Abstract
Background and Purpose
Previous work from the Framingham Heart Study suggests that brain changes due to arterial aging may begin in young adulthood and that such changes precede cognitive deficits. The objective of this study was to determine the association of arterial stiffness with measures of white matter and gray matter integrity in young adults.
Methods
1903 participants from the Framingham Heart Study Third Generation (mean age, 46±8.7 years) had complete tonometry measurements and brain MRI (T1-weighted and DTI). Tonometry measures included carotid-femoral pulse wave velocity (CFPWV), augmentation index, carotid-brachial pressure amplification and central pulse pressure. Fractional anisotropy (FA) and gray matter (GM) density images were computed from DTI and T1 images. Registration to a common anatomical template enabled voxel-based linear regressions relating measures of FA and GM to tonometry measures, adjusting for relevant covariables.
Results
Higher CFPWV was associated with lower regional FA, including the corpus callosum and the corona radiata (8.7 and 8.6cc respectively, p<0.001), as well as lower GM density in the thalamus region (0.9cc, p<0.001). Analyses did not reveal significant associations between other tonometry measures and FA or GM.
Conclusions
Among young healthy adults, higher aortic stiffness was associated with measures of reduced WM and GM integrity in areas implicated in cognitive decline and Alzheimer's disease. Greater aortic stiffness may result in subclinical vascular brain injury at ages much younger than previously described.
Keywords: Arterial stiffness, Pulse pressure, MRI, White matter, Diffusion tensor imaging
Introduction
With aging, the aorta stiffens and pressure pulsatility increases, inducing hemodynamic changes implicated in the development of cardiovascular diseases1, 2. Recent analyses from the Framingham Heart Study (FHS) and other cohorts suggest that, in older individuals, vascular remodeling may also play a central role in the development of structural brain injury3, 4, which have been associated with reduced cognitive ability5-7 and increased likelihood of incident dementia.8
The earliest differences in brain structure associated with increased pulsatility, however, have yet to be determined, and it is possible that differences could be identified even earlier in life, before symptomatic disease expression, should a suitably sized cohort and sensitive MRI measures be available. The FHS Third Generation (G3) is ideal to test this hypothesis as participants were recruited at an early age and were 40 years of age on average at the time of this study. Despite the relatively young age of this cohort, sensitive measures of white-matter injury from diffusion tensor imaging (DTI) identified subtle vascular brain injury in association with elevated systolic blood pressure9, suggesting that newer imaging techniques may be able to identify clinically silent structural brain differences in association with hemodynamic metrics.
Our aim was to extend our preliminary findings by exploring associations between tonometry measures and regional white matter injury, as estimated by DTI-derived fractional anisotropy, and gray matter atrophy in G3 participants. We postulated that increased arterial stiffness would be associated with lower fractional anisotropy and gray matter density, which would suggest that arterial stiffness can lead to vascular brain injury as early as the fifth decade of life.
Methods
Study sample
The design of the Framingham G3 Cohort study has been detailed previously10. Of the 3519 participants who attended Exam 2, 2034 underwent brain MRI between 2009 and 2013 and had successful arterial tonometry measures. Participants were excluded from the present analysis for the following reasons: prevalent stroke at the MRI evaluation, other neurologic disorders that might confound the assessment of brain volumes, because of bad or incomplete tonometry data or poor MRI quality, resulting in a sample of 1903 individuals. All protocols were approved by Boston University Medical Center's institutional review board, and participants provided written informed consent.
Clinical Evaluation and Definitions
Medical history, physical examination, and ECG were performed routinely at each FHS examination. Blood pressures represent the average of 2 auscultatory blood pressures obtained by the physician on seated participants at the time of each Framingham clinic examination with the use of a standardized measurement protocol.
