Abstract
Background
Little is known about outcomes of AML in adolescents and young adults (AYA). The purpose of this study is to determine the characteristics and outcomes of AYA AML patients in comparison to older adult patients with AML.
Patients and Methods
We retrospectively analyzed all AML patients treated at our institution from 1965 to 2009 aged 16 to 29 years.
Results
Among 3,922 adult AML patients treated during this period, 432 (11%) were identified as AYA. Median age was 23 years (range 16-29 years); 73 (17%) patients had Core Binding Factor (CBF)-AML [inv (16), t(8:21)] and 51 (12%) acute promyelocytic leukemia. Complete remission (CR) rates were 93% for CBF AML, 78% for APL, 77% with diploid karyotype and 68% for other AML. Univariate analysis demonstrated higher rates of complete remission (CR), CR duration, and overall survival (OS) in the AYA group compared to older patients. On multivariate analysis, AYA age group was independently associated with improved CR rate and CR duration, with a trend for longer OS (p-value=0.085).
Conclusion
Outcome of AYA AML patients is overall better than for older adults with AML. Despite improvements in treatments and outcomes over time, there is still need for improvement in AYA with AML particularly for those with AML other than CBF and APL.
Keywords: acute myeloid leukemia, AML, adolescent and young adult, AYA, outcomes
Introduction
Acute myeloid leukemia (AML) is typically a disease of older adults with an age-adjusted incidence of 3-4:100,000 in Western countries, with a median age at the time of diagnosis of 70 years 1. AML accounts for approximately one-third of all leukemia cases in the United States, with estimated incidence of 18,860 newly diagnosed patients and 10,460 patient deaths in the United States in 20142. Age at the time of diagnosis is one of the most important prognostic features, with the prognosis worsening with increasing age, even when accounting for cytogenetic risk groups 3. The impact of age has focused mostly on the older age groups which have suggested that outcomes have consistently been more unfavorable in patients older than age 60-65 years4. Some of these differences are related to patient characteristics (e.g., worse performance status among older patients, more co-morbidities, poor tolerance for intensive chemotherapy) and others due to disease biology (e.g., an increased incidence of high-risk chromosomal abnormalities and higher frequency of multi-drug resistance (MDR) expression)5.
Adolescents and young adults (AYA) with cancer represent a group of patients receiving growing attention mainly due to the striking lack of progress in treatments and outcomes compared to both younger pediatric and older patient populations 6,7. AYA with leukemia are a unique group of cancer patients that may exhibit distinctive patient and disease characteristics compared to other age groups and other cancer patients8-10. Much has been reported about the prognosis of AYA with acute lymphoblastic leukemia (ALL) 11,12. Among patients with ALL, analysis of the SEER database has shown a significant improvement in overall survival for adolescents, young adults and older adults over the past two decades, with a significant overall survival improvement in AYA (ages 15-19) whose 5-year relative survival improved from 41% to 62% 11,13,14.
Outcomes in patients focusing on pediatric AML have been reviewed by various groups, and demonstrate significant improvements over time 15-17. Little is known, however, about the outcomes of the specific subset of the AYA AML group, treated with adult-aimed chemotherapy. This study investigates the characteristics and outcomes of AML in AYA at our institution and compares outcomes to those of older patients the same chemotherapy regimens.
Methods
Patients
Patients with AML treated in the Department of Leukemia at MD Anderson Cancer Center (MDACC) from 1965 to 2009 were analyzed. Patients ages 16 to 29 years were defined as AYA and are the focus of this analysis. Among the 3,922 adult AML patients seen during this period, 432 (11%) were AYA, and were included in our analysis. Patient, disease, and treatment characteristics were analyzed, including: age at diagnosis, cytogenetics at time of diagnosis, history of antecedent hematologic disease (AHD), primary versus secondary (including treatment-related) AML, other disease characteristics, and treatment administered. When available, molecular testing for FLT3 mutations was also included. Only patients receiving their induction therapy at our institution were considered for this analysis. All patients were treated under protocols approved by the Institutional Review Board and all patients signed informed consent in accordance with rules and regulations of the Declaration of Helsinki. The study was approved in a separate protocol approved by the Institutional Review Board.
To compare outcomes over time, patients were grouped into 3 treatment eras: 1965-1984, 1985-2000, and 2001-2009. When divided by treatment era, AYA patients represented 41%, 26%, and 19%, respectively, of AML patients treated.
Response Criteria
Responses were defined as per the International Working Group criteria 18. For each treatment era and cytogenetic groups we investigated induction mortality, CR rate, and CR duration, and compared these values over time.
Statistical Methods
Kaplan-Meier curves were generated for assessment of overall survival with comparison of groups performed by log-rank testing. Fisher’s exact test or Mann-Whitney U test was employed for comparisons of non-parametric data with categorical or continuous variables, respectively. T-test was performed to analyze differences among subgroups by covariates for parametric data. Generalized linear modeling was used to determine potential predictive factors for CR. The statistical analysis was performed using STATA/SE version 13.1 statistical software (Stata Corporation, LP, College Station, TX). Both univariate (via log-rank testing) and multivariate analysis (via Hazard Cox Regression modeling with extraction of significant variables from the univariate testing) were performed. For this analysis, p-values < 0.05 were considered statistically significant for univariate analysis; p-values < 0.10 on univariate analysis were included in multivariate logistic regression modeling to assess for independent determinants of CR, CR duration, and OS.
Results
Patient characteristics are summarized in Table 1. The median age of the 432 AYA patients was 23 years (range 16-29). These included 73 (17%) patients with Core Binding Factor (CBF) and 51 (12%) patients with acute promyelocytic leukemia (APL). In addition, 97 (22%) patients had diploid cytogenetics and 19 patients (4%) had monosomy 5 (−5) and/or monosomy 7 (−7) with complex cytogenetics. Miscellaneous cytogenetic abnormalities were identified in 167 patients (39%): 47 patients (11%) with other non-complex cytogenetics(≤3 abnormalities), 29 (7%) with other complex cytogenetics (≥3 abnormalities), 12 (3%) with insufficient metaphases, and cytogenetics unknown or unavailable in 101 patients (23%). Antecedent hematologic disorders (AHD), defined as documented abnormalities in peripheral blood counts prior to the diagnosis of AML, were present in 74 patients (17%). A prior malignancy had been diagnosed in 26 patients (6%), and 21 (81%) had received chemotherapy for their prior malignancies. The most common prior tumors were sarcoma (n=6) and lymphoma (n=6; 4 Hodgkin’s lymphoma). Four patients had prior hematologic malignancies: 3 myelodysplastic syndrome (MDS) and one prior aplastic anemia.
