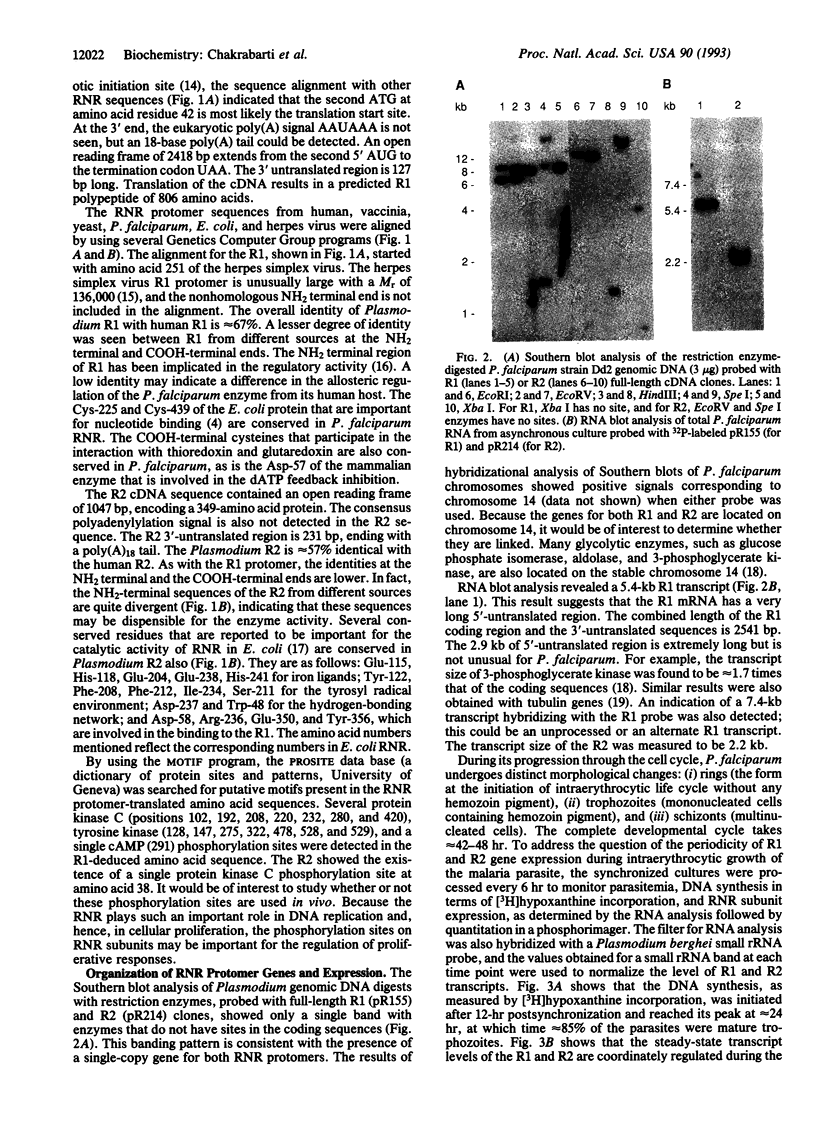
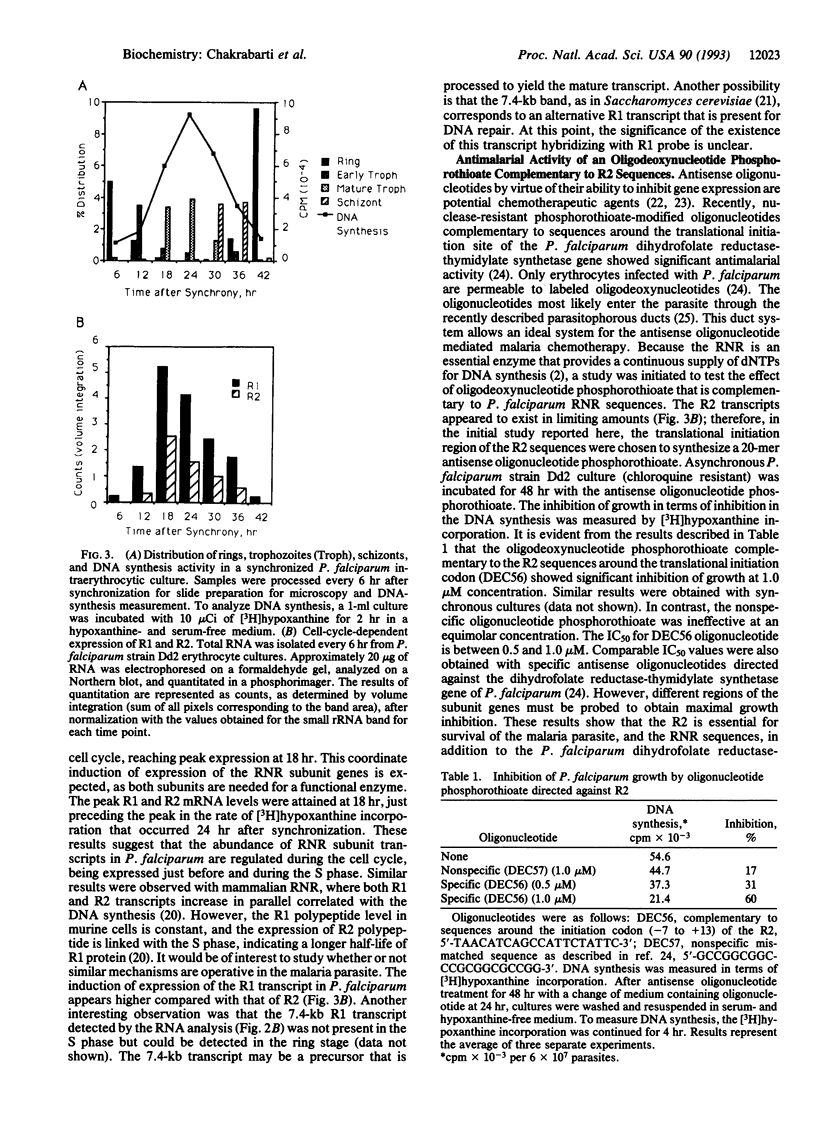
Abstract
Ribonucleotide reductase (EC 1.17.4.1; RNR), a cell-cycle-regulated enzyme, catalyzes the rate-limiting step in the de novo synthesis of deoxyribonucleotides by the reduction of the corresponding ribonucleotides. The important role of the RNR in DNA synthesis and cell division makes this enzyme an excellent target for chemotherapy. However, nothing is known about this enzyme from the malaria parasite Plasmodium falciparum. We have isolated cDNA clones encoding both the large and small RNR subunits. The sequences of full-length clones of the large and small RNR subunits revealed an open reading frame encoding 806 and 349 amino acids, respectively, and showed significant identity with other RNR sequences in the data base. RNA blot analysis showed that the size of the large and small RNR subunit transcripts are 5.4 kb and 2.2 kb, respectively. Both the RNR subunit transcripts fluctuate in level during the cell cycle, reaching a peak preceding maximal DNA synthesis activity. An oligodeoxynucleotide phosphorothioate that is complementary to sequences around the translational initiation codon of the small RNR subunit showed significant inhibition of growth, as measured by the inhibition in DNA synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S. Antisense oligonucleotides as antiviral agents. Trends Biotechnol. 1992 May;10(5):152–158. doi: 10.1016/0167-7799(92)90203-8. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Björklund S., Skog S., Tribukait B., Thelander L. S-phase-specific expression of mammalian ribonucleotide reductase R1 and R2 subunit mRNAs. Biochemistry. 1990 Jun 12;29(23):5452–5458. doi: 10.1021/bi00475a007. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Martin D. W., Jr Molecular cloning of the cDNA for a mutant mouse ribonucleotide reductase M1 that produces a dominant mutator phenotype in mammalian cells. Mol Cell Biol. 1988 Jul;8(7):2698–2704. doi: 10.1128/mcb.8.7.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cory J. G. Ribonucleotide reductase as a chemotherapeutic target. Adv Enzyme Regul. 1988;27:437–455. doi: 10.1016/0065-2571(88)90030-1. [DOI] [PubMed] [Google Scholar]