Tonometry Data Acquisition and Analysis
Noninvasive hemodynamic data acquisition is described in the Supplement file (please see http://stroke.ahajournals.org). Mean arterial pressure (MAP) was calculated by integration of the calibrated brachial pressure waveform. Central pulse pressure (CPP) was defined as the difference between the peak and trough of the calibrated carotid pressure waveform. Augmentation index (AI) was computed from the carotid pressure waveform as described previously.11 Briefly, AI is the augmentation pressure divided by pulse pressure, expressed as a percentage. Carotid-femoral pulse wave velocity (CFPWV) values were calculated from tonometry waveforms and body surface transit distance, which were adjusted for parallel transmission in the brachiocephalic artery and aortic arch with the use of the suprasternal notch as a fiducial point.12 The carotid-femoral transit path spans the descending aorta, making CFPWV a measure of aortic stiffness.
Brain MRI analysis
We used DTI measures of fractional anisotropy (FA) which is a sensitive indicator of WM integrity13. FA was computed from DTI using FSL software tools.14 Segmentation of GM, WM and total cranial volume (TCV) were performed from T1-weighted and FLAIR images by automated procedures previously described15. FA and GM maps were finally coregistered to a minimal deformation template (MDT)16, 17 for group statistical analyses (please see http://stroke.ahajournals.org).
Statistical analyses
Voxel-based regressions of FA and GM with components of hemodynamic load
The primary goal of the statistical analysis was to determine if, at the image voxel level, individual key components of hemodynamic load (CFPWV, AI, CPP and MAP) were associated with greater brain injury as indicated by lower FA or GM atrophy adjusting for a set of CVD risk factors. To achieve this goal, we used a linear regression (Model 1) with either measures of FA or GM density as the dependent variable and each of the four components as independent variables, adjusting for a reference set of standard risk factors including age, gender, use of antihypertensive therapy, total cholesterol, current smoking status, presence of diabetes mellitus, TCV and time between clinical and MRI exams. CFPWV was inverted in order to reduce heteroscedasticity and normalize the distribution; the value was multiplied by −1000 in order to convert units to ms/m and restore directionality of effects.
In a second model (Model 2), we then tested whether relations of CFPWV, AI, CPP and MAP with FA and GM may be modulated by age, sex, and antihypertensive treatment therapy. We assessed this goal by performing regressions as described in Model 1, including additional interactions of the component of hemodynamic load with the 3 covariates, i.e. age, sex, and antihypertensive therapy.
The T-map obtained for each comparison was evaluated for statistical significance using threshold free cluster enhancement (TFCE) at the p < 0.05 level (please see http://stroke.ahajournals.org) and corrected for multiple comparison using permutation-based correction (N=1000)18. We then overlaid the thresholded T-maps with the Johns Hopkins University probabilistic fiber map atlases19 and GM atlas, warped to the MDT space, to provide a post hoc regional description of significant voxels in terms of the WM tracts and GM structures to which they most likely belonged. For each significant region, the mean FA (respectively mean GM density) was computed for each person by superimposing the mask of the corresponding region to each individual's FA (respectively GM) images. This enabled us to perform linear regressions as described above but at region level.
Modulation of hemodynamic load components effects by other components
To determine whether significant regional relations between one hemodynamic component and FA or GM were mediated in part by the other hemodynamic variables, we performed, for that component, region-based level regressions as described in Model 1, including in addition the other hemodynamic variables as covariates.
Accelerated white matter aging related to hemodynamic load components
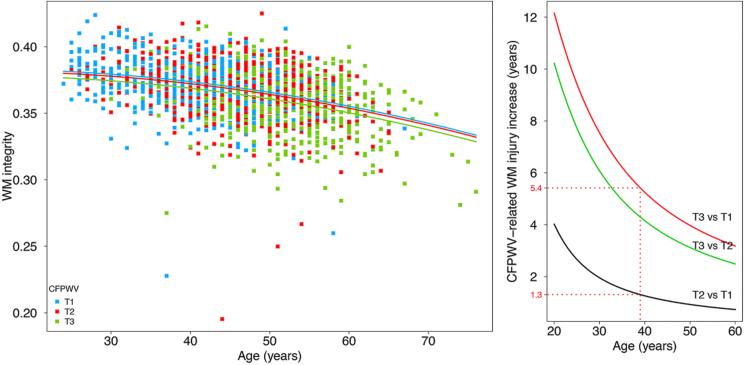
We computed the mean FA within regions identified in the voxel-based analyses as significantly associated with hemodynamic load components to be used as the dependent variable in a linear regression. The tonometry measures were categorized into 3 groups (higher, intermediate and lower tertile intervals), and used as the independent variable, adjusting for the reference set of standard risk factors. Because the mean FA measure appeared to be quadratically related to age on visual inspection (Figure 1A), we also created a model that included the quadratic effect of age.
