Abstract
Background and Purpose
In acute stroke patients the intensity of a fluid attenuated inversion recovery (FLAIR) lesion in the region of diffusion restriction is associated with time from symptom onset. We hypothesized that collateral status, as assessed by the hypoperfusion intensity ratio (HIR) could modify the association between time from stroke onset and FLAIR lesion intensity.
Methods
From the ‘AX200 for ischemic stroke trial’ 141 patients had appropriate FLAIR, diffusion (DWI) and perfusion (PWI) images. In the region of non-reperfused core we calculated voxel based relative FLAIR signal intensity (rFLAIR SI). The HIR was defined as the ratio of the Tmax >10 s lesion over the Tmax > 6 s lesion volume. A HIR-threshold of ≤ 0.4 was used to dichotomize good versus poor collaterals. We studied the interaction between collateral status on the association between time from symptom onset and FLAIR intensity.
Results
Time from symptom onset was associated with the relative FLAIR intensity in the region of non-reperfused core (B=1.05; 95%CI: 1.0-1.1). We identified an interaction between this association and collateral status; an association was present between time and rFLAIR intensity in patients with poor collaterals (r = 0.53), but absent in patients with good collaterals (r = 0.17, p=0.04).
Conclusions
Our findings show that the relationship between time from symptom onset and rFLAIR lesion intensity depends on collateral status. In patients with good collaterals the development of a rFLAIR positive lesion is less dependent on time from symptom onset compared to patients with poor collaterals.
Keywords: collateral circulation, diffusion-weighted imaging, perfusion imaging, pathophysiology, fluid attenuation inversion recovery imaging
Introduction
In 10-25% of acute stroke patients the exact time from symptom onset cannot be obtained.1–5 Most commonly these patients wake up with stroke symptoms or they are aphasic, hampering the ability to inform others on the exact onset time.
When duration of stroke symptoms is uncertain multiparametric magnetic resonance imaging (MRI) can be used to estimate the age of the lesion.6,7 Minutes after stroke onset cytotoxic edema can already develop causing a net decrease in water diffusion as visualized by an increased signal on diffusion-weighted imaging (DWI). Hours after stroke onset vasogenic edema gradually appears, causing a visible hyperintensity on fluid attenuated inversion recovery (FLAIR) imaging.8–10
Therefore in the first hours after stroke onset the difference in signal intensities between DWI and FLAIR, the DWI/FLAIR mismatch, is proposed as a predictor for time from symptom onset before 4.5h, the time-window for thrombolysis.11–14 The sensitivity and specificity of the DWI/FLAIR mismatch vary between studies with sensitivity ranging between 40 and 80% and specificity between 78 and 89%.7 These differences can be explained by various factors, e.g. variation in imaging techniques used to assess the mismatch or size of the diffusion lesions11, but other pathophysiological variables influencing the intensity of the FLAIR lesion have not been extensively studied. Collateral circulation plays an important role in timely progression of infarcted tissue and response to reperfusion.15,16 Digital subtraction angiography remains the gold standard for collateral grading17, but non-invasive methods have been developed using computed tomography angiography (CTA), dynamic susceptibility contrast-enhanced magnetic resonance perfusion (DSC) and arterial spin labeling (ASL).18–20 Recently the severity of perfusion-weighted imaging (PWI) abnormalities, defined by the hypoperfusion intensity ratio (HIR), was shown to be a good predictor of poor collaterals.21 We hypothesized that collateral status, as assessed by HIR, could modify the association between time from stroke onset and FLAIR lesion intensity.
Materials and Methods
Clinical and neuroimaging data from the ‘AX200 for ischemic stroke’ trial (AXIS 2) were used.22 This was a large Phase IIb, multicenter, placebo-controlled, randomized and double-blinded trial. The clinical efficacy of recombinant Granulocyte Colony Stimulating Factor (rhG-CSF, AX200) in acute ischemic stroke patients was tested.
