Abstract
The purpose of this study was to evaluate a simplified method of walking track analysis to assess treatment outcome in canine spinal cord injury. Measurements of stride length (SL) and base of support (BS) were made using a ‘finger painting’ technique for footprint analysis in all limbs of 20 normal dogs and 27 dogs with 28 episodes of acute thoracolumbar spinal cord injury (SCI) caused by spontaneous intervertebral disc extrusion. Measurements were determined at three separate time points in normal dogs and on day 3, 10 and 30 following decompressive surgery in dogs with SCI. Values for SL, BS and coefficient of variance (COV) for each parameter were compared between groups at each time point.
Mean SL was significantly shorter in all four limbs of SCI-affected dogs at days 3, 10, and 30 compared to normal dogs. SL gradually increased toward normal in the 30 days following surgery. As measured by this technique, the COV-SL was significantly higher in SCI-affected dogs than normal dogs in both thoracic limbs (TL) and pelvic limbs (PL) only at day 3 after surgery. BS-TL was significantly wider in SCI-affected dogs at days 3, 10 and 30 following surgery compared to normal dogs. These findings support the use of footprint parameters to compare locomotor differences between normal and SCI-affected dogs, and to assess recovery from SCI. Additionally, our results underscore important changes in TL locomotion in thoracolumbar SCI-affected dogs.
Keywords: Spinal cord injury, Intervertebral disc disease, Dog, Locomotor recovery, Outcome assessment
Introduction
Intervertebral disc extrusion (IVDE) is the most common cause of acute spinal cord injury (SCI) in dogs, and chondrodystrophic breeds such as the Dachshund, Cocker spaniel, Basset hound, Beagle, Pekingese, Shih Tzu, Miniature poodle and Bichon frise are commonly represented in the literature (Olby et al., 2003; Ito et al., 2005; Levine et al., 2011; Aikawa et al., 2012; Bergknut et al., 2012; Packer et al., 2013). The high incidence of spontaneous SCI in dogs makes them an important animal model for human SCI (Rice et al., 2009). Dogs offer a genetically similar, but environmentally heterogeneous study population, with comparable mechanisms of injury and resultant pathology to that in humans, which can bridge the gap between experimental rodent models and the human SCI population (Borgens et al., 1999; Laverty et al., 2004; Olby et al., 2004; Jeffery et al., 2006). Successful clinical trials in dogs with spontaneous SCI may lead to the development of interventional therapies that can help both dogs and humans.
Walking track analysis has been used previously to assess return of pelvic limb function following animal models of nerve injury and traumatic SCI (de Medinaceli et al., 1982; Kunkel-Bagden et al., 1993; Cheng et al., 1997; Klapdor et al., 1997; Varejao et al., 2004; Hamers et al., 2001; Hamers et al., 2006; Gordon-Evans et al., 2009, Rangasamy, 2013). Measurements such as base of support (BS), stride length (SL), inter-limb coordination, regularity of step patterns and paw position can provide valuable information regarding the animal's pattern of locomotion in both the thoracic limbs (TL) and pelvic limbs (PL) which may reflect the injury type, severity of injury, and specific spinal tracts affected by the lesion (Kunkel-Bagden et al., 1993; Klapdor et al., 1997; Hamers et al., 2001; Hamers et al., 2006; Rangasamy, 2013). Dogs with spinal cord disease exhibit an uncoordinated gait that is quantifiably different using instrumented gait analysis from dogs with lameness due to orthopedic disease (Gordon-Evans et al., 2009). Coefficients of variance (COV) for SL, swing time and lateral paw positioning have previously been shown to differ in dogs with neurologic disease when compared to normal dogs (Hamilton et al., 2008; Gordon-Evans et al., 2009). Analysis of footprints recorded during walking track assessments may reveal gait deficits that can be objectively measured, and not readily detected through visual assessment only (McEwen and Springer, 2006).
