Abstract
Background and Purpose
Stroke mimics (SM) challenge the initial assessment of patients presenting with possible acute ischemic stroke (AIS). When SM is deemed likely, IV tPA may be withheld, risking an opportunity to treat AIS. While CT is routinely utilized for tPA decision-making, MRI may diagnose AIS when SM is favored but not certain. We hypothesized that a hyperacute MRI (hMRI) protocol would identify tPA-eligible AIS patients among those initially favored to have SM.
Methods
A streamlined hMRI protocol was designed based upon barriers to rapid patient transport, MRI acquisition, and post-MRI tPA delivery. Neurologists were trained to order hMRI when SM was favored and tPA was being withheld. Use of hMRI for tPA decision-making, door-to-needle times, and outcomes were compared before hMRI implementation (“Pre-hMRI”: 8/1/2011-7/31/2013) and after (“Post-hMRI”: 8/1/13-1/15/15).
Results
Post-hMRI, 57 patients with suspected SM underwent hMRI (median MRI-order-to-start time 29 min), of whom, 11 (19%) were diagnosed with AIS and 7 (12%) received tPA. Pre-hMRI, no tPA-treated patients were screened with hMRI. Post-hMRI, 7 of 106 (6.6%) tPA-treated patients underwent hMRI to aid in decision-making due to suspected SM (0 vs 6.6%, p=0.001). To ensure standard care was maintained after implementing the hMRI protocol, pre- vs post-hMRI tPA-treated cohorts were compared and did not differ: door-to-needle time (39 vs 37 min, p=0.63), symptomatic hemorrhage rate (4.5 vs 1.9% (p=0.32), and favorable discharge location (85 vs 89%, p=0.37).
Conclusions
A streamlined hMRI protocol permitted tPA administration to a small, but significant, subset of AIS patients initially deemed to have SM.
Keywords: MRI, acute ischemic stroke, hyperacute MRI, thrombolysis
Subject Terms: Ischemic stroke, Magnetic Resonance Imaging, Cerebrovascular Disease/Stroke
Background and Purpose
Rapid thrombolysis improves clinical outcomes in acute ischemic stroke.1–3 While the diagnostic accuracy of MRI for ischemic stroke is far superior to CT,4, 5 it is unknown whether hyperacute MRI (hMRI) improves clinical outcomes or accuracy of IV tPA decision-making.6 Beyond longer scanning times than CT, several practical challenges of hMRI include: access to MRI at all hours, physical location of MRI outside the Emergency Department, safety contraindications, and access to rapid MRI interpretation. Therefore, using hMRI instead of non-contrast CT as a general screening tool for thrombolytic decision-making should be approached cautiously due to its potential to delay treatment until improved outcomes can be demonstrated.7
While hMRI may be impractical for all patients presenting with possible stroke, we hypothesized that hMRI may benefit a subset of stroke patients who are deprived of thrombolytic therapy due to an initial diagnosis of stroke mimic. Stroke mimics commonly challenge the initial clinical assessment of patients presenting with possible stroke.8–11 In such cases, CT is inadequate to exclude ischemic stroke, as it is typically normal in the first few hours after symptom onset.4, 5 When stroke mimic is deemed likely, IV tPA may be withheld, risking an opportunity to treat an acute ischemic stroke.
Prior to 2013, hMRI for acute ischemic stroke was rarely used at our institution due to process delays that would prevent IV tPA administration within the treatment window. We have previously applied lean manufacturing principles and value stream analysis (a lean method developed by companies to improve speed and quality in industrial processes) to successfully reduce our door-to-needle times for ischemic stroke patients eligible for IV tPA12. In 2013, we utilized a similar lean process to create a streamlined hMRI protocol. We hypothesized that the streamlined hMRI protocol would identify a small, but important group of IV tPA-eligible patients among those initially favored to have stroke mimic.
