables and median [interquartile range] for continuous variables.
N=21,712;
N=21,700;
N=21,614;
N=15,825.
Based on these trends and prior data indicating a positive relationship between PAWP or PVR and mortality in selected PH populations,20 additional analyses were performed to determine if borderline PH patients remained at risk for adverse clinical outcome after excluding these high-risk subgroups, as well as inpatients at the time of RHC (Table 4). However, we observed an increase in the adjusted hazard for mortality in borderline PH patients with PAWP ≤15 mmHg (N=10744; HR 1.31, 95% CI [1.18–1.45], P<0.0001), PVR <3.0 Wood units (N=16389, HR 1.17, 95% CI [1.05–1.30], P=0.0036), or outpatient status at the time of RHC (N=12306; HR 1.20, 95% CI [1.05–1.38], P=0.0075) compared to patients in the referent group. Directionally similar findings were observed for adjusted hospitalization risk in borderline PH patients with PAWP ≤15 mmHg (HR 1.05, 95% CI [1.00–1.11], P=0.0761), PVR <3.0 Wood units (HR 1.07, 95% CI [1.01–1.13], P=0.0141), or outpatient status (HR 1.07, 95% CI [1.01–1.14], P=0.0327).
Table 4.
Adjusted hazard ratio for mortality and hospitalization among patients assigned to the borderline PH and PH groups compared to the referent group after excluding high-risk patient subgroups.
| Subgroup Inclusion Criteria | ||||||
|---|---|---|---|---|---|---|
| PVR <3.0 Wood units (N=16,389) | PAWP ≤15 mmHg (N=10,744) | Outpatient RHC (N=12,306) | ||||
| mPAP (mmHg) | Adjusted Mortality | P Value | Adjusted Mortality | P Value | Adjusted Morality | P Value |
| ≤18 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | |||
| 19–24 | 1.17 (1.05–1.30) | 0.0036 | 1.31 (1.18–1.45) | <0.0001 | 1.20 (1.05–1.38) | 0.0075 |
| ≥25 | 1.73 (1.54–1.95) | <0.0001 | 2.31 (2.08–2.56) | <0.0001 | 2.17 (1.90–2.49) | <0.0001 |
| mPAP (mmHg) | Adjusted Hospitalization | P Value | Adjusted Hospitalization | P Value | Adjusted Hospitalization | P Value |
| ≤18 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | |||
| 19–24 | 1.07 (1.01–1.13) | 0.0141 | 1.05 (1.00–1.11) | 0.0761 | 1.07 (1.01–1.14) | 0.0327 |
| ≥25 | 1.15 (1.07–1.23) | <0.0001 | 1.19 (1.12–1.27) | <0.0001 | 1.18 (1.10–1.27) | <0.0001 |
mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; PAWP, pulmonary artery wedge pressure; RHC, right heart catheterization. Adjusted models for the subgroup analyses includes the same covariates as in the model described in Table 2, with the exception that PAWP >15 was excluded from the PAWP subgroup analysis and inpatient status was excluded from the Outpatient subgroup analysis as these variables were the basis for subgroup selection.
DISCUSSION
This analysis of invasive hemodynamic data from a large national cohort demonstrates for the first time that mPAP levels below the current PH diagnostic threshold are independently associated with increased all-cause mortality and hospitalization. These data on clinically relevant outcomes in a large patient cohort provide evidence to support expanding hemodynamic criteria that define increased clinical risk associated with mPAP, and, therefore, have important implications for identifying at-risk patients categorized currently as normal using the present hemodynamic definition of PH.
The current study shows that mortality risk increases independently with mPAP levels within the borderline PH range, defined in this study as 19–24 mmHg, and that borderline PH is independently associated with a modest increase in hospitalization and the combined end-point of mortality and hospitalization. Importantly, this relationship was maintained after excluding inpatients at the time of RHC, or those with hemodynamic evidence of left heart disease or severe pulmonary vascular disease. These findings provide further evidence that the association between mPAP and adverse outcome was not contingent on other high-risk characteristics in borderline PH patients.
Compared to data from reports on the clinical profile of non-VA populations referred for RHC,21 the PH and borderline PH groups in our study were characterized by elevated rates of classical PH risk factors,22 including systemic hypertension, coronary artery disease, and COPD, among others. These data suggest consistency between the frequency of PH risk factors and PH-associated hazard for adverse outcome. Longitudinal studies are needed to determine rates, timing, and predictors of progression in PA pressures, as well as their implications for prognosis.