Walking track analysis can be performed in a research setting using specialized equipment such as the Tekscan or Catwalk (Hamers et al., 2001; Hamers et al., 2006; Gordon-Evans et al., 2009). However, this equipment is costly and not universally available, hindering its use in multi-center veterinary clinical trials for SCI.
The primary goal of this study was to evaluate a simplified ‘finger painting’ method of walking track analysis using inexpensive and universally available materials to compare footprint parameters between normal dogs and dogs with acute thoracolumbar SCI caused by IVDE. We also aimed to document the change in measurable footprint parameters in SCI-affected dogs over a 30-day recovery period, specifically SL and BS. We hypothesized that footprint analysis using this method would produce reliable measures of SL and BS such as those obtained via more expensive commercially available equipment; that SL and BS would differ between normal dogs and SCI-affected dogs; and that these parameters would improve towards normal during recovery after SCI.
Materials and methods
The study was conducted in accordance with the guidelines and approval of The Ohio State University Clinical Research Advisory Committee and the Institutional Animal Care and Use Committee (Approval No. 2012A00000149). Written owner consent was obtained prior to study enrollment for all dogs.
Normal and SCI-affected dogs
Normal, skeletally mature, behaviorally amenable small breed dogs (≤ 20 kg bodyweight) were recruited as controls from the pet population of The Ohio State University Veterinary Medical Center. Dogs were determined to be neurologically and orthopedically normal via clinical evaluation by two investigators (RBS - residency trained in neurology, and SAM, a board certified neurologist) and had no history of neurologic or orthopedic disease. Valgus and varus conformational limb variations typical for chondrodystrophic breeds were considered acceptable for enrollment to facilitate generalization of results across a realistic clinical population.
SCI-affected dogs from the same institution were prospectively and consecutively enrolled if they met the following criteria: (1) clinical localization of a T3-L3 myelopathy caused by acute IVDE determined by computed tomography with or without myelography, or magnetic resonance imaging; (2) intact nociception of both pelvic limbs and tail; (3) small-breed ≤ 20 kg; and (4) behaviorally amenable. All dogs underwent surgical decompression for their IVDE.
Footprint acquisition
Different colored non-toxic, washable paints were applied to each paw. Dogs were then walked with a leash at a natural, consistent pace by the same investigator (RBS) down 3 m of butcher paper. Five walking trials were collected during each testing session. Dogs that were reluctant to walk were encouraged with treats and verbal cues by a second investigator at the opposite end of the butcher paper. Notations on the butcher paper were made during testing to indicate if dogs stopped or deviated from the butcher paper path. Control dogs were tested on three different days, separated by at least 48 h. SCI-affected dogs were tested at days 3, 10 and 30 following decompressive surgery. Preliminary evaluation of this technique indicated that only dogs that were ambulatory without assistance could successfully perform the task sufficient for paw print acquisition.
Footprint analysis
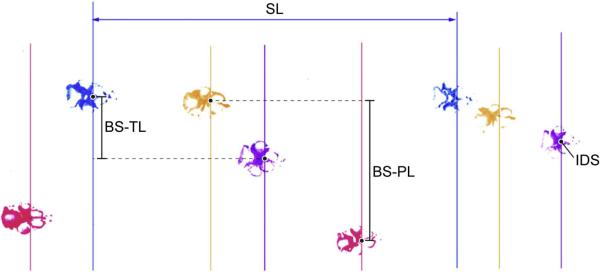
The first and last steps per trial for each paw were excluded from analysis to account for the animal's adjustment to a unique walking surface. A single investigator (MSO) performed all measurements. Consistent with previous reports, a reference point for each paw print was located at the intersection of the intermetacarpophalangeal space and the P3-P4 inter-digital space (Kunkel-Bagden et al., 1993). For partial prints, a template indicating the inter-digital space was made using a complete print from the same testing session and overlaid using landmarks on the partial print to determine the inter-digital space. To correct for rotational variation of the paw, lines were drawn perpendicular to the edge of the walking track through each print at the inter-digital space as previously described (Kunkel-Bagden et al., 1993). The distance between these lines was measured for each step per paw, and designated as the SL. The distance between the inter-digital space on the right and left thoracic limbs was designated as the thoracic limb base of support (BS-TL). The same method was used to measure the pelvic limb base of support (BS-PL; Fig. 1).