Methods
Value Stream Analysis for Streamlined hMRI Protocol
In April 2013, a two-day multidisciplinary meeting in the form of a value stream analysis (VSA) was held, comprised of the following individuals: neurology, radiology, and emergency medicine physicians, radiology technologists and managers, emergency medicine nurses, an emergency medicine pharmacist, and a process engineer. The goal of this team event was to apply value stream mapping techniques to understand current MRI process, identify barriers and plan appropriate process improvements, enabling rapid hMRI without compromising safety. The team set a goal door-to-MRI time of less than 45 minutes. The current state map revealed numerous barriers to patient flow including delays due to inefficient: MRI ordering and safety screening, patient and staff preparation for MRI (de-metal), patient transport, MRI acquisition, MRI interpretation, and post-MRI tPA delivery. A planned future state was mapped which included target metrics for door-to- MRI and door-to-needle times for each segment of the process (Supplemental Figures I and II).
Major sources of delay and their respective solutions included: (1) a scanning prioritization list for MRI technicians was created allowing hMRI to signify highest priority with the exception of ICU or anesthetized patients who were already being scanned; (2) the MRI screening sheet was streamlined and placed near trauma bays where the acute stroke patients are evaluated, (3) MRI-compatible cardiac leads and patient gowns without snaps were purchased and placed near treatment rooms, (4) the hMRI imaging protocol was condensed to < 6 min (Table 1), and (5) a Pyxis Medstation was placed in MRI and stocked with tPA and antihypertensive medications. The final step prior to implementing the streamlined hMRI protocol was providing training to all team members. The hMRI protocol went live 8/1/2013, approximately four months after the two day VSA was held.
Table 1.
Streamlined hyperacute MRI imaging protocol.
| Sequence | min:sec | |
|---|---|---|
|
| ||
| LOCALIZER | 0:13 | |
| DWI / ADC | 2:06 | |
| FLAIR | 3:20 | |
| T2* GRE | 0:09 | |
|
| ||
| TOTAL | 5:48 | |
|
| ||
| Optional Sequences | ||
|
| ||
| Time-of-Flight MRA | 2:26 | |
| Perfusion Weighted Imaging | 2:06 | |
DWI=diffusion-weighted imaging; ADC=apparent diffusion coefficient; FLAIR=fluid attenuated inversion recovery; GRE=gradient echo; MRA=magnetic resonance angiography
Decision-making for hMRI
Treating neurologists were trained to order hMRI when the initial diagnostic impression was likely stroke mimic, but when ischemic stroke could not be entirely ruled out and when the patient was otherwise tPA-eligible. Neurologists were specifically instructed to ask themselves whether they would give the patient tPA if MRI was not available at their institution: if ‘Yes’, they were instructed to proceed with tPA administration without hMRI; if “No”, they were instructed to proceed with hMRI to aid treatment decision-making (Figure 1). This instruction was important to avoid overutilization of hMRI which could inappropriately delay tPA treatment in patients for whom hMRI would be unlikely to alter decision-making. We hypothesized that our stroke mimic rate should not change significantly after initiating this protocol if neurologists were only proceeding to hMRI for patients they would not have treated with IV tPA under the previous protocol.
Figure 1. Hyperacute MRI decision algorithm for treating neurologists.