G-CSF failed to meet the primary and secondary endpoints of the trial. The full methodology of the trial has been described previously.22 Patients were included in a time window of ≤9h after the patient was last seen normal. MRI (FLAIR, T2, T2*, DWI, time-of-flight magnetic resonance angiography, PWI) was mandatory before inclusion. A minimum DWI lesion size of 15 mL was required. Prior treatment with intravenous tissue plasminogen activator (IV tPA) was allowed if patients fulfilled all criteria to be eligible for thrombolysis. The main exclusion criteria were signs of severe stroke on imaging (carotid T occlusion, ischemic lesions larger than two-thirds of the middle cerebral artery territory, signs of midline shift), hemorrhagic, and lacunar strokes.
For this study we only included patients in whom PWI, DWI and FLAIR lesion volumes could be obtained. Additionally a perfusion deficit within a DWI lesion needed to be present for this analysis of the non-reperfused core. Quantitative relative FLAIR maps (rFLAIR) were calculated in a voxel-based manner using in house developed software. For each voxel the relative signal intensity (rSI) was determined by the ratio of the signal intensity in that voxel and the median of the signal intensity in a sphere with radius 15 mm positioned around the homologue voxel in the other hemisphere. The midplane used for mirroring every voxel to the contralateral hemisphere was semi-automatically defined. To achieve accurate mirroring, points were manually chosen and were used to define the three-dimensional midplane. A secondary analysis was done with a radius of 10 and 20 mm to investigate if the chosen radius would influence our results (Supplementary figure I).
RAPID software23 was used to calculate PWI and DWI lesion volumes. Tmax (time to the maximum of the residue function obtained by deconvolution) was used to define regions of hypoperfusion. The irreversibly injured tissue (‘core’) was estimated based on an apparent diffusion coefficient of less than 620 × 10-6 mm2/s. Artifacts were manually removed blinded to clinical data. The non-reperfused core (DWI lesion volume within a region of Tmax > 6s perfusion deficit) was selected as the region of interest to study the pathophysiology of FLAIR signal intensity and calculate the mean FLAIR rSI (Figure 1). We compared our results with a more commonly used non-voxelbased technique to determine the mean FLAIR rSI in the non-reperfused core. First, this region was mirrored to the contralateral hemisphere. Second, the relative signal intensity of the ROI was defined by the ratio of the mean signal intensity of the ROI over the mean signal intensity of the mirrored ROI. Patients with white matter lesions in more than 1/3 of the contralateral ROI, as visually assessed by an individual rater (A.W.) blinded to the clinical information, were excluded from the analysis. Collateral status was assessed by using the hypoperfusion intensity ratio (HIR) which is defined as the proportion of the Tmax >6s lesion volume with Tmax >10s delay. Good and poor collateral circulation were dichotomized based on the predefined threshold of 0.421, with poor collateral status defined by a HIR>0.4.
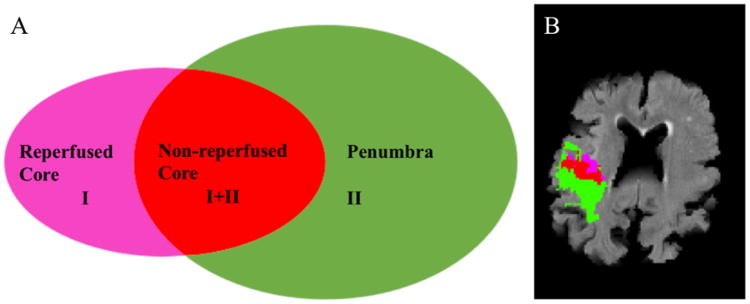
Figure 1.
Regions of interest.
A. Presentation of the different regions of interest.
I = region of ADC < 620 mm2/s.
II = region of TMax > 6s.
Green: region of TMax >6s without diffusion restriction = Penumbra
Red: region of diffusion restriction with TMax > 6s = Non-reperfused core
Pink: the area of diffusion restriction without hypoperfusion = Reperfused core
B. 82 year old patient with right-sided stroke. The image shows the different regions of interest.
We assessed the interaction between collateral status and the association between time from symptom onset and FLAIR intensity in a linear regression model with rFLAIR as dependent variable. As additional variables age, sex, NIHSS score, DWI lesion volume and arterial occlusion site were tested in univariate linear regression. We postulated variables with an α < 0.1 to be included in the multivariate model. Collateral status (dichotomized in poor versus good collaterals defined by the HIR), time from symptom onset and an interaction term where included as independent variables. In a second approach the collateral status in the regression model defined by the HIR was replaced by the mean Tmax of the whole region of hypoperfusion, assuming higher values to be associated with poorer collateral status.