Table 1.
Patient Characteristics by Age*
| Age (years) |
p-value | ||||||
|---|---|---|---|---|---|---|---|
| ≥30 | 16-29 | ||||||
|
|
|||||||
| WBC (K/uL) | <0.001 | ||||||
| N | 3488 | 430 | |||||
| Median | 8.9 | 15.6 | |||||
| Range | .2 - 760 | .2 - 516 | |||||
| Platelet count (K/uL) | 0.501 | ||||||
| N | 3488 | 430 | |||||
| Median | 47 | 46 | |||||
| Range | 1 -2292 | 2 - 865 | |||||
| Hemoglobin (g/dL) | 0.007 | ||||||
| N | 3488 | 430 | |||||
| Median | 8.5 | 8.9 | |||||
| Range | 2 - 16.1 | 2.9 14.7 | |||||
| Peripheral blood blast (%) | <0.001 | ||||||
| N | 2679 | 376 | |||||
| Median | 32 | 51 | |||||
| Range | 0 - 99 | 0 - 99 | |||||
| Bone marrow blast (%) | <0.001 | ||||||
| N | 3450 | 423 | |||||
| Median | 54 | 70 | |||||
| Range | 0 - 98 | 0 - 98 | |||||
| N | % | N | % | ||||
| Diagnosis groups ** | <0.001 | ||||||
| APL | 244 | 7 | 51 | 12 | |||
| CBF | 246 | 7 | 73 | 17 | |||
| All other groups | 2998 | 86 | 306 | 71 | |||
| Complete remission | <0.001 | ||||||
| No | 1483 | 42.5 | 105 | 24.4 | |||
| Yes | 2005 | 57.5 | 325 | 75.6 | |||
| Treatment | <0.001 | ||||||
| 1022 | 29.3 | 97 | 22.6 | ||||
| High-dose | ARA-C | (HDAC)+ | |||||
| Anthracycline +/− other agents | |||||||
| Fludarabine-based | 567 | 16.3 | 48 | 11.2 | |||
|
HDAC, no anthracycline +/− other
agents |
558 | 16.0 | 58 | 13.5 | |||
| ARA-C, not high dose | 892 | 25.6 | 179 | 41.6 | |||
| Other | 293 | 8.4 | 22 | 5.1 | |||
| ATRA based therapy (APL patients) | 156 | 4.5 | 26 | 6.1 | |||
| Prior Malignancy | <0.001 | ||||||
| No | 2746 | 78.7 | 406 | 94.4 | |||
| Yes | 741 | 21.3 | 24 | 5.6 | |||
| Prior Chemotherapy or Radiation | <0.001 | ||||||
| No | 3016 | 86.5 | 411 | 95.6 | |||
| Yes | 472 | 13.5 | 19 | 4.4 | |||
| Performance Status (PS) | 0.015 | ||||||
| 0 | 501 | 14.4 | 81 | 18.9 | |||
| 1 | 1960 | 56.3 | 249 | 58 | |||
| 2 | 694 | 19.9 | 70 | 16.3 | |||
| 3 | 214 | 6.1 | 23 | 5.4 | |||
| 4 | 111 | 3.2 | 6 | 1.4 | |||
| NPM1 | 0.481 | ||||||
| Negative | 233 | 80.1 | 17 | 73.9 | |||
| Positive | 58 | 19.9 | 6 | 26.1 | |||
| FLT3-ITD | 0.393 | ||||||
| Negative | 878 | 81.8 | 60 | 77.9 | |||
| Positive | 195 | 18.2 | 17 | 22.1 | |||
| FLT3-D835 | 0.206 | ||||||
| Negative | 1002 | 93.5 | 70 | 89.7 | |||
| Positive | 70 | 6.5 | 8 | 10.3 | |||
Age groups: Among n=3,922 total, four patients had two different AML diagnoses at two different time points; therefore, for baseline characteristics, these four patients have only been listed once, hence n=3,918 for Table 1.
Abbreviations: Cytogenetic group: CBF = core-binding factor AML [includes inv(16), t:(16;16) and t(8/21)]; APL=acute promyelocytic leukemia
Seventy-eight AYA patients were evaluated for FLT3, with mutations identified in 28%: 17 (22%) patients had internal tandem duplication 19, 8 (10%) had D835 point mutation and 3 (4%) had both FLT3-ITD and D835 abnormalities. An NPM1 mutation was identified in 6 of 23 evaluable patients (26%).
Response to treatment
A complete remission (CR) was achieved in 329 (76%) patients (including 2 with CR with incomplete recovery of platelets). The CR rates were 68/73 (93%) for CBF AYA AML, 40/51 (78%) for AYA APL, [7/8 (88%) for AYA APL treated with All-trans retinoic acid (ATRA) plus arsenic as frontline therapy] and 77/97 (78%) for AYA patients with diploid cytogenetics.
Since the focus of this analysis was on younger AML patients, for reference we also classified the patients according to the Revised MRC criteria20. The CR rates by this classification were: 110/128 (86%) in the favorable group, 144/192 (75%) in the intermediate group, 30/49 (61%) in the adverse group, and 43/63 (68%) in the unknown cytogenetics group.
During the study period, 123 (28%) AYA patients underwent stem cell transplantation (SCT) as part of their treatment, 22 of them in first complete remission (CR1) (6 favorable, 14 intermediate and 4 adverse MRC cytogenetic group). Of the 22 patients, 9 (40%) died in CR1, 3 relapsed, and 10 (45%) are still alive and in CR at a median of 80 months (range, 6 months-25 years). The median remission duration for the total population was 16 months, with 31% patients maintaining CR1 after 5 years. This includes 307 treated with chemotherapy only and 22 patients who received a SCT in CR1. The median CR duration was not reached for those receiving SCT and 14 months for those without SCT. We then compared OS among the 123 AYA patients who underwent SCT versus the 309 patients who did not undergo SCT; there was a statistically significant difference found favoring those who went to SCT (p=0.04). However, the 5-year OS rate was identical (28% for both groups).