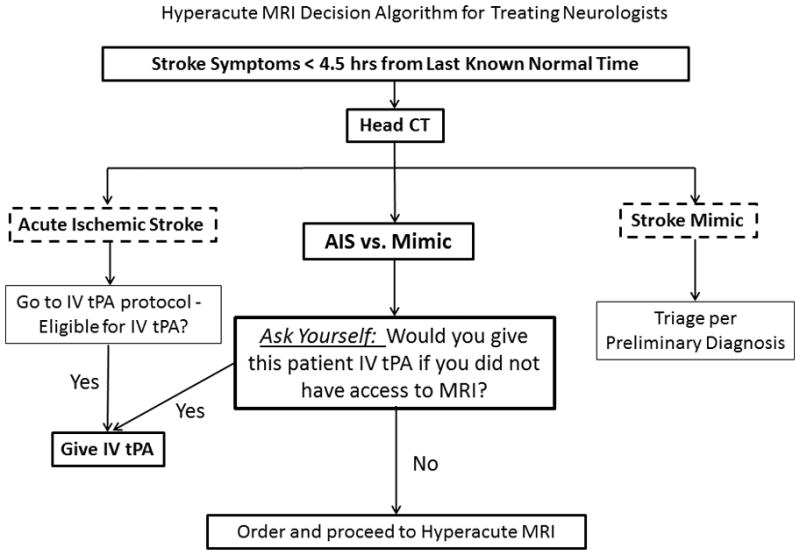
Prior to implementing the hMRI protocol, treating neurologists were trained according to a “hMRI decision algorithm” to prevent over-utilization of hMRI and to prevent inappropriate delays of tPA treatment in patients who would have received tPA if hMRI were not available.
Data Analysis
Before and after the streamlined hMRI protocol was implemented, we prospectively collected baseline characteristics, discharge location, protocol metrics such as tPA administration time and door-to-needle time, according to the routine quality and safety monitoring practices at our institution for all tPA-treated patients. Additional outcomes including discharge diagnosis and symptomatic intracerebral hemorrhage (defined as any clinically-identified neurological worsening within 36 hours of stroke onset associated with acute blood on brain imaging)13 were obtained by chart review. After hMRI protocol implementation in 8/2013, we collected baseline characteristics and protocol metrics for all non-tPA treated patients going to hMRI. The use of hMRI prior to hMRI protocol implementation was only tracked in tPA-treated patients and therefore its utilization or baseline characteristics in non-tPA treated patients going to hMRI were not collected. In order to ensure that the new protocol was not affecting standard of care tPA administration in patients more likely to have ischemic stroke on initial evaluation, we compared overall door-to-needle times and outcomes for all patients receiving tPA regardless of hMRI use, prior to (08/01/2011 – 07/31/2013) and after (08/01/2013 - 01/15/2015) protocol implementation. Student’s T test (parametric) and Mann Whitney U test (non-parametric) were used for analysis of continuous variables. Fisher Exact test was used for analysis of binary outcome variables. P<0.05 was required for significance. SPSS v. 22 was utilized for all statistical analyses.
Results
Patient Characteristics and Protocol Metrics Post-hMRI
In the Pre-hMRI epoch, hMRI was rarely used because an adequately rapid hMRI protocol was not available. Therefore, no tPA-treated patients were screened with hMRI prior to tPA delivery. In the Post-hMRI epoch, 57 patients underwent hMRI for evaluation of suspected stroke mimic and to rule out ischemic stroke. Age was 55 ± 14 years, 39 (68%) were women, and median NIHSS was 4 [2, 10]. Median time of hMRI order to hMRI begin was 29 [17, 45] min and median time of patient arrival to hMRI begin was 61 [ 52, 94] min (Table 2).
Table 2.
Baseline Characteristics, MRI positivity, and Discharge Diagnoses in Suspected Stroke Mimics Taken to hMRI After Initiation of Streamlined Protocol †
| Post-hMRI (8/2013-1/2015) | N=57 |
|---|---|
| Baseline Characteristics | |
| Age, years | 55 ± 14 |
| Gender, Female | 39 (68%) |
| Race, African-American | 24 (42%) |
| Baseline NIHSS | 4 [2, 10] |
|
| |
| MRI with DWI restriction consistent with clinical presentation, n (%) | 11 (19%) |
| Treated with IV tPA, n (%) | 7 (12%) |
|
| |
| Protocol Metrics | |
| Door-to-CT, time, min | 12 [8, 16] |
| CT done to MRI order, min | 21 [13, 39] |
| MRI order to MRI Begin, min | 29 [17, 45] |
| Door-to-MRI Begin, min | 61 [52, 94] |
|
| |
| Discharge Diagnoses in Suspected Stroke Mimics Taken to hMRI | |
| Conversion Disorder | 14 (25%) |
| Acute Ischemic Stroke | 11 (19%) |
| Seizures | 11 (19%) |
| Other * | 11 (19%) |
| Migraine with Prolonged Aura | 6 (11%) |
| TIA | 4 (7%) |
hMRI utilization was not tracked within the pre-hMRI epoch (8/11-7/13) except for tPA-treated patients in whom none were screened with hMRI prior to tPA.