The qualitative DWI/FLAIR mismatch was visually rated by two readers (A.W., V.T.) blinded to clinical information as previously described.24 The Youden Index defined the most optimal threshold of rFLAIR to predict symptom onset before 4.5h. We compared the sensitivity and specificity for symptom onset before 4.5h between rFLAIR and the qualitative DWI/FLAIR mismatch.
Clinical characteristics between different groups were compared using the Mann-Whitney-U test for continuous variables and a Chi-Square test for categorical variables. A value of p < 0.05 was considered significant. Statistical testing was performed with IBM SPSS Statistics software version 22.
Results
Patient population and clinical characteristics
From the original 323 patients of the AXIS 2 trial, in 206 (63.8 %) all imaging sequences were available and of sufficient quality for analysis. Furthermore, 12 patients (5.2 %) were excluded because of confluent or large FLAIR lesions in the contralateral hemisphere or leukoaraiosis overlapping the acute lesion; in 53 (25.7%) patients the core was reperfused and could therefore not be analyzed. Here we report the results of the total of 141 included patients. The interobserver reliability for visual DWI/FLAIR mismatch was good (kappa=0.70). Collateral status, assessed by the HIR, was poor in 87 patients (61.7%) and good in 54 patients (38.3%). Patients with poor collaterals had more severe stroke symptoms at baseline as determined by the National Institute Stroke Scale (NIHSS 14 vs NIHSS 11, p= 0.01), larger DWI lesion volumes (47.2 vs 14.6 ml, p= < 0.01) and larger perfusion (TMax > 6s) volumes (91.5 vs 45.8 ml, p= 0.01). The rate of excellent functional outcome at 90 days, Modified Rankin Scale (mRS) 0-1, was higher in patients with good collaterals (31%) vs patients with poor collaterals (17%; p= 0.05; Table 1).
Table 1.
Characteristics comparison by collateral quality.
| Good Collaterals (n=54) | Poor collaterals (n=87) | P-value | ||
|---|---|---|---|---|
| Age (y) | 68 (61.8-77) | 72 (62-76) | 0.40 | |
| Gender (f) | 27 (50.0%) | 39 (44.8%) | 0.60 | |
| Time from symptom onset (min) | 326.5 (258.3-413) | 317 (229-381) | 0.10 | |
| IV tPA | 34 (63.0%) | 56 (64.4%) | 0.90 | |
| NIHSS | 11 (8-14.3) | 14 (10-18) | 0.01 | |
| DWI volume (ml) | 14.6 (8.6-26.3) | 47.2 (24.6-87.8) | < 0.01 | |
| Tmax >6s volume (ml) | 45.8 (24.2-99.9) | 91.5 (42.2-146.4) | 0.01 | |
| DWI/FLAIR mismatch | 19 (35.2%) | 41 (47.1%) | 0.20 | |
| rFLAIR | 1.15 (1.11-1.23) | 1.14 (1.09-1.22) | 0.20 | |
| mRS D90 (0-1) | 17 (31.0%) | 15 (17.0%) | 0.05 |
Data are median (IQR) or n (%). Collateral grading is based on Hypoperfusion Intensity Ratio (HIR). HIR >0.40 represents poor collateral circulation. Group comparison was done by Chi Square test for categorical variables and Mann-Withney-U for continuous variables. IV tPA = intravenous tissue plasminogen activator, NIHSS= National Institute Stroke Scale, DWI = Diffusion Weighted Imaging, FLAIR= Fluid Attenuated Inversion Imaging, rFLAIR = relative FLAIR calculated with the voxel-based method (sphere diameter 15 mm), mRS = Modified Rankin Scale.