Figure 1.
Regression curves relating white matter (WM) integrity as a function of the carotid-femoral pulse wave velocity (CFPWV) category (Lower tertile interval, T1: blue; middle tertile interval, T2: red; higher tertile interval, T3: green), and the age of the participant (A) and their difference between CFPWV categories according to age (B).
Results
Demographics
Compared with the remainder of the G3 cohort (Table 1) without MRI, individuals included in this study were on average significantly younger (p=0.02) with significantly lower SBP (p<0.001), CFPWV (p=0.008), AI (p=0.016), MAP (p=0.007) and CPP (p=0.036). In addition, they were less likely to smoke (p=0.004), to receive treatment for hypertension (p=0.007) or have diabetes (p=0.006).
Table 1.
Participants’ characteristics.
| Study sample | Sample without MRI | P value | |
|---|---|---|---|
| N | 1903 | 1485* | |
| Age, years | 46.2 (8.7), [24; 76] | 46.9 (8.8), [24; 78] | 0.020 |
| Women, n (%) | 1005 (53) | 790 (53) | 0.832 |
| Systolic blood pressure, mm Hg | 115 (13), [80; 207] | 117 (15), [84; 188] | <0.001 |
| Antihypertensive therapy, n (%) | 328 (17) | 309 (21) | 0.007 |
| Total cholesterol, mg/dL | 186 (34), [78; 564] | 188 (37), [86; 568] | 0.081 |
| Smoker, n (%) | 170 (9) | 178 (12) | 0.004 |
| Diabetes mellitus, n (%) | 77 (4) | 90 (6) | 0.006 |
| Carotid-femoral pulse wave velocity, m/s* | 7.1 (1.4), [4.3; 18.8] | 7.2 (1.5), [4.5; 17.0] | 0.008 |
| Augmentation index, % | 9.4 (13), [−33.6; 53.0] | 10.6 (13.4), [−32.1; 55.4] | 0.016 |
| Mean arterial pressure, mm Hg | 86.6 (10.8), [56; 168] | 87.6 (11.3), [60; 134] | 0.007 |
| Central pulse pressure, mm Hg | 52.6 (13.1), [19.8; 130.2] | 53.7 (14.5), [22.1; 135.5] | 0.036 |
| Time between clinical and MRI exams, years | 1.69 (0.95), [−0.68; 5.17] | ||
| Mean fractional anisotropy | 0.35 (0.02), [0.20; 0.40] | ||