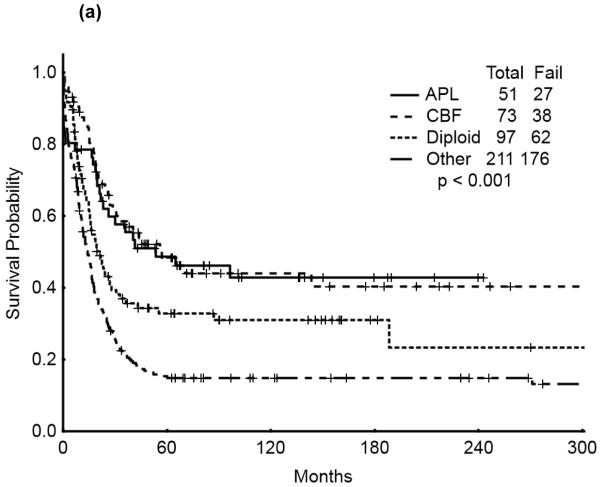
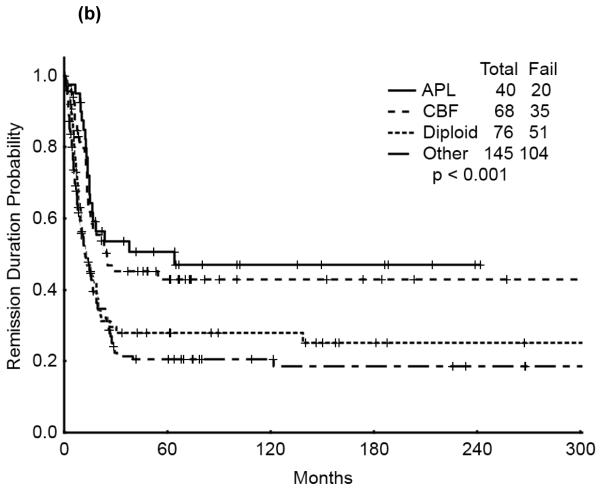
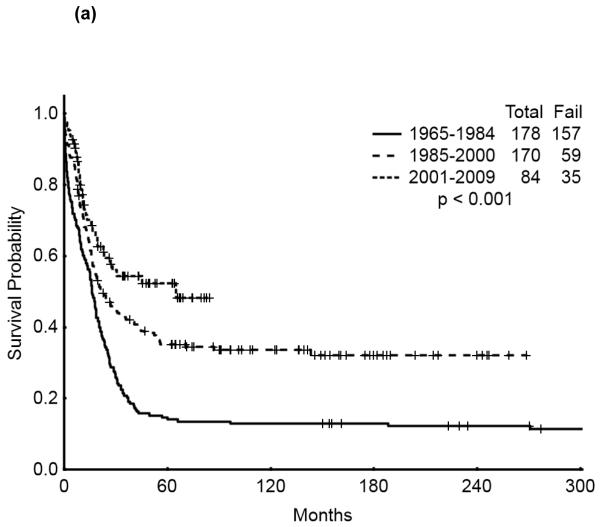
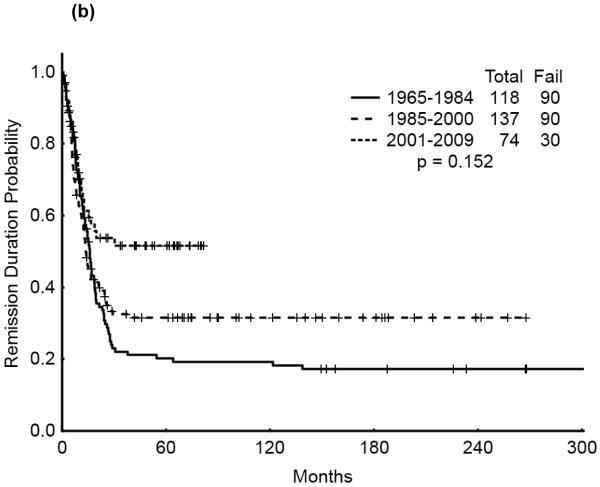
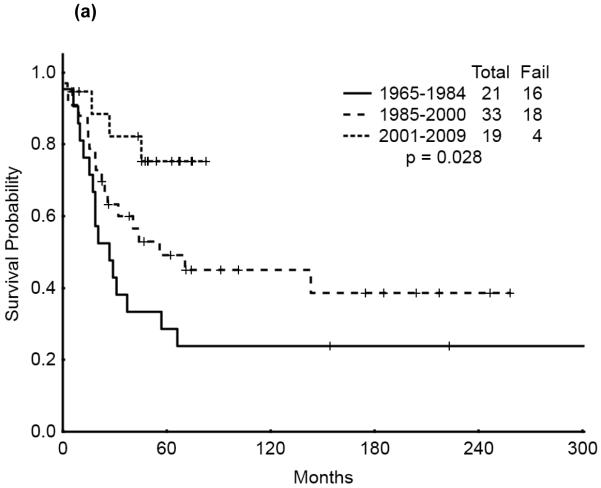
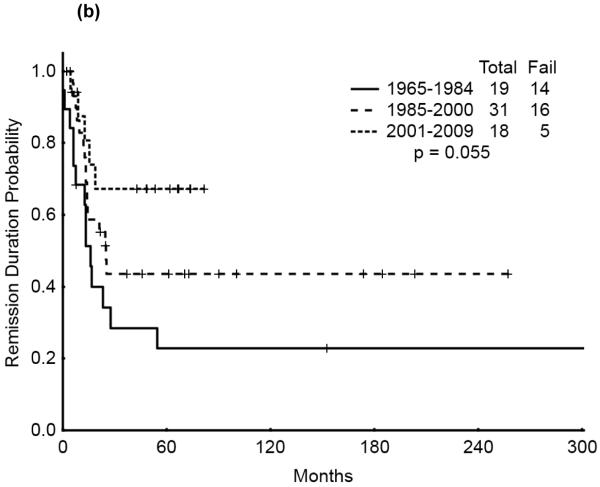
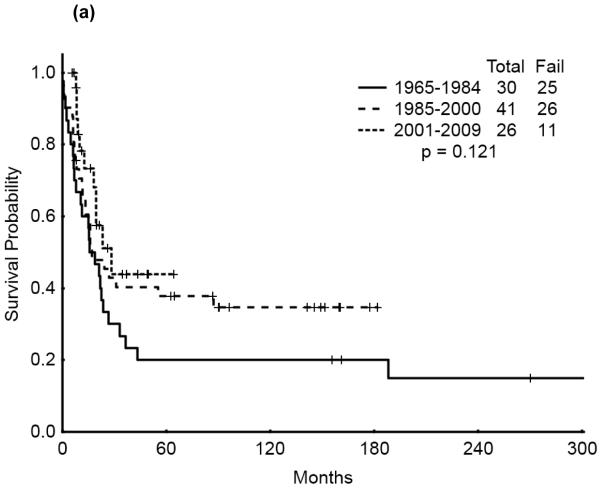
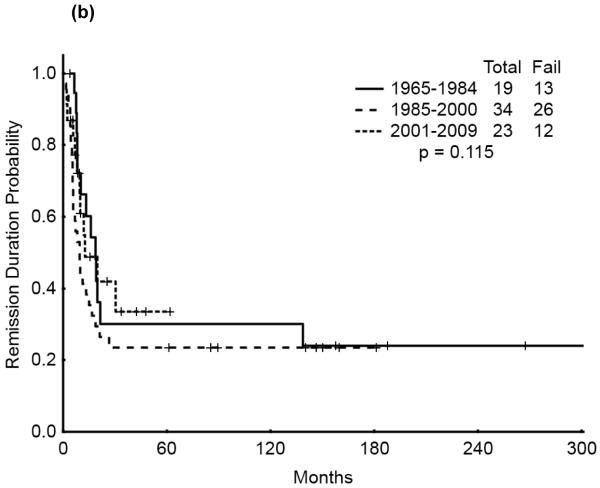
The 5-year survival probability for all AYA patients was 28% and the 5-year probability of sustained remission 31%. Long-term outcomes were better for patients with CBF-AML (5-year survival 49%, probability of sustained CR , 43%) and APL (5-year survival 47%, sustained CR, 50%) compared with diploid (5-year survival 32%, probability of sustained CR, 29%) and other AML (5-year survival 16%, sustained CR, 21%), p<0.001 (Figure 1a,b). Overall survival among AYA patients improved significantly over time with 5-year survival rates of 15%, 35%, and 53%, respectively, for the three time periods analyzed (p<0.001). Similarly, the 5-year CR duration was 20%, 32% and 52%, respectively (Figures 2a,b).
Figure 1.
(a) Overall Survival of AYA patients by cyotogenetic group
(b) Remission Duration of AYA patients by cyotogenetic group
Figure 2.
(a) Overall Survival of AYA patients by Treatment Era
(b) Remission Duration of AYA patients by Treatment Era
Table 1S (overall survival 5-year) and Table 2S (complete remission duration 5-year) of the Supplemental section contain data on distribution by cytogenetic group by era.
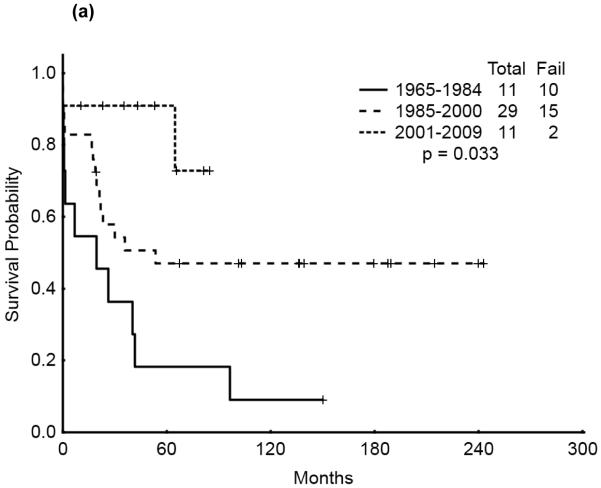
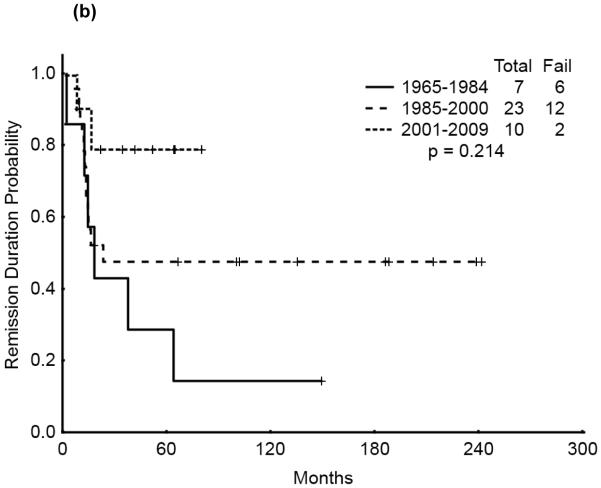