Comparison of quantitative versus visual DWI/FLAIR mismatch
The correlation between rFLAIR and time from symptom onset was moderate (r=0.4, p<0.01). The optimal threshold of rFLAIR to predict symptom onset before 4.5h was 1.16. Using the threshold of 1.16, rFLAIR had a sensitivity of 88% (95%CI 75-95%) and a specificity of 57% (95%CI 46-67%) to predict symptom onset before 4.5h. The visual DWI/FLAIR mismatch had a sensitivity of 66% (95%CI 51-78%) and a specificity of 70% (95% CI 60-79%) to predict symptom onset before 4.5h. The Pearson Correlation coefficient between the quantitative and visual mismatch was 0.5.
Effect of collateral status on the association between time from symptom onset and FLAIR intensity
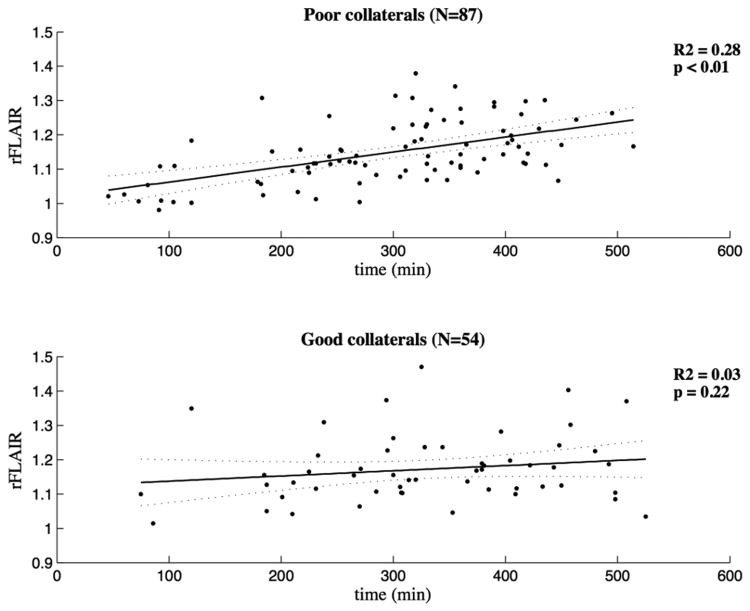
The predictive value of time for rFLAIR intensity was moderate in patients with poor collateral circulation (R2 = 0.28), but poor in patients with a good collateral circulation (R2 = 0.03) (Figure 2). From all other variables only the DWI lesion volume was associated with rFLAIR in univariate linear regression analysis (p = 0.04), but was excluded from the model because of its poor explanatory value (R2 = 0.03).
Figure 2.
Linear regression model (HIR).
Linear regression lines are presented and accompanied by the 95% confidence interval. The upper panel represents the group with poor collateral circulation (n=87) (HIR > 0.4) and the lower panel the patients with good collaterals (n=54) (HIR ≤ 0.4).
The relation between time from symptom onset and rFLAIR was stronger in patients with poor collaterals compared to patients with good collateral status as measured by HIR (p for interaction = 0.04) (Table 2). When modifying the sphere diameter from 15mm to 10 or 20 mm to calculate the rFLAIR, the results remained unaltered (Table 2). Using the non-voxel based approach for calculating FLAIR rSI no interaction was identified (p=0.12).
Table 2.
Results of the multivariate linear regression model.
| Radius | B (time) 95% CI | B (HIR) 95% CI | p-value of interaction term |
|---|---|---|---|
| 10mm | 0.03 (0.02-0.04)* | 0.01 (-0.02-0.05) | 0.04 |
| 15mm | 0.03 (0.02-0.04)* | 0.02 (-0.02-0.04) | 0.04 |
| 20mm | 0.03 (0.02-0.04)* | 0.02 (-0.01-0.05) | 0.05 |
This table shows the results of the multivariate linear regression model with mean qFLAIR in the non-reperfused core as dependent variable and time (per hour) and the quality of collaterals (defined by HIR) as explanatory variables. An interaction term of the two independent variables is included. The first two columns represent the B, the variable estimate, with the corresponding 95% Confidence Interval. The last column shows the p-value of this interaction. Radius represents the sphere-radius used to calculate the rFLAIR value.
Corresponding p-value < 0.01.
HIR = Hypoperfusion Intensity Ratio.