Other Diagnoses included: encephalitis, peripheral neuropathy, syncope, recrudescence
DWI=Diffusion Weighted Imaging
TIA=Transient Ischemic Attack
Of the 57 patients, 11 (19%) and 4 (9%) were discharged with diagnoses of acute ischemic stroke and TIA, respectively. Remaining discharge diagnoses included conversion disorder in 14 (25%), seizures in 11 (19%), complicated migraine in 6 (11%) and other in 11 (19%) including diagnoses of encephalitis, peripheral neuropathy, syncope, and stroke recrudescence. In five of the 11 patients who were diagnosed with seizures, hMRI demonstrated findings supportive of seizures including gyriform diffusion restriction across vascular territories or an underlying structural lesion. In the remaining non-stroke etiologies, MRIs did not yield additional diagnostic information beyond being negative for stroke or other acute pathology. Due to the hyperacute nature of these scans, contrast was administered in a minority of patients.
IV tPA-treated Patients Pre- and Post-hMRI
To confirm that implementation of a streamlined hMRI protocol changed patient management, hMRI utilization was compared pre- and post-hMRI in tPA-treated patients. In the Pre-hMRI epoch, 159 patients (~6.6 patients/month) received IV tPA, of whom, none underwent hMRI prior to treatment. In the Post-HRMI epoch, 106 patients (~6.6 patients/month) received IV tPA, of whom 7 (6.6%) patients underwent hMRI prior to IV tPA (0 vs. 6.6%, p=0.001) (Table 3). This increase in tPA treatments screened with hMRI in the post-hMRI epoch was not unexpected as hMRI was rarely used prior to its implementation. Four of the 11 patients with acute ischemic stroke on MRI did not receive tPA despite the MRI DWI positivity due to elevation of blood pressure, the presence of a subacute appearing stroke/change in last known normal time, and two patients with rapidly improving stroke/minor deficits. Clinical histories and DWI of the 7 patients who received IV tPA are shown (Figure 2) demonstrating: (1) clinical histories which were consistent with possible stroke mimics suggesting appropriate utilization of hMRI and use of the decision algorithm in the Methods/Figure 1, (2) the relatively small size of the stroke lesions and, in some, lesion locations known to cause non-focal deficits (i.e. thalamus causing cognitive changes), and (3) DWI-positive lesions were only subtly hyperintense due to the hyperacute timing of imaging, whereas hypointensity on ADC was more useful for diagnosis.
Table 3.