We then analyzed the outcome over time by cytogenetic group. Significant improvement in overall survival was observed among patients with CBF (p=0.028) and APL (p=0.033) but not among those with diploid cytogenetics (p=0.121). There was a similarly notable but not statistically significant trend for improvement in CR duration over time for the CBF and APL groups (p=0.055 and p=0.214, respectively). (Figures 3a & b, 4a & b, 5a & b). No discernible improvement was seen for the diploid group.
Figure 3.
(a) CBF AYA by treatment era: Overall Survival
(b) CBF AYA by treatment era: Remission Duration
Figure 4.
(a) APL AYA by treatment era: Overall Survival
(b) APL AYA by treatment era: Remission Duration
Figure 5.
(a) Diploid AYA by treatment era: Overall Survival
(b) Diploid AYA by treatment era: Remission Duration
Among the 17 AYA patients with FLT3-ITD mutation, one had APL. Among the 16 remaining non-APL patients with FLT3-ITD mutation, 11 (69%) achieved CR. One patient is alive and in remission (after SCT and in CR1) after 5 years; the other 9 relapsed and 7 died, 4 after SCT.
Comparative analysis with older patients
We then analyzed the AYA patient characteristics and outcome compared to those of patients of other age groups treated in the same time period. (Table 1) AYA patients were more likely to have higher white blood cell (WBC) count, higher bone marrow and peripheral blood blast percent, higher hemoglobin levels at baseline, more likely to have CBF AML and APL, more likely to have received SCT in 1st CR, and more commonly diagnosed in 2001-2009. CR for patients ages 16-29 years was 76% with 5-year survival of 33%; for ages 30-59 years, CR was 74% and 5-year survival 31%; for ages 60-65 years CR was 64% with 5-year survival 16%, and for patients age greater than 65 years, CR was 56% with 5-year survival of 10%.