We performed an additional analysis with the mean Tmax in the region of the perfusion deficit as a measurement of hypoperfusion severity. A strong interaction between this severity on the association between time and rFLAIR intensity was identified (p=0.001), confirming the initial results that collateral status modifies the relationship between time from symptom onset and rFLAIR intensity.
Discussion
This study shows that the relationship between time from symptom onset and rFLAIR lesion intensity is dependent on the severity of hypoperfusion (which is a reflection of collateral status). In this study population no association was identified in individuals with good collaterals between time from symptom onset and rFLAIR lesion intensity. In contrast, in patients with poor collaterals, FLAIR lesion intensity increased over time.
Animal and human studies have shown that rFLAIR lesion intensity gradually increases with time after stroke onset.8,9,12,14,25,26 The temporal difference between the presentation of a DWI lesion and FLAIR intensity after stroke onset, the DWI/FLAIR mismatch, has been proposed to determine eligibility for thrombolysis in patients presenting with unknown time from stroke onset.11,27,28 One of the limitations of this imaging pattern is the inter-subject variability when determining a FLAIR positive lesion.29 In acute ischemic stroke due to large vessel occlusion, collateral status is a known modifier of infarct growth and is associated with NIHSS at presentation and outcome after reperfusion therapy.16,30–33 Here, we show that collateral status is a predictor of rFLAIR intensity over time. In severely hypoperfused lesions the development of vasogenic edema appears to have a strict relationship with time as opposed to in patients with good collaterals. These findings could be clinical relevant when FLAIR intensity34 is used to select patients for thrombolysis (e.g. in clinical trials) since patients with good collaterals might be selected after the 4.5 h time window from stroke onset. It is unclear whether these patients, i.e. patients with good collaterals in an extended time-window, will have the same benefit from thrombolysis, as patients with poor collaterals but within 4.5 hours.
The optimal threshold of rFLAIR to predict symptom onset before 4.5h was 1.16. This value was higher than previously reported, which is most likely related to the different method used to determine the rFLAIR intensity.35 The prediction of stroke onset time using the quantitative method vs the visual assessment of the DWI/FLAIR mismatch didn't improve which is a confirmation of previous findings.35
Our study has certain limitations. First, in this dataset digital substraction angiography (DSA) was lacking. Therefore collateral status was indirectly obtained by collateral grading based on HIR. This technique has a good correlation with DSA-assessed collateral circulation21. Second, the HIR is used as a dichotomized scale, defining good vs bad collaterals, which could have resulted in loss of power to detect an interaction. To strengthen our findings we performed an additional analysis with the mean Tmax in the perfusion deficit as a marker of hypoperfusion severity, which confirmed the interaction between severity of hypoperfusion on the association between time from symptom onset and rFLAIR lesion intensity. Third, a new method to calculate rFLAIR was used. Previous studies used the contralateral lesion to calculate the relative ratio.35,36 We believe our voxel-based approach to be more accurate. To strengthen our results we showed our findings not to be critically dependent on the sphere radius chosen. Finally, the exact time from onset of symptoms was not known for all patients and in these cases, time since last seen normal was used instead.
We conclude that collateral status rated by severity of hypoperfusion modifies the association between time from symptom onset and rFLAIR signal intensity. This could influence the accuracy of FLAIR signal intensity to predict stroke onset in patients with unknown time from onset. Clinical trials using the DWI/FLAIR mismatch to determine stroke onset ≤ 4.5h might therefore be enrolling a larger proportion of patients with good collateral status.
Supplementary Material
Acknowledgments
RL is a Senior Clinical Investigator of FWO Flanders
Sources of Funding: Dr. Albers received grant funding from the NIH.
Footnotes
Disclosures: Anke Wouters – grants from European Union.
Patrick Dupont – none
Soren Christensen - consultant of iSchemaView.
Bo Norrving - fees paid to the institution from SYGNIS for steering committee work in the AXIS2 trial.
Rico Laage – none
Götz Thomalla – receiving grants from European Union.
Greg Albers - consultant of iSchemaView, Covidien and Lundbeck. He has an equity interest in iSchemaview.
Vincent Thijs - receiving fees for serving on the steering committee of the AXIS 2 trial.