Comparison of baseline variables, protocol metrics, and safety outcomes pre- and post-hMRI for all tPA-treated patients (left) and comparison of post-hMRI tPA-treated patients screened with CT alone vs. CT and hMRI (right)
| Pre-hMRI 8/11-7/13 N=159 |
Post-hMRI 8/13-1/15 N=106 |
P-Value | Post-hMRI (8/13-1/15) N=106 |
|||
|---|---|---|---|---|---|---|
| Screened with CT Alone N= 99 | Screened with CT and hMRI N = 7 | P-Value | ||||
| Age, years | 62 [54, 75] | 67 [56, 75] | 0.10 | 66 [56, 75] | 72 [64, 76] | 0.41 |
| Gender, Female | 78 (49%) | 55 (52%) | 0.71 | 49 (50%) | 6 (86%) | 0.11 |
| Race, African-American | 90 (57%) | 55 (52%) | 0.45 | 50 (50%) | 5 (71%) | 0.44 |
| Baseline NIHSS | 7 [4, 12] | 8 [4, 15] | 0.23 | 8 [4, 14] | 4 [2, 12] | 0.22 |
|
| ||||||
| Screened with hMRI prior to tPA | 0 (0%) | 7 (7%) | 0.001 | 0 (0%) | 7 (100%) | -- |
| Door-to-Needle Time, min | 39 [28, 58] | 37 [25, 66] | 0.63 | 37 [28, 52] | 112 [100, 116] | <0.001 |
| Onset-to-Needle Time, min | 116 [85, 155] | 130 [84, 172] | 0.81 | 102 [70, 159] | 143 [133, 180] | 0.14 |
|
| ||||||
| Favorable Discharge Location+ | 132 (85%) | 94 (89%) | 0.37 | 88 (89%) | 6 (85%) | 0.58 |
| Symptomatic ICH++ | 7 (4.5%) | 2 (1.9%) | 0.32 | 2 (2%) | 0 (0%) | 1.0 |
| Stroke Mimic+++ | 21 (13.2%) | 12 (11.3%) | 0.71 | 12 (12%) | 0 (0%) | 1.0 |
Defined as Discharge to Home or Inpatient Rehab.
Defined as any clinically-identified neurological worsening < 36 hours of onset associated with blood on head CT.13
Defined as any discharge diagnosis other than stroke.
Figure 2. Hyperacute MRI diffusion weighted imaging for patients receiving IV tPA.
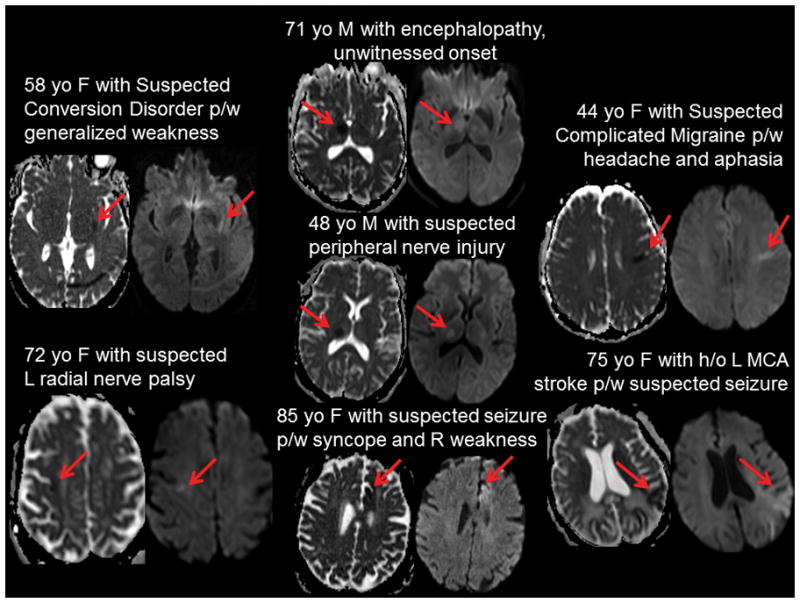
Brief clinical histories and diffusion weighted imaging (DWI) are shown for the 7 patients who were screened with hMRI to aid tPA-decision making and who received IV tPA. Clinical histories are consistent with suspected stroke mimics suggesting likely appropriate utilization of hMRI. Acute ischemic stroke lesions (red arrows) shown as hypointensity on apparent diffusion coefficient (ADC) maps (left) and hyperintensity on diffusion weighted imaging (right) for each patient demonstrate small lesion areas that appear to be of recent onset (given mild hyperintensity on DWI).
To ensure that hMRI protocol implementation did not adversely affect the standard care for the majority of tPA-treated patients screened with CT alone, we evaluated protocol metrics and clinical and safety outcomes pre- and post-hMRI for all tPA-treated patients. There were no differences in door-to-needle, onset-to-needle, or door-to-arrival times pre- and post-hMRI (Table 3). Furthermore, there were no statistically significant differences in favorable discharge location, symptomatic intracerebral hemorrhage rate, or stroke mimic rates between the two epochs.