Predictors of outcome
To define whether AYA age group was independently associated with outcome, we analyzed the characteristics associated with outcome compared to older patients. For this purpose we included 3918 (99.8%) of the 3922 patients evaluated within 4 weeks of diagnosis,(four patients had occurrence of two separate AML diagnoses during their treatment history, and were counted only once for comparative analysis).
In univariate analysis for CR, CR duration, and OS, AYA patients had significantly greater rates of all three outcome measures compared to older patients. Specifically, for the AYA group, there was an increased CR rate (76 vs. 58% for older patients; odds ratio(OR) 2.29, 95% confidence interval(CI) for OR 1.82-2.88, p-value <0.001); longer CR duration (months) (median CR duration 13.6 months [range, 0.2-405.7 months] vs. 11.5 months [range, 0-424.2 months] (p 0.003); and longer OS (median, 19.61 months vs. 10.09 months), HR 0.64 (95% CI for HR 0.57-0.73) (p <0.001).
Multivariate analysis was then performed for CR, CR duration, and OS. AYA was independently associated with improved CR rate (OR 1.67, 95% CI 1.10-2.53) (p 0.016); and longer CR duration [mean CR duration (β) = 12.65, 95% CI for β 5.04-20.26, p = 0.001]. Other factors associated with improved CR rate were: a trend towards improved CR rate with ATRA based chemotherapy (for APL patients, but not statistically significant) and no prior chemotherapy/radiation; factors associated with inferior CR rate were: prior AHD, and other non-complex cytogenetics. Other factors associated with longer CR duration were: treatment with ATRA based therapy (APL patients) and factors associated with shorter CR duration were: trend towards other non-complex cytogenetics (p-value=0.092).
For OS the multivariate analysis identified the following factors to be independently predictive of inferior outcome: unfavorable cytogenetic abnormalities (−5/−7(HR 2.42, p-value <0.001) and other complex cytogenetics (HR 1.37, p-value =0.001)); prior chemotherapy (HR 1.54, p-value <0.001), FLT3-ITD positivity (HR 1.75, p-value <0.001) and chemotherapy treatment of HDAC+ no anthracycline +/− other agents, (HR 1.36, p-value =0.010) and “other” chemotherapies (HR 2.25, p-value <0.001), as compared to HDAC + anthracycline. There was a trend towards improved OS for the AYA group, but this was not statistically significant (HR 0.74, 95% CI for HR 0.53-1.04, p-value 0.085). Table 2 (2a, 2b) contains data for the univariate and multivariate analyses for the overall group.
| Table 2a. Univariate Analysis, Overall group | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| CR | CR duration | OS | ||||
|
| ||||||
| Variable | OR |
p- value |
Median (mo) |
p- value |
HR |
p- value |
|
| ||||||
| Age, years | ||||||
| ≥30 | 11.5 | |||||
| 16-29 | 2.29 | <0.001 | 13.6 | 0.003 | 0.64 | <0.001 |
|
| ||||||
|
Prior
chemo/Radiation |
12.25 | |||||
| No | 0.52 | <0.001 | 8.45 | 0.012 | 1.48 | <0.001 |
| Yes | ||||||
|
| ||||||
| Treatment | ||||||
| HDAC+Anthracycline | 10.25 | |||||
| Fludarabine-based | 0.94 | 0.562 | 11.1 | 1.08 | 0.190 | |
|
HDAC, no
anthracycline |
0.80 | 0.031 | 10.1 | 1.20 | <0.001 | |
|
ARAC, not high
dose |
0.76 | 0.002 | 12.2 | 1.22 | <0.001 | |
| Other | 0.25 | <0.001 | 9.80 | 2.03 | <0.001 | |
|
ATRA based
therapy- APL patients |
2.43 | <0.001 | 57.05 | <0.001 | 0.29 | <0.001 |
|
| ||||||
| Cytogenetic group | ||||||
| Diploid | 12.0 | |||||
| −5/−7 | 0.32 | <0.001 | 5.7 | 2.14 | <0.001 | |
| Other non-complex | 1.20 | 0.038 | 18.50 | 0.75 | <0.001 | |
| Other complex | 0.70 | <0.001 | 8.90 | <0.001 | 1.25 | <0.001 |
|
| ||||||
| SCT | ||||||
| No | 1.00 | 11.80 | ||||
| Yes | 8.26 | <0.001 | 17.20 | 0.278 | 0.45 | <0.001 |
| b. Multivariate Analysis, Overall Group | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| CR | CR duration | OS | ||||
|
| ||||||
| Variable | HR |
p- value |
β |
p- value |
HR |
p- value |
|
| ||||||
| Age, years | ||||||
| ≥30 | ||||||
| 16-29 | 1.67 | 0.016 | 12.65 | 0.001 | 0.74 | 0.085 |
|
| ||||||
|
Prior
chemo/Radiation |
||||||
| No | 0.46 | 0.005 | −2.69 | 0.530 | 1.54 | <0.001 |
| Yes | ||||||
|
| ||||||
| Treatment | ||||||
| HDAC+Anthracycline | ||||||
| Fludarabine-based | 0.92 | 0.607 | 15.44 | 0.001 | 1.23 | 0.135 |
|
HDAC, no
anthracycline |
0.71 | 0.041 | 10.87 | 0.094 | 1.36 | 0.010 |
|
ARAC, not high
dose |
0.39 | <0.001 | 6.04 | 0.020 | 0.88 | 0.304 |
| Other | 0.28 | <0.001 | 6.58 | 0.201 | 2.25 | <0.001 |
|
ATRA based
therapy- APL patients |
1.33 | 0.639 | 58.28 | <0.001 | 0.28 | 0.002 |
|
| ||||||
| Cytogenetic group | ||||||
| Diploid | ||||||
| −5/−7 | 0.37 | 0.125 | −4.78 | 0.157 | 2.42 | <0.001 |
| Other non-complex | 0.41 | 0.045 | −8.04 | 0.092 | 0.49 | <0.001 |
| Other complex | 0.35 | 0.387 | 1.29 | 0.646 | 1.37 | <0.001 |
|
| ||||||
| SCT | ||||||
| No | ||||||
| Yes | 8.88 | 3.76 | 7.61 | 2.83 | 0.37 | 0.26 |
To account for the higher incidence of adverse chromosomal abnormalities in older patients, we then performed a subset analysis of only patients with diploid cytogenetics. Among 1,231 patients of all ages who exhibited diploid cytogenetics, 97 (8%) were AYA. In the univariate analysis, CR and OS were significantly better for AYA (n= 97) as compared to the older patients (n= 1,134) with diploid cytogenetics, but no significant difference was observed with CR duration. By multivariate analysis, there was a trend in favor of the AYA group for CR (p = 0.07) and OS (p = 0.052). Table 3S and 4S of the supplemental section contain the univariate and multivariate analysis for the diploid cytogenetics group.