- Crooke S. T. Progress toward oligonucleotide therapeutics: pharmacodynamic properties. FASEB J. 1993 Apr 1;7(6):533–539. doi: 10.1096/fasebj.7.6.7682523. [DOI] [PubMed] [Google Scholar]
- Delves C. J., Alano P., Ridley R. G., Goman M., Holloway S. P., Hyde J. E., Scaife J. G. Expression of alpha and beta tubulin genes during the asexual and sexual blood stages of Plasmodium falciparum. Mol Biochem Parasitol. 1990 Dec;43(2):271–278. doi: 10.1016/0166-6851(90)90151-b. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Davis R. W. Two genes differentially regulated in the cell cycle and by DNA-damaging agents encode alternative regulatory subunits of ribonucleotide reductase. Genes Dev. 1990 May;4(5):740–751. doi: 10.1101/gad.4.5.740. [DOI] [PubMed] [Google Scholar]
- Frame M. C., Marsden H. S., Dutia B. M. The ribonucleotide reductase induced by herpes simplex virus type 1 involves minimally a complex of two polypeptides (136K and 38K). J Gen Virol. 1985 Jul;66(Pt 7):1581–1587. doi: 10.1099/0022-1317-66-7-1581. [DOI] [PubMed] [Google Scholar]
- Hicks K. E., Read M., Holloway S. P., Sims P. F., Hyde J. E. Glycolytic pathway of the human malaria parasite Plasmodium falciparum: primary sequence analysis of the gene encoding 3-phosphoglycerate kinase and chromosomal mapping studies. Gene. 1991 Apr;100:123–129. doi: 10.1016/0378-1119(91)90357-h. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Pouvelle B., Spiegel R., Hsiao L., Howard R. J., Morris R. L., Thomas A. P., Taraschi T. F. Direct access to serum macromolecules by intraerythrocytic malaria parasites. Nature. 1991 Sep 5;353(6339):73–75. doi: 10.1038/353073a0. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Misiura K., Agrawal S., Zamecnik P. Antimalarial activities of oligodeoxynucleotide phosphorothioates in chloroquine-resistant Plasmodium falciparum. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8577–8580. doi: 10.1073/pnas.89.18.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg B. M., Gräslund A. Ribonucleotide reductase. Adv Inorg Biochem. 1983;5:87–110. [PubMed] [Google Scholar]
- Stubbe J. Ribonucleotide reductases. Adv Enzymol Relat Areas Mol Biol. 1990;63:349–419. doi: 10.1002/9780470123096.ch6. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]