Robin Lemmens – none
References
- 1.Fink JN, Kumar S, Horkan C, Linfante I, Selim MH, Caplan LR, et al. The Stroke Patient Who Woke Up: Clinical and Radiological Features, Including Diffusion and Perfusion MRI. Stroke. 2002;33:988–993. doi: 10.1161/01.str.0000014585.17714.67. [DOI] [PubMed] [Google Scholar]
- 2.Barreto AD, Martin-Schild S, Hallevi H, Morales MM, Abraham AT, Gonzales NR, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40:827–832. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadeau JO, Fang J, Kapral MK, Silver FL, Hill MD. Outcome after stroke upon awakening. Can J Neurol Sci. 2005;32:232–236. doi: 10.1017/s0317167100004029. [DOI] [PubMed] [Google Scholar]
- 4.Silva GS, Lima FO, Camargo ECS, Smith WS, Singhal AB, Greer DM, et al. Wake-Up Stroke: Clinical and Neuroimaging Characteristics. Cerebrovasc Dis. 2010;29:336–342. doi: 10.1159/000278929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackey J, Kleindorfer D, Sucharew H, Moomaw CJ, Kissela BM, Alwell K, et al. Population-based study of wake-up strokes. Neurology. 2011;76:1662–1667. doi: 10.1212/WNL.0b013e318219fb30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimmele DL, Thomalla G. Wake-Up Stroke: Clinical Characteristics, Imaging Findings, and Treatment Option - an Update. Front Neurol. 2014;5:35. doi: 10.3389/fneur.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wouters A, Lemmens R, Dupont P, Thijs V. Wake-up stroke and stroke of unknown onset: a critical review. Front Neurol. 2014;5:153. doi: 10.3389/fneur.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, Silva MD, Sotak CH, Fisher M. Temporal evolution of ischemic injury evaluated with diffusion-, perfusion-, and T2-weighted MRI. Neurology. 2000;54:689–696. doi: 10.1212/wnl.54.3.689. [DOI] [PubMed] [Google Scholar]
- 9.Chen F, Suzuki Y, Nagai N, Jin L, Yu J, Wang H, et al. Rodent stroke induced by photochemical occlusion of proximal middle cerebral artery: Evolution monitored with MR imaging and histopathology. Eur J Radiol. 2007;63:68–75. doi: 10.1016/j.ejrad.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Xu XQ, Cheng QG, Zu QQ, Lu SS, Yu J, Sheng Y, et al. Comparative study of the relative signal intensity on DWI, FLAIR, and T2 images in identifying the onset time of stroke in an embolic canine model. Neurol Sci. 2014;35:1059–1065. doi: 10.1007/s10072-014-1643-6. [DOI] [PubMed] [Google Scholar]
- 11.Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet. 2011;10:978–986. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 12.Petkova M, Rodrigo S, Lamy C, Oppenheim G, Touzé E, Mas J, et al. MR Imaging Helps Predict Time from Symptom Onset in Patients with Acute Stroke : Implications for Patients with Unknown Onset Time. Radiology. 2010;257:782–792. doi: 10.1148/radiol.10100461. [DOI] [PubMed] [Google Scholar]
- 13.Ebinger M, Scheitz JF, Kufner A, Endres M, Fiebach JB, Nolte CH. MRI-based intravenous thrombolysis in stroke patients with unknown time of symptom onset. Eur J Neurol. 2012;19:348–350. doi: 10.1111/j.1468-1331.2011.03504.x. [DOI] [PubMed] [Google Scholar]
- 14.Aoki J, Kimura K, Iguchi Y, Shibazaki K, Sakai K, Iwanaga T. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39–44. doi: 10.1016/j.jns.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell BCV, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1168–1172. doi: 10.1038/jcbfm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial Design and Reporting Standards for Intra-Arterial Cerebral Thrombolysis for Acute Ischemic. Stroke. 2003;34:e109–137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Son JP, Ryoo S, Lee MJ, Cha J, Kim KH, et al. A novel magnetic resonance imaging approach to collateral flow imaging in ischemic stroke. Ann Neurol. 2014;76:356–369. doi: 10.1002/ana.24211. [DOI] [PubMed] [Google Scholar]