There are many potential ramifications on clinical practice of expanding the abnormal mPAP pressure range. Most importantly, our findings demonstrate that the current approach in which PH is diagnosed as present or absent by virtue of mPAP above or below 25 mmHg, respectively,1,2 is an insufficient descriptor of the continuum between mPAP level and risk of mortality or hospitalization. Along these lines, our findings suggest that risk prognostication for other conditions may improve if elevated levels of mPAP below the current diagnostic threshold for PH are taken into consideration. For a number of cardiopulmonary diseases represented in the database,5,6,23 the development of increased PA pressure is strongly associated with adverse outcomes, and it may be the case that a lower diagnostic standard for PH could improve calculation of risk for patients with these associated conditions, as well.
This study adds to a growing body of literature on the importance of borderline PH, which has emerged as a key area of investigation in the field of cardiopulmonary medicine but thus far has been evaluated mostly in single-center small cohort studies involving selected PH subgroups, such as scleroderma or sickle cell disease.7,14,24–27 While an increase in mortality and heart failure hospitalizations has been reported in large population cohort studies at PA systolic pressure below abnormal when assessed echocardiographically,9,12,28 considering the limited precision of this method for determining cardiopulmonary hemodynamics13,29 and the requirement of mPAP to diagnose PH, the clinical relevance of these findings remained unclear. Our study confirms the relationship between mPAP and outcomes in a medically complex patient cohort using the gold-standard method of diagnosis, invasive hemodynamic monitoring, in a large national sample. Thus, while these data do not demonstrate causality between borderline PH and outcome or potential pathogenetic mechanism(s) by which to account for this association, and, therefore, do not provide evidence in support of initiating treatment based on mPAP 19–24 mmHg per se, our findings provide a good rationale to prospectively investigate preventive and therapeutic strategies for improving on elevated adverse clinical event rates in borderline PH patients.
Certain limitations merit consideration when interpreting our findings. First, this is a retrospective study involving a referral population of U.S. veteran patients, who are characterized by elevated rates of cardiopulmonary diseases and hospital admissions compared to the general population.30 In this study, data were acquired from RHCs performed based on clinician preference and clinical indication, and we were unable to include truly normal patients owing to ethical concerns that prohibit invasive testing in the absence of a clinical indication. Based on these reasons, the distribution of mPAP values in this cohort is unlikely to be representative of a purely general population and, as a result, the effect size for borderline PH or PH in this study could be overestimated compared to non-Veteran patients or individuals with similar cardiopulmonary hemodynamics without an evident indication for RHC. Thus, the generalizability of our data to community-based practice requires further analysis in unselected populations that are younger, female, outside the VA system, asymptomatic or controlled for disease severity, and comprised of PH subtypes that may have been under-represented in the current study cohort.31 Second, while borderline PH in this study was defined as mPAP 19–24 mmHg based on our analysis suggesting an increase in risk for adverse outcomes beginning at mPAP levels between 19 and 20 mmHg, alternative lower limits or referent mPAP values could have been selected. Finally, practical limitations prevented standardization of RHC technique32 and hemodynamic acquisition software systems at participating centers, although the overall size of the cohort would seem to compensate for this and some other patient selection issues in this study.
In summary, analysis of the VA-CART national hemodynamic database demonstrates that borderline PH, defined as mPAP 19–24 mmHg, is a common and independent risk factor for adverse clinical outcomes in a large cohort of patients with underlying cardiopulmonary disease, particularly left heart dysfunction or parenchymal lung disease, who are referred for invasive hemodynamic testing. Overall, these data illustrate the continuum of PH risk on mortality and hospitalization, and support future prospective studies that investigate the significance of borderline PH in other patient populations, as well as the consequences of treatment on clinical end-points in this cohort of at-risk patients.
Supplementary Material
Clinical Perspectives.