The 7 patients who received IV tPA following hMRI had subsequent outcomes and discharge locations similar to our other patients receiving intravenous thrombolysis without MRI, and none suffered hemorrhagic conversion. The median door-to-MRI begin and door-to-needle times were 55 [49, 67] and 112 [100, 116] min, respectively, for the 7 patients treated with intravenous thrombolysis.
Patient characteristics and Protocol Metrics for IV tPA-treated patients screened by hMRI compared to those screened by CT alone
To evaluate the impact of hMRI on protocol metrics and potential for adding delays to tPA-treatment, door-to-needle and onset-to-needle times were compared. For the 99 tPA-treated patients screened with CT alone, door-to-needle times were much shorter than for the 7 patients screened with CT and sent to hMRI prior to IV tPA (37 [28, 52] vs. 112 [100, 116] min, p < 0.001). Furthermore, there was a trend towards longer onset-to-needle times when utilizing hMRI prior to IV tPA (102 [70, 159] vs. 143 [133, 180] min, p=0.14). Patients screened with hMRI compared to CT alone showed trends towards a lower NIHSS (8 [4, 14] vs. 4 [2, 12], p=0.22) and were more likely female (50% vs. 86%, p=0.11), the latter characteristics which have been associated with stroke mimics in other studies.14 Clinical outcome measures, symptomatic hemorrhage rates, and stroke mimic rates did not differ between those screened with hMRI vs. CT alone (Table 3).
Discussion
Our results demonstrate that hMRI identifies ischemic stroke in a subset of patients initially thought to have stroke mimic, and further that it can be performed sufficiently quickly to enable treatment with intravenous thrombolysis when appropriate. Given these findings of seven treatable patients, we suspect that there were AIS patients in the pre-hMRI epoch who were not offered tPA treatment due to being initially diagnosed as stroke mimics. It is possible that this AIS patient subgroup is being overlooked for tPA treatment at many centers. An acute ischemic stroke was only found in a minority of the 57 patients evaluated with hMRI, in accordance with our instructions to only include patients who would not normally receive IV tPA if hMRI were not available. At our high volume stroke center several patients received improved care over a 16 month period directly as a result of implementing hMRI. Also, we could not detect any evidence that hMRI worsened care of patients with initial clinical suspicious for stroke who did not undergo hMRI.
The door-to-needle times for the 7 patients treated with IV tPA were considerably longer than for patients clinically suspected to have an acute stroke initially and who did not undergo hMRI, thus reinforcing cautious use of hMRI in suspected stroke patients at an institution such as ours where MRI is physically distant from the ED and hMRI is performed in a minority of patients. However, the lengthy door-to-needle times are permissible compared to not receiving intravenous tPA at all, as would have been the case prior to implementation of the hMRI protocol. The long door-to-MRI begin and MRI-to-needle times suggest that improvements can be made. We used a value stream based analysis to create the hyperacute stroke MRI protocol at an institution where the MRI scanner is separated from the Emergency Department by several floors and hallways. Applying a similar analysis to improve the hMRI protocol may help reduce treatment times. Shah et al. recently reported the use of lean improvement methods to improve door-to-needle times using a hMRI protocol in the vast majority of patients.6 Over two years, door-to-needle times were reduced from median of 93 to 55 minutes.
While our study was not designed to assess cost-effectiveness, we note that approximately 1 in 8 patients were screened with hMRI to be treated with IV tPA, raising the question of whether this protocol is sufficiently cost-effective to warrant its use more universally, particularly in low volume stroke centers. Furthermore, stroke lesion size in suspected stroke mimics who had ischemic stroke on MRI tended to be smaller with lower NIHSS scores, for which net benefit of tPA may be debated. Recent analysis of combined randomized clinical trial data testing the efficacy of IV tPA demonstrate significant benefit of IV tPA in patients with low NIHSS scores (0–4).1 The value of hyperacute stroke MRI may extend beyond the opportunity to treat with intravenous tPA in that it may result in more rapid diagnosis, i.e. stroke or otherwise, and thereby reduce length of stay, though this was not evaluated in the current study.