- 19.Menon BK, O'Brien B, Bivard A, Spratt NJ, Demchuk AM, Miteff F, et al. Assessment of leptomeningeal collaterals using dynamic CT angiography in patients with acute ischemic stroke. J Cereb Blood Flow Metab. 2013;33:365–371. doi: 10.1038/jcbfm.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaharchuk G. Arterial spin-labeled perfusion imaging in acute ischemic stroke. Stroke. 2014;45:1202–1207. doi: 10.1161/STROKEAHA.113.003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olivot JM, Mlynash M, Inoue M, Marks MP, Wheeler HM, Kemp S, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke. 2014;45:1018–1023. doi: 10.1161/STROKEAHA.113.003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ringelstein EB, Thijs V, Norrving B, Chamorro A, Aichner F, Grond M, et al. Granulocyte colony-stimulating factor in patients with acute ischemic stroke: results of the AX200 for Ischemic Stroke trial. Stroke. 2013;44:2681–2687. doi: 10.1161/STROKEAHA.113.001531. [DOI] [PubMed] [Google Scholar]
- 23.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wouters A, Dupont P, Ringelstein EB, Norrving B, Chamorro A, Grond M, et al. Association between the perfusion/diffusion and diffusion/FLAIR mismatch: data from the AXIS2 trial. J Cereb Blood Flow Metab. 2015;35:1681–1686. doi: 10.1038/jcbfm.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moseley E. Serial MRI After Transient Focal Cerebral Ischemia in Rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000:1965–1972. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- 26.Moseley ME, Kucharczyk J, Mintorovitch J, Cohen Y, Kurhanewicz J, Derugin N, et al. Diffusion-weighted MR imaging of acute stroke: Correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. Am J Neuroradiol. 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- 27.Breuer L, Schellinger PD, Huttner HB, Halwachs R, Engelhorn T, Doerfler A, et al. Feasibility and safety of magnetic resonance imaging-based thrombolysis in patients with stroke on awakening: initial single-centre experience. Int J stroke. 2010;5:68–73. doi: 10.1111/j.1747-4949.2010.00410.x. [DOI] [PubMed] [Google Scholar]
- 28.Aoki J, Kimura K, Iguchi Y, Shibazaki K, Iwanaga T, Watanabe M, et al. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovasc Dis. 2011;31:435–441. doi: 10.1159/000323850. [DOI] [PubMed] [Google Scholar]
- 29.Ebinger M, Galinovic I, Rozanski M, Brunecker P, Endres M, Fiebach JB. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock? Stroke. 2010;41:250–255. doi: 10.1161/STROKEAHA.109.568410. [DOI] [PubMed] [Google Scholar]
- 30.Miteff F, Levi CR, Bateman Ga, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 31.Marks MP, Lansberg MG, Mlynash M, Olivot JM, Straka M, Kemp S, et al. Effect of Collateral Blood Flow on Patients Undergoing Endovascular Therapy for Acute Ischemic Stroke. Stroke. 2014;45:1035–1039. doi: 10.1161/STROKEAHA.113.004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liebeskind DS, Cotsonis Ga, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomalla G, Fiebach JB, Ostergaard L, Pedraza S, Thijs V, Nighoghossian N, et al. A multicenter randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP) Int J stroke. 2014;9:829–836. doi: 10.1111/ijs.12011. [DOI] [PubMed] [Google Scholar]
- 35.Cheng B, Brinkmann M, Forkert ND, Treszl A, Ebinger M, Köhrmann M, et al. Quantitative measurements of relative fluid-attenuated inversion recovery (FLAIR) signal intensities in acute stroke for the prediction of time from symptom onset. J Cereb Blood Flow Metab. 2013;33:76–84. doi: 10.1038/jcbfm.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostwaldt AC, Galinovic I, Hotter B, Grittner U, Nolte CH, Audebert HJ, et al. Relative FLAIR Signal Intensities over Time in Acute Ischemic Stroke: Comparison of Two Methods. J Neuroimaging. 2015;25:964–968. doi: 10.1111/jon.12224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.