Elevated pulmonary artery pressure is an established risk factor for adverse clinical outcome across the gamut of cardiopulmonary disease, as well as numerous other conditions. The current definition of pulmonary hypertension requires a mean pulmonary artery pressure (mPAP) ≥25 mmHg measured during right heart catheterization. However, this diagnostic threshold is based largely on expert consensus opinion, while definitive data establishing the spectrum of clinical risk associated with mPAP are lacking. In the current report, invasive hemodynamic, clinical, and outcome data from 76 cardiac catheterization centers nationally in the Veterans Affairs healthcare system were assembled for 21,727 patients, resulting in the largest database to date analyzing the relationship between mPAP and hard clinical end-points. Our findings demonstrate a progressive increase in risk for mortality and hospitalization for mPAP that is observed beginning at approximately 19 mmHg. We demonstrate that patients with mPAP of 19–24 mmHg, a subgroup classified currently as normal, are at increased risk of hospitalization and/or mortality. This effect was observed after adjusting for clinical variables or excluding patients with hemodynamic evidence of left heart failure. These data support expanding the cardiopulmonary hemodynamic range associated with increased clinical risk, and suggest that mPAP ≥19 mmHg can be used for risk-stratification of patients with underlying cardiopulmonary disease. Furthermore, these data lay the framework for future prospective investigations examining pulmonary hypertension diagnostic criteria, treatment timing, and prevention in at-risk patients to improve on elevated clinical event rates in patients with pulmonary vascular disease.
Acknowledgments
The authors wish to acknowledge Dr. Evan Brittain for his expert review of this manuscript, and Ms. Stephanie Tribuna for her technical assistance in preparing this manuscript.
Funding Sources: B.A.M.: (NIH) 1K08HL11207-01A1, American Heart Association (AHA 15GRNT25080016), Pulmonary Hypertension Association, Center for Integration of Medicine & Innovative Technology (CIMIT) foundation, Cardiovascular Medical Research and Education Fund (CMREF), and Klarman Foundation at Brigham and Women’s Hospital, Gilead Young Scholars Foundation (Gilead Sciences, Inc.); T.M.M.: VA Health Services Research and Development Career Development Award (CDA 08-021); A.R.O: Dunlevie Family Fund; T. L.: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Biomedical Laboratory Research and Development Service (MERIT Review Award, 1I01BX002042-01A2), and Catherine and Lowe Berger and Pauline L. Ford Scholarship in Pulmonary Medicine, Department of Medicine, Indiana University; R.G.H.: VA Merit Award from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Clinical Science Research and Development Service; J.A.L: (NIH/NHLBI) 1U01HL125215-01; R.T.Z.: Junior Faculty Endowment by Vera Moulton Wall Center for Pulmonary Vascular Disease and NIH/NHLBI/NIAID (PO1 HL108797, UO1 HL107393, R24 HL123767, N01 HV00242, ASCO1); G.C.: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Biomedical Laboratory Research and Development Service (MERIT Review Award, IBX000711A) and support from the Department of Medicine, Alpert Medical School.
Footnotes
Journal Subject Terms: Pulmonary Hypertension; Heart Failure
Disclosures: B.A.M reports investigator initiated research supported by Gilead Sciences Inc. A.R.O. reports investigator initiated Research supported by Actelion Pharmaceuticals. RTZ reports investigator initiated research support from United Therapeutics and is a consultant for Therametrics, Bayer, Gilead, and United Therapeutics; and has stock options in Seltan Pharma Inc. J.M.E. reports research support from Actelion, Novartis, Arena, AIRES, Bayer, Gilead, Reata, United Therapeutics, Bellerphon, GeNo, Lung LLC, and serves on the consultant or advisory board of Gilead, Bayer, Actelion, United Therapeutics, Lung LLC. GC reports investigator initiated research supported by Novartis.
All other authors report no relevant conflicts of interest. The views expressed are those of the authors alone and do not represent the views of the Veterans Affairs or other Federal government agencies.