| Mean gray matter density | 0.62 (0.02), [0.47; 0.69] | ||
| White matter hyperintensities volumes, cc | 0.84 (2.2), [0.003; 67.82] | ||
| White matter volume, cc | 513 (118), [303; 762] | ||
| Gray matter volume, cc | 621 (134), [441; 814] | ||
| Intra cranial volume, cc | 1445 (142), [1079; 1947] |
Data presented as mean (SD) and range for continuous variables
Among the 1485 individuals without MRI, 1376 have non-missing values for carotid-femoral pulse wave velocity, 1461 for augmentation index, 1478 for mean arterial pressure and 1461 for central pulse pressure
Associations of FA and GM with components of hemodynamic load
Voxel-based regressions of FA and GM with components of hemodynamic load
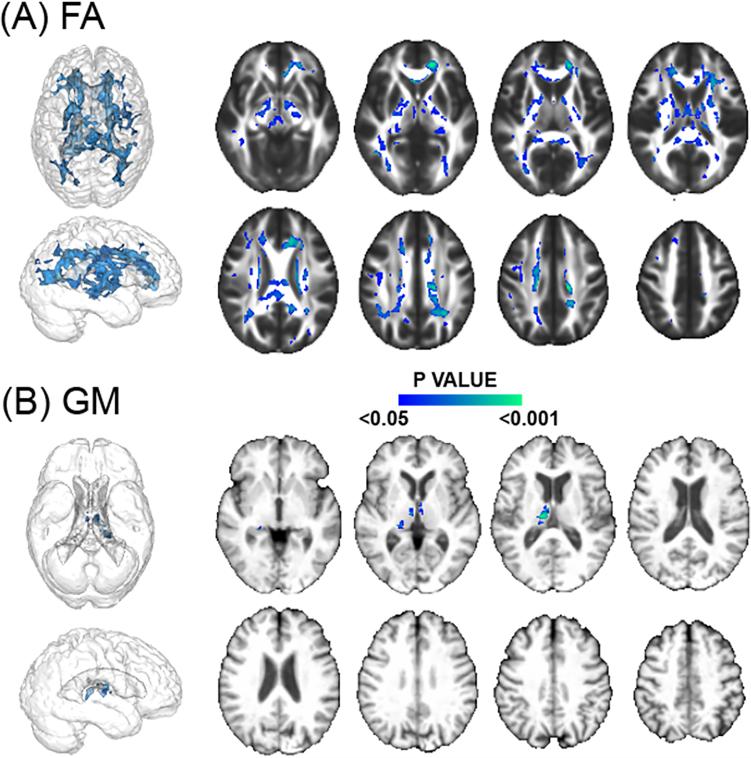
In Model 1, higher CFPWV was associated with lower FA within voxels that covered 25.5cc of the WM (Table I, please see http://stroke.ahajournals.org). WM tracts most implicated included the splenium, body and genu of corpus callosum region (2.77, 3.78 and 2.05cc respectively) and the anterior, superior and posterior part of the corona radiata (4.13, 2.55 and 1.86cc respectively, see Figure 2A).
Figure 2.
Regions of the cerebral white matter and gray matter (GM) in which lower fractional anisotropy (FA) and lower GM are significantly associated with higher carotid- femoral pulse wave velocity (CFPWV). Color scale indicates the P value.
Higher CFPWV was also associated with lower GM density but within voxels that covered only 0.93cc of the GM (Table I, please see http://stroke.ahajournals.org), implicating the thalamic region (see Figure 2B).
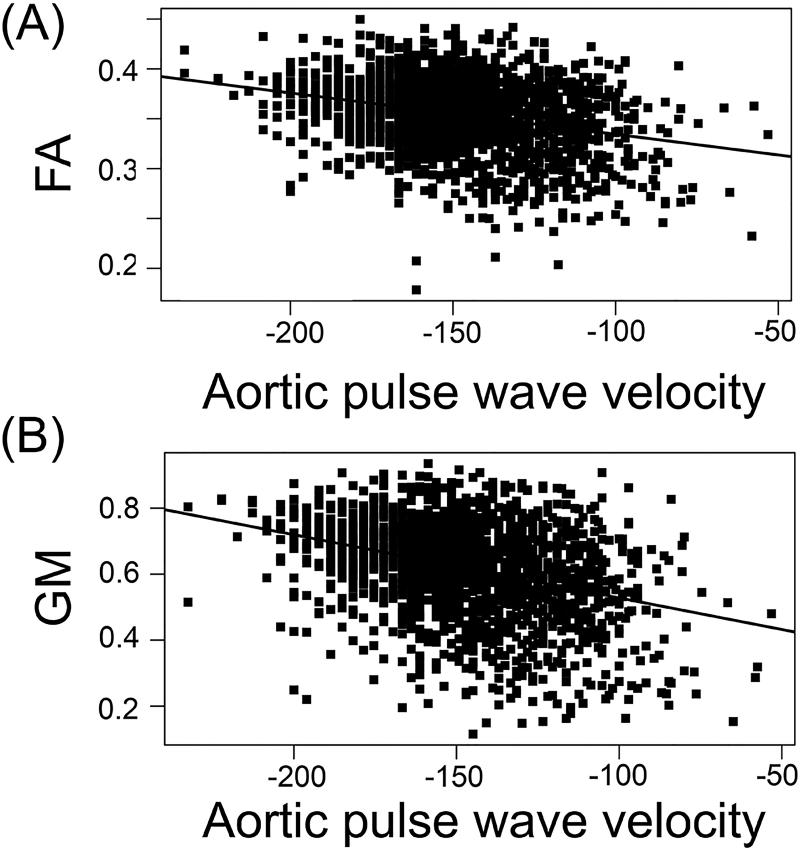
Figure 3 illustrates regression curves relating CFPWV with the mean FA and mean GM density, within the respective significant voxels for the largest identified regions.