Discussion
Although AML is typically a disease of older patients, (median age 65-70 years) it still accounts for 15-20% of childhood leukemias 21 and 33% of adolescent leukemias 22-24. In this single-institution analysis the outcome of AYA patients with AML was significantly better than for older adults with AML, with improved CR and CR duration rates, and a trend towards improved overall survival., There has also been an improvement of outcomes over time for AYA patients with AML, likely the result of a variety of factors including improvements in supportive care, intensity of treatments, and application of specific, targeted therapies to certain subsets of patients (e.g. APL).
There is growing recognition that AYA patients with cancer may represent a unique cohort with special needs25. This “in-between” age group has age-specific pharmacological26, toxicity profile27, psychosocial28, adherence29, fertility30, socio-economic31, access to and the cost of healthcare32 and palliative care needs33 that may set it apart from other age groups of patients dealing with cancer. In leukemias, more is known about the incidence and outcomes in AYAs with ALL, as this malignancy occurs more frequently in younger age groups than its myeloid counterparts9,34. Notably, for both solid and hematologic cancer patients, the AYA population has been underrepresented in clinical trials35,36 Access to and availability of new therapeutic options can also be improved for these young patients.
In response to the growing awareness of AYA oncology patients, the National Comprehensive Cancer Network (NCCN) has recently published AYA oncology guidelines37. The age range that defines AYA has varied; the NCCN has set age range for its current guidelines to include ages 15-39 as AYA but others have used other age ranges including 15-29 years 38,39. In this analysis we focused on patients age 16-29 as this age group has been used extensively in the AYA literature 34,38.
The effect of age on outcome of patients with AML has been extensively studied, but most analyses have focused on the older age groups. Appelbaum and colleagues analyzed 968 adults with AML and demonstrated that outcomes were significantly worse for older versus younger patients, arguing for age-specific assessments in the therapeutic evaluation of AML patients3. The recently proposed cytogenetic classification for younger patients with AML includes patients younger than 60 years 20.
Some population series have analyzed the outcome of AYA patients with AML. Based on analysis of the SEER database, Pulte and colleagues demonstrated increasing relative survival rates in younger AML patients and continued improved expected survival rates in this younger population40. In this study, the authors devised a validation model to project relative overall survival in AML patients in the United States during 2006-2010, with 5-year relative survival estimates of 21.4% and 10-year relative survival estimates of 18.7% for all ages; among those ages 25-34 years, the 5- and 10-year relative survival rates were 62.2% and 57.4%, respectively. In another epidemiological study, Pulte and coauthors13 examined the relative survival of AYA with hematologic malignancies, including AML patients, in the United States (based on SEER data). This analysis demonstrated that survival improved over time compared to prior treatment eras in all five hematologic malignancies reviewed (Hodgkin’s lymphoma, Non-Hodgkin’s lymphoma, ALL, AML, chronic myeloid leukemia (CML). Specifically, AYA patients with AML (ages 15-24) experienced an improvement in 10-year relative survival from 15.2% in 1981-1985 to 45.1% in 2001-2005. The authors concluded that even with these improvements, survival levels had stabilized at relatively low levels 13.
Another population-based Swedish study, published by Derolf and colleagues1, analyzed data during time period 1973-2005. It demonstrated an improved survival among almost every age group over time. The progressive increase in outcomes was attributed to a variety of factors including more intensified chemotherapy, improvement in supportive care and transfusions, and improvements in allogeneic SCT. Still the authors remarked that although AML survival has improved over time for all age groups, the majority of patients still die of their AML and that age continues to persist as an important prognostic predictor1.
The outcome of AYA patients (ages 16-24) in the AML10 and AML 12 trials conducted by the MRC showed 10-year OS of 47%, relapse rate of 47%, 14% deaths in CR, with 61% patients considered good-risk (MRC AML 10, 12 trials)12. In a Japanese study of greater than 1000 patients with AML age 1-29 years, the 7-year EFS was 32% among patients ages 15-1941,42. Similar findings were found by other groups , again concluding that even among young patients, increasing age still confers an unfavorable prognostic factor in AML patients22(38)43. Among all age groups, there are many factors that have led to the improvement in outcome of AML patients, including better supportive care, the availability of clinical trials, and the incorporation of targeted therapies, especially for many high risk sub-groups 44,45.The reasons in particular for some of the improved outcomes among AYA patients with AML as compared to their older counterparts over time likely includes a multifactorial explanation, encompassing both patient/host and AML disease biology. In terms of host biology, some of these factors include the overall likelihood of the AYA patient to better tolerate AML chemotherapy and for AYA patients to be better suited for more dose intense regimens. Other host factors include that most AYA patients have less co-morbid conditions at baseline and are taking less concomitant medications (therefore resulting in less drug-drug interactions and toxicities) as compared to their older counterparts. In terms of disease biology,,AYA patients tend to have lower incidence of abnormal/complex cytogenetics and reduced occurrence of secondary/therapy-related AML as compared to older AML patients.
In our study, among patients treated in an adult setting with adult-type chemotherapy, we demonstrate significantly better outcomes of AYA patients over time with CR duration reaching 81% and overall survival 53% in the most recent decade analyzed. In our series the most important improvements occurred in the CBF and APL groups, where 5-year survival rates are 49% and 47%, respectively. Patients with diploid cytogenetics lag behind significantly with rate of only 32%, and even worse for those with adverse/other cytogenetic features, with rate of only 16%; one possible explanation for this observation includes the recent elucidation of poor prognostic molecular mutations (e.g. FLT3-ITD, DNMT3a) that transform an intermediate-risk patient to high/poor risk status. Clearly better therapies, including targeted therapies aimed at particular molecular mutations, are needed in AYA patients with these features.