Our data demonstrate that stroke mimic rates in tPA-treated patients were unchanged comparing rates before and after implementation of the hMRI protocol. If our hMRI protocol had been utilized in the majority of tPA-treated patients, we would have expected our stroke mimic rate to decrease after implementing the hMRI protocol. However, as we only utilized hMRI to a minority of subjects, our stroke mimic rate was statistically unchanged. The potential benefit of the streamlined hMRI protocol favored inclusion of more patients for IV tPA rather than reducing potential harm of tPA by reducing the rate of tPA treatment in stroke mimics. If hMRI protocols could truly be streamlined and offered to a majority of patients as has been successful at select centers, tPA treatment within stroke mimics could be greatly reduced which may be cost effective given the cost of tPA and inpatient monitoring required for 24 hours post-tPA administration.6, 15
There are several limitations to this study. This was not a randomized trial and differences between cohorts separated in time could be due to a variety of factors including differences in treatment protocols. While the majority of data were collected prospectively, analysis was retrospective and unblinded. The sample size is small and limited to a single institution. While treating neurologists were trained to follow a decision-algorithm when considering hMRI, we did not track actual use of this algorithm and therefore, it is possible that patients were taken to hMRI who would have otherwise been given IV tPA had they not had access to MRI. Given the clinical histories, low NIHSS, and minority of strokes in the hMRI group, it appears likely that the neurologists did reserve hMRI for suspected mimics who were otherwise tPA eligible with a goal of ruling-out ischemic stroke. Furthermore, DWI is falsely negative in ~5–10% of cases, especially early after symptom onset16, 17, raising the possibility that hMRI failed to identify additional stroke patients among the cohort of 57 patients reported here.
Conclusions
A hyperacute MRI stroke protocol, designed by multi-disciplinary value stream analysis, was effective in identifying a small subset of stroke patients eligible for IV tPA who were initially suspected to have a stroke mimic. Door-to-needle times were long in those patients treated with IV tPA due to hMRI, suggesting that ongoing process improvements are required to enhance the effectiveness of hMRI.
Supplementary Material
Acknowledgments
We would like to acknowledge the Washington University neurology and emergency medicine residents, Barnes-Jewish Hospital ED nurses, radiology and patient care technicians.
Sources of Funding
This study was supported by grants from National Institute of Health K23 NS069807 (to AF) and NIH CTSA UL1 TR000448 from the Washington University Institute of Clinical and Translational Sciences.