Figure 3.
Regression curves relating carotid-femoral pulse wave velocity (CFPWV) and mean fractional anisotropy (FA) measures (A) and gray matter (GM) density (B) within the significant voxels for the respective largest identified regions (splenium of the corpus callosum and thalamus for the white and GM tissues respectively).
Voxel-based analyses did not reveal significant association between FA and GM with MAP, AI or CPP measures.
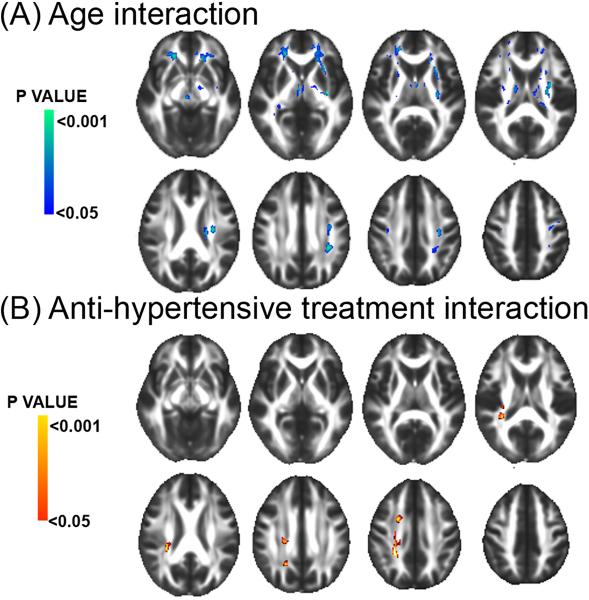
Modulation of hemodynamic load associations by sex, gender and antihypertensive treatment therapy
Model 2 revealed a significant interaction between CFPWV and age on FA (see Figure 4A) with increasing age being associated with a larger negative relation of CFPWV with FA measures within voxels that covered 9.22cc of the WM. WM tracts most affected included the anterior corona radiata (3.44cc) and the external capsule (1.19cc, Table II, please see http://stroke.ahajournals.org). The model also revealed a significant interaction of CFPWV with antihypertensive treatment therapy, indicating an attenuated relation of CFPWV with FA in individuals receiving antihypertensive treatment as compared with individuals with no treatment. This modeled interaction implicated voxels that covered 0.86cc of the WM, mostly in the posterior corona radiata region (0.53cc, see Figure 4B and Table II, please see http://stroke.ahajournals.org).
Figure 4.
Regions of the cerebral white matter in which an interaction between carotid-femoral pulse wave velocity (CFPWV) with age (A) and anti-hypertensive treatment (B) was significantly associated with fractional anisotropy (FA). Cold and warm scales indicate P value corresponding to negative and positive associations respectively.
Similar analysis of relations of CFPWV with GM density at voxel-based level failed to find any significant effects.
Finally, the lack of significant associations between FA and GM with MAP, AI and CPP was not found to be modulated by age, gender or antihypertensive treatment.
Modulation of hemodynamic load components effects by other components
Adding MAP, AI and CPP as covariates in Model 1 with CFPWV as variable of interest and regional FA and GM measures as dependent variables did not significantly change the association between CFPWV and FA and GM (Table I, please see http://stroke.ahajournals.org).
Accelerated white matter aging related to hemodynamic load components
CFPWV tertile intervals were T1=[4.3; 6.3], T2=[6.3; 7.3] and T3=[7.3; 18.8] (m/s). On average, WM integrity (i.e. mean FA) was found to be negatively associated with CFPWV tertile groups (p=0.016). Posthoc analyses, corrected for multiple comparisons using Bonferroni correction, revealed that individuals of T3 group had, on average, lower FA as compared with T2 (β=−2.6×10−3, p=0.037) and T1 (β=−3.4×10−3, p=0.013) groups. Mean FA was not found to differ between the T1 and T2 groups (p=0.88). WM integrity was found to decrease with increasing age in an accelerating fashion, as evidenced by significant linear and quadratic terms (β=−8.3×10−4, p<0.001 and β=−1.2×10−5, p=0.012 respectively, see Figure 1A), independently of CFPWV (p=0.64 and p=0.33 for linear and quadratic interactions respectively).