On multivariate analysis modeling, AYA age group was significantly associated with increased CR rate and CR duration, and a trend towards survival improvement (p-value 0.085).
One limitation of the current study is the lack of molecular data on all patients analyzed, due to the historical nature of our analysis, when molecular abnormalities well recognized today had not yet been identified. Several studies have demonstrated the poorer prognosis in FLT3-ITD AML is sustained even in AYA patients46-48. In our study, among the 78 AYA patients evaluated for FLT3 mutations, 28% had FLT3 mutations. Dohner and colleagues 49, recently reported that mutant NPM1 confers a favorable prognosis in AML AYA patients, particularly when in the absence of any concomitant FLT3-ITD 21,49. Since FLT3 and other molecular makers have only been widely available and part of the AML diagnostic workup over the most recent time period studied (2000-2009)50, our analysis focused on cytogenetic subgrouping, as this was available on the majority of patients included in the study period. Future studies will further define the impact of molecular characterization in the AYA population.
In conclusion, patients with AYA constitute a unique subset of patients with AML with an improved prognosis compared to other age groups. However, despite the notable improvements in outcomes over time, there is still significant need for improvement in this patient population, including greater emphasis on availability, access to, and enrollment on clinical trials.
Supplementary Material
Table 1S: Overall Survival- Distribution by Cytogenetic Group by Treatment Era for AML AYA group
Table 2S: Complete Remission Duration-- Distribution by Cytogenetic Group by Treatment Era AML AYA group
Table 3S: Univariate Analysis, Diploid cytogenetics group
Table 4S: Multivariate Analysis, Diploid cytogenetic group
Clinical Practice Points.
Adolescents and young adults (AYA) with cancer represent a potentially vulnerable subgroup of patients with unique features and diverse needs
Little is known about characteristics and outcomes of AYA patients with leukemia outside of the field of acute lymphoblastic leukemia (ALL), particularly for AYA AML patients
On multivariate analysis, AYA AML age group was independently associated with improved CR rate and CR duration, with a trend for longer OS (p-value=0.085)
Outcomes for AYA AML patients have improved over time, as compared to older adults with AML. Despite improvements in treatments and outcomes over time, there is still need for improvement in AYA with AML particularly for those with AML other than core-binding factor (CBF) AML and acute promyelocytic leukemia (APL)
Microabstract.
Little is known about outcomes of AML in adolescents and young adults (AYA), a unique subgroup of AML patients. We retrospectively analyzed all AML patients (n=3,922) treated at our institution from 1965 to 2009 aged 16 to 29 years and found 432 (11%) AYA AML. Over time, outcomes for AYA AML patients have improved compared to older adults with AML.
Acknowledgements
This research is supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship contributions: NP and JC designed the study. NP and JC wrote the manuscript. GNG, XH, SP provided statistical support and data analysis. NP, HK, FR, SO, WW, DT, GGM, GB, SV, JC evaluated and treated the patients that were part of the study. All authors reviewed and approved the final manuscript prior to publication.
Disclosures/conflicts of interest: The authors have no conflicts of interest to disclose with regards to this manuscript.
References
- 1.Derolf AR, Kristinsson SY, Andersson TM, Landgren O, Dickman PW, Bjorkholm M. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009;113:3666–72. doi: 10.1182/blood-2008-09-179341. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravandi F, Burnett AK, Agura ED, Kantarjian HM. Progress in the treatment of acute myeloid leukemia. Cancer. 2007;110:1900–10. doi: 10.1002/cncr.23000. [DOI] [PubMed] [Google Scholar]
- 5.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–94. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 6.Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nature reviews. 2008;8:288–98. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt C. Lack of progress in teen and young adult cancers concerns researchers, prompts study. Journal of the National Cancer Institute. 2006;98:1760–3. doi: 10.1093/jnci/djj517. [DOI] [PubMed] [Google Scholar]
- 8.Bleyer A, Siegel SE, Coccia PF, Stock W, Seibel NL. Children, adolescents, and young adults with leukemia: the empty half of the glass is growing. J Clin Oncol. 2012;30:4037–8. doi: 10.1200/JCO.2012.44.7466. author reply 8-9. [DOI] [PubMed] [Google Scholar]
- 9.Wood WA, Lee SJ. Malignant hematologic diseases in adolescents and young adults. Blood. 2011;117:5803–15. doi: 10.1182/blood-2010-12-283093. [DOI] [PubMed] [Google Scholar]
- 10.Ofran Y, Rowe JM. Acute myeloid leukemia in adolescents and young adults: challenging aspects. Acta Haematol. 2014;132:292–7. doi: 10.1159/000360200. [DOI] [PubMed] [Google Scholar]
- 11.Stock W. Adolescents and young adults with acute lymphoblastic leukemia. Hematology / the Education Program of the American Society of Hematology American Society of Hematology. 2010:21–9. doi: 10.1182/asheducation-2010.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Advani AS, Hunger SP, Burnett AK. Acute leukemia in adolescents and young adults. Semin Oncol. 2009;36:213–26. doi: 10.1053/j.seminoncol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Pulte D, Gondos A, Brenner H. Trends in survival after diagnosis with hematologic malignancy in adolescence or young adulthood in the United States, 1981-2005. Cancer. 2009;115:4973–9. doi: 10.1002/cncr.24548. [DOI] [PubMed] [Google Scholar]
- 14.Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–11. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- 15.Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120:3187–205. doi: 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- 16.Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Hematol Oncol Clin North Am. 2010;24:35–63. doi: 10.1016/j.hoc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Kaspers GJ, Zwaan CM. Pediatric acute myeloid leukemia: towards high-quality cure of all patients. Haematologica. 2007;92:1519–32. doi: 10.3324/haematol.11203. [DOI] [PubMed] [Google Scholar]