Footnotes
Conflicts of Interest / Disclosures:
Manu S. Goyal, MD, MSc: None
Brian G. Hoff, MSc: None
Jennifer Williams, RN, PhD: None
Naim Khoury, MD: None
Rebecca Wiesehan, BA: None
Laura Heitsch, MD: Speakers' Bureau: Entity: Genentech; Relationship: Myself; Explanation: Speaker's Bureau; Compensation: Significant (>$10K or 5%); Consultant or Advisory Board; Entity: Genentech; Relationship: Myself; Explanation: Advisory Board; Compensation: Modest (<$10K or <5%)
Peter Panagos, MD: Honoraria: Entity: Genentech; Relationship: Myself; Explanation: Speakers Bureau; Compensation: Significant (>$10K or 5%)
Katie D. Vo, MD: None
Tammie Benzinger, MD, PhD: Other Research Support; Yes, I have a other research support to disclose. Entity: NIH; Relationship: Myself; Explanation: Research grants Compensation: No Compensation; Entity: Avid/Lilly; Relationship: Myself; Explanation: Research grants; Compensation: No Compensation; Entity: Roche, Lilly, Avid; Relationship: Myself; Explanation: Clinical Trials; Compensation: No Compensation; Entity: DOD; Relationship: Myself; Explanation: Grant review; Compensation: Modest (<$10K or <5%); Entity: National MS Society; Relationship: Myself; Explanation: Grant review; Compensation: No Compensation; Entity: Foundation for the NIH; Relationship: Myself; Explanation: Research grant; Compensation: No Compensation
Colin P. Derdeyn, MD: None
Jin-Moo Lee, MD, PhD: None
Andria L. Ford, MD, MSc: Research Grant: Entity: NIH NINDS; Relationship: Myself; Explanation: 5K23NS069807-05; Compensation: Significant (>$10K or 5%)
References
- 1.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 3.Lansberg MG, Schrooten M, Bluhmki E, Thijs VN, Saver JL. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified rankin scale. Stroke. 2009;40:2079–2084. doi: 10.1161/STROKEAHA.108.540708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, et al. Ct and diffusion-weighted mr imaging in randomized order: Diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002;33:2206–2210. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 5.Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet. 2007;369:293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S, Luby M, Poole K, Morella T, Keller E, Benson RT, et al. Screening with mri for accurate and rapid stroke treatment: Smart. Neurology. 2015;84:2438–2444. doi: 10.1212/WNL.0000000000001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford AL, Leker RR. Mri in acute stroke: Good times are coming. Neurology. 2015;84:2394–2395. doi: 10.1212/WNL.0000000000001690. [DOI] [PubMed] [Google Scholar]
- 8.Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: The brain attack study. Stroke. 2006;37:769–775. doi: 10.1161/01.STR.0000204041.13466.4c. [DOI] [PubMed] [Google Scholar]
- 9.Libman RB, Wirkowski E, Alvir J, Rao TH. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119–1122. doi: 10.1001/archneur.1995.00540350113023. [DOI] [PubMed] [Google Scholar]
- 10.Winkler DT, Fluri F, Fuhr P, Wetzel SG, Lyrer PA, Ruegg S, et al. Thrombolysis in stroke mimics: Frequency, clinical characteristics, and outcome. Stroke. 2009;40:1522–1525. doi: 10.1161/STROKEAHA.108.530352. [DOI] [PubMed] [Google Scholar]
- 11.Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951–964. doi: 10.1016/S1474-4422(08)70216-3. [DOI] [PubMed] [Google Scholar]
- 12.Ford AL, Williams JA, Spencer M, McCammon C, Khoury N, Sampson TR, et al. Reducing door-to-needle times using toyota's lean manufacturing principles and value stream analysis. Stroke. 2012;43:3395–3398. doi: 10.1161/STROKEAHA.112.670687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 14.Zinkstok SM, Engelter ST, Gensicke H, Lyrer PA, Ringleb PA, Artto V, et al. Safety of thrombolysis in stroke mimics: Results from a multicenter cohort study. Stroke. 2013;44:1080–1084. doi: 10.1161/STROKEAHA.111.000126. [DOI] [PubMed] [Google Scholar]
- 15.Leker RR, Keigler G, Eichel R, Ben Hur T, Gomori JM, Cohen JE. Should dwi mri be the primary screening test for stroke? International journal of stroke : official journal of the International Stroke Society. 2014;9:696–697. doi: 10.1111/ijs.12316. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez RG, Schaefer PW, Buonanno FS, Schwamm LH, Budzik RF, Rordorf G, et al. Diffusion-weighted mr imaging: Diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology. 1999;210:155–162. doi: 10.1148/radiology.210.1.r99ja02155. [DOI] [PubMed] [Google Scholar]
- 17.Lovblad KO, Laubach HJ, Baird AE, Curtin F, Schlaug G, Edelman RR, et al. Clinical experience with diffusion-weighted mr in patients with acute stroke. AJNR Am J Neuroradiol. 1998;19:1061–1066. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