Figure 1B illustrates differences in WM integrity between CFPWV groups as a function of age. For example, the WM integrity of a 40 year-old individual with CFPWV in the higher range (T3) corresponded to that of a T2 group 1.3 years older, whereas this difference for T1 group was 5.4 years.
Discussion
Results from this study indicated that, among a sample of young to middle-aged adults representative of the community, CFPWV, the reference standard non-invasive measure of aortic stiffness, was associated with injury to WM and regional GM atrophy that worsened continuously with increasing CFPWV. Such associations were found to be accentuated by age and attenuated by anti-hypertensive treatment. A second finding was that, in our community-based sample, CPP, AI and MAP were not associated with measures of WM and GM integrity. These data indicate that cerebral microvascular damage and brain atrophy, associated with elevated aortic stiffness, which has been recently found to manifest in subclinical cognitive dysfunction4 in the FHS Offspring cohort (mean ± SD age: 61 ± 9 years old), is present and discernable even amongst younger individuals.
Biological mechanisms
The biological mechanisms triggered by increased arterial stiffness are complex. With aging, stiffening of the large arteries causes aortic systolic BP to increase, which contributes to loading of stiff components of the arterial wall and subsequently further increases arterial stiffness. Structural changes that increase aortic stiffness are precipitated by a progressive increase in collagen, ground substance, and calcium deposition, coupled with the degradation and fragmentation of elastin fibers20. These irregular physical forces in the arteries trigger atherogenic, hypertrophic and inflammatory responses in small vessels that may contribute to reduce microvascular reactivity. Arterial stiffness may contribute to microvascular brain injury by exposing the small vessels of the cerebral vasculature to high pressure fluctuations and flow pulsatility21. Central artery stiffness may also increase short-term variability in blood pressure, which, in the presence of impaired microvascular reactivity, may impact supply of oxygen and nutrients to the brain22. The impact of the reduction in microvascular reactivity on the cross-talk between large and small arteries may also play a role in brain injury23, 24, with the cross-talk between increased stiffness in large arteries and related microvascular dysfunction resulting in increased susceptibility to intermittent hypotension and relative ischemia, particularly in the periventricular WM watershed region25.
Impact of arterial stiffness on white matter integrity
Abnormal elevations in central and cerebral hemodynamic pulsatility promote the development of WMH and lacunar infarcts and increase the risk for a first cardiovascular disease event.1, 26 In particular, the association between central artery stiffness and WMH burden has been frequently and consistently reported among older individuals3, 4, 27. In the FHS Offspring cohort of older individuals, recent findings suggest that only CFPWV and MAP are associated with WMH burden. But recent DTI studies suggest that WMH may only represent the extreme foci of a more widespread, continuous WM injury process that progresses insidiously during aging13, 28, suggesting that subtle aortic stiffness-related brain injury may occur before evident injury events, like WMH, occur, even when the overall vascular burden is low. This hypothesis is supported by the present work using DTI in a young adult sample, wherein we found continuous associations between CFPWV and microstructural WM injury in several tracts (particularly the corpus callosum) that have been reported to be affected in elderly people with Alzheimer disease29. Our finding also extends observations from a recent tract-based spatial statistic DTI study3 that identified similar associations in a sample of 55 older individuals. Despite the fact that voxel-based and tract-based studies are two methods using a fairly different approach, the prior study and the current data both identified the corpus callosum, corona radiata and internal capsule as regions that are particularly vulnerable to increased aortic stiffness.