- 18.Daver N, Liu Dumlao T, Ravandi F, et al. Effect of NPM1 and FLT3 Mutations on the Outcomes of Elderly Patients With Acute Myeloid Leukemia Receiving Standard Chemotherapy. Clin Lymphoma Myeloma Leuk. 2013;13:435–40. doi: 10.1016/j.clml.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uitdehaag JC, Verkaar F, Alwan H, de Man J, Buijsman RC, Zaman GJ. A guide to picking the most selective kinase inhibitor tool compounds for pharmacological validation of drug targets. Br J Pharmacol. 2012;166:858–76. doi: 10.1111/j.1476-5381.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 21.Brown P, McIntyre E, Rau R, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007;110:979–85. doi: 10.1182/blood-2007-02-076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creutzig U, Buchner T, Sauerland MC, et al. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer. 2008;112:562–71. doi: 10.1002/cncr.23220. [DOI] [PubMed] [Google Scholar]
- 23.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 24.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 25.Morgan S, Davies S, Palmer S, Plaster M. Sex, drugs, and rock 'n' roll: caring for adolescents and young adults with cancer. J Clin Oncol. 2010;28:4825–30. doi: 10.1200/JCO.2009.22.5474. [DOI] [PubMed] [Google Scholar]
- 26.Veal GJ, Hartford CM, Stewart CF. Clinical pharmacology in the adolescent oncology patient. J Clin Oncol. 2010;28:4790–9. doi: 10.1200/JCO.2010.28.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gramatges MM, Rabin KR. The adolescent and young adult with cancer: state of the art-- acute leukemias. Current oncology reports. 2013;15:317–24. doi: 10.1007/s11912-013-0325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treadgold CL, Kuperberg A. Been there, done that, wrote the blog: the choices and challenges of supporting adolescents and young adults with cancer. J Clin Oncol. 2010;28:4842–9. doi: 10.1200/JCO.2009.23.0516. [DOI] [PubMed] [Google Scholar]
- 29.Butow P, Palmer S, Pai A, Goodenough B, Luckett T, King M. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4800–9. doi: 10.1200/JCO.2009.22.2802. [DOI] [PubMed] [Google Scholar]
- 30.Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4831–41. doi: 10.1200/JCO.2009.22.8312. [DOI] [PubMed] [Google Scholar]
- 31.Kent EE, Sender LS, Morris RA, et al. Multilevel socioeconomic effects on quality of life in adolescent and young adult survivors of leukemia and lymphoma. Qual Life Res. 2012 doi: 10.1007/s11136-012-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pemmaraju N, Cortes J. Chronic myeloid leukemia in adolescents and young adults: patient characteristics, outcomes and review of the literature. Acta haematologica. 2014;132:298–306. doi: 10.1159/000363434. [DOI] [PubMed] [Google Scholar]
- 33.Wein S, Pery S, Zer A. Role of palliative care in adolescent and young adult oncology. J Clin Oncol. 2010;28:4819–24. doi: 10.1200/JCO.2009.22.4543. [DOI] [PubMed] [Google Scholar]
- 34.Pemmaraju N, Kantarjian H, Shan J, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012;97:1029–35. doi: 10.3324/haematol.2011.056721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrari A, Montello M, Budd T, Bleyer A. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer. 2008;50:1101–4. doi: 10.1002/pbc.21459. [DOI] [PubMed] [Google Scholar]
- 36.Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110:2385–93. doi: 10.1002/cncr.23060. [DOI] [PubMed] [Google Scholar]
- 37.Coccia PF, Pappo AS, Altman J, et al. Adolescent and young adult oncology, version 2.2014. J Natl Compr Canc Netw. 2014;12:21–32. doi: 10.6004/jnccn.2014.0004. quiz. [DOI] [PubMed] [Google Scholar]
- 38.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107:1645–55. doi: 10.1002/cncr.22102. [DOI] [PubMed] [Google Scholar]
- 39.Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist. 2006;11:590–601. doi: 10.1634/theoncologist.11-6-590. [DOI] [PubMed] [Google Scholar]
- 40.Pulte D, Gondos A, Brenner H. Expected long-term survival of patients diagnosed with acute myeloblastic leukemia during 2006-2010. Ann Oncol. 21:335–41. doi: 10.1093/annonc/mdp309. [DOI] [PubMed] [Google Scholar]
- 41.Horibe K, Tsukimoto I, Ohno R. Clinicopathologic characteristics of leukemia in Japanese children and young adults. Leukemia. 2001;15:1256–61. doi: 10.1038/sj.leu.2402194. [DOI] [PubMed] [Google Scholar]
- 42.Canner J, Alonzo TA, Franklin J, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children's Oncology Group. Cancer. 2013;119:4162–9. doi: 10.1002/cncr.28342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods WG, Franklin AR, Alonzo TA, et al. Outcome of adolescents and young adults with acute myeloid leukemia treated on COG trials compared to CALGB and SWOG trials. Cancer. 2013;119:4170–9. doi: 10.1002/cncr.28344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi K, Kantarjian H, Pemmaraju N, et al. Salvage therapy using FLT3 inhibitors may improve long-term outcome of relapsed or refractory AML in patients with FLT3-ITD. Br J Haematol. 2013;161:659–66. doi: 10.1111/bjh.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pemmaraju N, Kantarjian H, Garcia-Manero G, et al. Improving outcomes for patients with acute myeloid leukemia in first relapse: a single center experience. Am J Hematol. 2015;90:27–30. doi: 10.1002/ajh.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Duhier FM, Goodeve AC, Wilson GA, et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol. 2000;111:190–5. doi: 10.1046/j.1365-2141.2000.02317.x. [DOI] [PubMed] [Google Scholar]
- 47.Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–80. [PubMed] [Google Scholar]
- 48.Rombouts WJ, Blokland I, Lowenberg B, Ploemacher RE. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the Flt3 gene. Leukemia. 2000;14:675–83. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- 49.Dohner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–6. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 50.Pemmaraju N, Kantarjian H, Andreeff M, Cortes J, Ravandi F. Investigational FMS-like tyrosine kinase 3 inhibitors in treatment of acute myeloid leukemia. Expert Opin Investig Drugs. 2014 doi: 10.1517/13543784.2014.911839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1S: Overall Survival- Distribution by Cytogenetic Group by Treatment Era for AML AYA group
Table 2S: Complete Remission Duration-- Distribution by Cytogenetic Group by Treatment Era AML AYA group
Table 3S: Univariate Analysis, Diploid cytogenetics group
Table 4S: Multivariate Analysis, Diploid cytogenetic group