References
- 1.Shah SJ. Pulmonary hypertension. JAMA. 2012;308:1366–74. doi: 10.1001/jama.2012.12347. [DOI] [PubMed] [Google Scholar]
- 2.Rich JD, Rich S. Clinical diagnosis of pulmonary hypertension. Circulation. 2014;130:1820–30. doi: 10.1161/CIRCULATIONAHA.114.006971. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Cahciery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peackock A, Vonk Noordegraf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Kleptko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Eur Respir J. 2015;46:1855–6. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 4.Hatano S, Strasser T. World Health Organization. 1975 [Google Scholar]
- 5.Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail. 2011;4:644–50. doi: 10.1161/CIRCHEARTFAILURE.110.960864. [DOI] [PubMed] [Google Scholar]
- 6.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 7.Hoeper MM. The new definition of pulmonary hypertension. Eur Respir J. 2009;34:790–1. doi: 10.1183/09031936.00056809. [DOI] [PubMed] [Google Scholar]
- 8.Heresi GA, Minai OA, Tonelli AR, Hammel JP, Farha S, Parambil JG, Dweik RA. Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ. 2013;3:916–925. doi: 10.1086/674756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam CSP, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–70. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maron BA, Choudhary G, Kahn UA, Jankowich MD, McChesney H, Ferrazzani SJ, Gaddam S, Sharma S, Opotwosky AR, Bhatt DL, Rocco TP, Aragam JR. The clinical profile and under-diagnosis of pulmonary hypertension in U.S. Veterans. Circ Heart Fail. 2013;6:906–12. doi: 10.1161/CIRCHEARTFAILURE.112.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kingrey JF, Panos RJ, Ying J, Meganathan K, Vandivier R, Elwing JM. Provider recognition and response to echocardiographic findings indicating pulmonary hypertension in the Veterans affairs medical center population. Pulm Circ. 2013;3:389–95. doi: 10.4103/2045-8932.113184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhary G, Jankowich M, Wu WC. Elevated pulmonary artery systolic pressure predicts heart failure admissions in African Americans: Jackson Heart Study. Circ Heart Fail. 2014;7:558–64. doi: 10.1161/CIRCHEARTFAILURE.114.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farber WH, Foreman AJ, Miller DP, McGoon MD. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56–64. doi: 10.1111/j.1751-7133.2010.00202.x. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs G, Avian A, Tscherner M, Foris V, Bachmaier G, Olschewski A, Olschewski H. Characterization of patients with borderline pulmonary artery pressure. Chest. 2014;146:1486–93. doi: 10.1378/chest.14-0194. [DOI] [PubMed] [Google Scholar]
- 15.Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, Gillespie B, Box T, Fihn SD, Jesse RL, Rumsfeld JS. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program) Am J Cardiol. 2014;114:1750–1757. doi: 10.1016/j.amjcard.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 16.Anderson CB, Codd JR, Graff RA, Groce MA, Harter HR, Newton WT. Cardiac failure and upper extremity arteriovenous dialysis fistulas. Case reports and review of the literature. Arch Intern Med. 1976;136:292–7. [PubMed] [Google Scholar]
- 17.Barst RJ, Rubin LJ, Long WA, Lewy PS. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Eng J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 18.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer-Verlag; 2000. [Google Scholar]
- 20.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failue. JACC Heart Fail. 2013;1:290–9. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Goonewardena SN, Blair JEA, Manuchehry A, Brennan JM, Keller M, Reeves R, Price A, Spencer KT, Puthumana J, Gehorghiade A. Use of hand carried ultrasound, B-type natriuretic peptide, and clinical assessment in identifying abnormal left ventricular filling pressures in patients referred for right heart catheterization. J Cardiac Fail. 2010;16:69–75. doi: 10.1016/j.cardfail.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 23.Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB, Systrom DM. A central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: implications for heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:278–85. doi: 10.1161/CIRCHEARTFAILURE.114.001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonneau G, Robins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langelben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs G, Berghold A, Scheidl S, Olshewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Resp J. 2009;34:888–94. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 27.Heresi GA, Minai OA, Tonelli AR, Hammel JP, Farha S, Parambil JG, Dweik RA. Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ. 2013;3:916–25. doi: 10.1086/674756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhary G, Jankowich M, Wu WC. Prevalence and clinical characteristics associated with pulmonary hypertension in African-Americans. PLoS One. 2013;8:e84264. doi: 10.1371/journal.pone.0084264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich JD. Counterpoint: can Doppler echocardiography estimates of pulmonary artery systolic pressure be relied upon to accurately make the diagnosis of pulmonary hypertension? No. Chest. 2013;143:1536–9. doi: 10.1378/chest.13-0297. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Ravelo A, Wagner TH, Phibbs CS, Bhandari A, Chen S, Barnett PG. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3 Suppl):146S–167S. doi: 10.1177/1077558703257000. [DOI] [PubMed] [Google Scholar]
- 31.Davis KK, Lilienfeld DE, Doyle RL. Increased mortality in African Americans with idiopathic pulmonary arterial hypertension. J Natl Med Assoc. 2008;100:69–72. doi: 10.1016/s0027-9684(15)31177-9. [DOI] [PubMed] [Google Scholar]
- 32.Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012;163:589–94. doi: 10.1016/j.ahj.2012.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