Impact of arterial stiffness on gray matter integrity
Our study also suggests a localized impact of CFPWV on GM integrity exclusively in the bilateral thalamic regions, covering only 0.9cc, and may be explained by the location and blood supply of this region30. At regional level, one study examined correlation between CFPWV and regional cerebral perfusion, using arterial spin labeling, within GM regions including hippocampus, thalamus and caudate nucleus in 35 middle-aged adults31 but did not find evidence of significant associations of CFPWV with any of the GM structures. While the ability of this study to detect associations may have been hindered by low statistical power, a potential hypothesis explaining the lack of associations is that the impact of arterial stiffness on GM integrity may be very slow, subtle and hardly perceptible during early and mid-adult life, but exacerbated later in the aging process, suggesting that cortical atrophy may be the end stage of advanced vascular disease. Longitudinal analysis of the effect of CFPWW on GM integrity may clarify this issue.
Implications of Hypertension Treatment
There is now substantial evidence that elevated arterial stiffness is the earliest manifestation of systolic hypertension32. For example, in the Framingham Heart Study, the baseline measure of CFPWV was found to be strongly associated with incident hypertension33. However, after controlling for baseline measure of CFPWV, no blood pressure component (systolic, diastolic or mean) entered the model for future stiffness, supporting the hypothesis that aortic stiffness may antedate and contribute to the development of hypertension32. This hypothesis is also supported by recent basic and clinical studies that find associations between altered CFPWV, through the disruption of elastin in the aortic wall, and subsequent development of hypertension34. Finally, it should be noted that results from studies that seek to show that elevation in blood pressure precedes increase in CFPWV, are more controversial32. Arterial stiffness, which is easily measurable, may therefore serve to identify individuals at greatest risk of hypertension progression. Our results find that treated hypertension is associated with more normal CFPWV values. This finding suggests that the elastic nature of the aortic vasculature may be more resilient and amenable at younger ages indicating that early life treatment of hypertension may be substantially more beneficial. Such a hypothesis deserves further testing.
Absence of association between brain integrity measures and augmentation index, central pulse pressure and mean arterial pressure
Overall, our findings do not suggest significant relations of AI and CPP with WM and GM integrity. Previous work from the FHS has identified CFPWV as a significant predictor of a first major CVD event1. In contrast, neither carotid-radial PWV, AI or CPP were related to CVD events in risk factor–adjusted models that included peripheral systolic blood pressure. Thus, CFPWV may be superior to other hemodynamic measures as a marker of risk that is distinct from standard vascular risk factors; the present analysis suggests that attention should be focused on aortic PWV as a biomarker of microstructural WM integrity, even in younger individuals.
Strengths and limitations
The cross-sectional design limits our ability to establish causal relations between arterial stiffness and brain aging measures. In addition, the FHS cohort is mostly of white descent and therefore does not fully represent the general population of the USA. Third, we referred to our sample as an adult healthy population although 73 participants were 60 years old and older. Nonetheless, excluding these older participants from the analyses did not change significantly the findings reported in the present study. Fourth, we did not include systolic blood pressure (SBP) in our regression analyses to avoid introducing collinearity in the models. Similarly, we did not include measures of dietary or exercise, which both have been found to have beneficial effects on cognition35, as recent studies have reported a significant impact of these non-drug interventions on arterial stiffness by several mechanisms36.
Conclusion
In conclusion, CFPWV, a non-invasive measure of arterial stiffness, was associated with deterioration of WM integrity in young to middle-aged adults likely years before overt WMH appear. With the accumulating evidence that arterial stiffness impacts the trajectory of cognition later in life, the public health implications of our results emphasize the need for primary and secondary prevention of vascular stiffness and remodeling as early as the fifth decade. Use of tonometric measures may provide a relatively simple measure to identify higher risk young individuals who may most benefit from hypertension or other vascular risk factor treatment.
Supplementary Material
Acknowledgments
SOURCE OF FUNDING
The study was supported by NIH grants to CD R01 AG033040, P30 AG010129, by NIH to CWT K23 LH118529 and by the NHLBI, Framingham Heart Study, NHLBI/NIH Contract #N01-HC-25195, and HHSN268201500001I (RSV) and the Boston University School of Medicine and by HL076784, G028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, and 2-K24-HL04334.
Footnotes
DISCLOSURE
Dr. Maillard, Dr. Seshadri, Dr. Beiser, Dr. Satizabal, Dr. Himali and Dr. Vasan report no disclosures.
Dr. DeCarli is a consultant to Novartis, Pharmaceuticals.
Dr. Pase is funded by an Australian National Health and Medical Research Council Early Career Fellowship (APP1089698).
Dr. Tsao is partially supported by an award from the American Heart Association (11CRP4930004)
Dr. Mitchell is owner of Cardiovascular Engineering Inc (a company that develops and manufactures devices to measure vascular stiffness) and is a consultant to Novartis, Merck and Servier.
References
- 1.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 3.Tarumi T, de Jong DL, Zhu DC, Tseng BY, Liu J, Hill C, et al. Central artery stiffness, baroreflex sensitivity, and brain white matter neuronal fiber integrity in older adults. Neuroimage. 2015;110:162–170. doi: 10.1016/j.neuroimage.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: The framingham offspring study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 6.Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: The framingham heart study. Arch.Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 7.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the framingham heart study: A cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The third generation cohort of the national heart, lung, and blood institute's framingham heart study: Design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 11.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: Relationship to pressure wave forms. Circulation. 1980;62:105–116. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The framingham heart study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 13.Maillard P, Fletcher E, Harvey D, Carmichael O, Reed B, Mungas D, et al. White matter hyperintensity penumbra. Stroke. 2011;42:1917–1922. doi: 10.1161/STROKEAHA.110.609768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher E, Singh B, Harvey D, Carmichael O, DeCarli C. Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:5319–5322. doi: 10.1109/EMBC.2012.6347195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, et al. Regional pattern of white matter microstructural changes in normal aging, mci, and ad. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, et al. Regional spatial normalization: Toward an optimal target. J Comput Assist Tomogr. 2001;25:805–816. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52:1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pase MP. Modifiable vascular markers for cognitive decline and dementia: The importance of arterial aging and hemodynamic factors. J Alzheimers Dis. 2012;32:653–663. doi: 10.3233/JAD-2012-120565. [DOI] [PubMed] [Google Scholar]
- 21.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 22.Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, et al. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: Findings from 2 large databases. Hypertension. 2012;60:369–377. doi: 10.1161/HYPERTENSIONAHA.112.197491. [DOI] [PubMed] [Google Scholar]
- 23.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: An anatomic study. AJNR Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 24.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;233:883–890. doi: 10.1148/radiol.2333020981. [DOI] [PubMed] [Google Scholar]
- 25.Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: Pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- 26.Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E. Arterial stiffness, the brain and cognition: A systematic review. Ageing research reviews. 2014;15:16–27. doi: 10.1016/j.arr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Jochemsen HM, Muller M, Bots ML, Scheltens P, Vincken KL, Mali WP, et al. Arterial stiffness and progression of structural brain changes: The smart-mr study. Neurology. 2015;84:448–455. doi: 10.1212/WNL.0000000000001201. [DOI] [PubMed] [Google Scholar]
- 28.Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, et al. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721–1726. doi: 10.1161/STROKEAHA.113.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nir TM, Jahanshad N, Villalon-Reina JE, Toga AW, Jack CR, Weiner MW, et al. Effectiveness of regional dti measures in distinguishing alzheimer's disease, mci, and normal aging. NeuroImage. Clinical. 2013;3:180–195. doi: 10.1016/j.nicl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34:2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- 31.Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens. 2011;24:1108–1113. doi: 10.1038/ajh.2011.101. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell GF. Arterial stiffness and hypertension: Chicken or egg? Hypertension. 2014;64:210–214. doi: 10.1161/HYPERTENSIONAHA.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol. 2011;301:H221–229. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middleton LE, Yaffe K. Targets for the prevention of dementia. J Alzheimers Dis. 2010;20:915–924. doi: 10.3233/JAD-2010-091657. [DOI] [PubMed] [Google Scholar]
- 36.Raymond AR, Norton GR, Woodiwiss AJ, Brooksbank RL. Impact of gender and menopausal status on relationships between biological aging, as indexed by telomere length, and aortic stiffness. Am J Hypertens. 2015;28:623–630. doi: 10.1093/ajh/hpu212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.