Fig. 1.
Footprints were acquired using a simplified method of walking track analysis. Different colors of washable non-toxic paint were applied to each limb (blue, left thoracic limb; purple, right thoracic limb; pink, right pelvic limb; yellow, left pelvic limb). A reference point at the inter-digital space (IDS) was identified and marked as shown (black circle). Methods used to obtain stride length (SL), and base of support of the thoracic limbs (BS-TL) and pelvic limbs (BS-PL) are shown.
Prints were excluded from analysis if the inter-digital space could not be identified either directly or by extrapolation, the dog stopped or slowed walking in the middle of a trial, or notations made during the test indicated an abrupt alteration in step cycle. When a print was excluded, the entire step cycle including that print was excluded from analysis. Mean SL, coefficient of variance (COV) of SL, mean BS, and COV BS were calculated for each limb per testing session for control and SCI-affected dogs using all measurable prints from that session.
Statistical analysis
Summary statistics including mean ± standard deviation, or median and range where appropriate, were calculated for clinical data on all dogs and for SL, BS, and COV for all testing sessions. Mean and COV of SL and BS were compared between normal dogs at session 1 and SCI-affected dogs at days 3, 10 and 30 using two sample t-tests. Paired t-tests were used to test for improvement in SL of each limb between days 10 and 30 for all SCI-affected dogs that had measurements obtained at both time points. A mixed effect model incorporating repeated measures was used to test the trends in SL and BS for each limb across three sessions for SCI-affected dogs that had measurements at all three time points. A mixed effect model was used to evaluate differences between normal dogs across three testing sessions. Analyses were conducted using commercially available software (SAS). A P value of < 0.05 was considered statistically significant for all analyses.
Results
Normal dogs
Twenty normal dogs were recruited for the study. Ages ranged from 8 months to 6.5 years (median 3 years) and weight ranged from 3.7-17.2 kg (median 9.4 kg). There were eight spayed females and 12 castrated males. Breeds included mixed breed dogs (n=6), Dachshund (n=4), Miniature schnauzer (n=2), Sealyham terrier (n=2), and one each of the following: Beagle, Bichon frise, Cocker spaniel, Pembroke Welsh corgi, Miniature pinscher, and Shih Tzu. All normal dogs enrolled were able to participate in footprint collection. Time period between each testing session for normal dogs ranged from 2-27 days (median, 6 days). The median total number of steps recorded from an individual dog per session was 74 (range, 41-147). The median % excluded steps for an individual dog per session was 2% (range, 0-27).
Affected dogs
A total of 27 dogs with 28 discrete episodes of acute SCI caused by IVDE were enrolled. Ages ranged from 2-11 years (median, 6 years) and weight ranged from 3.9 kg to 17.0 kg (median, 9.8 kg). There was not a significant difference in bodyweight between normal and SCI-affected dogs (P=0.73). There were 13 spayed females, 12 castrated males, and two intact males. Breeds included Dachshund (n=12), mixed breed dogs (n=6), French bulldog (n=4), Beagle (n=2), and one each of the following: Pembroke Welsh corgi, Shih Tzu, and Cocker spaniel.
All dogs underwent decompressive hemilaminectomy or pediculectomy at one or multiple sites between T10-11 and L3-4, with or without lateral disc fenestrations. Post-operative analgesic treatment was dependent upon the surgeons’ preference. All SCI-affected dogs enrolled were able to participate in footprint collection at one or more time points during recovery, based on the requirement to ambulate without assistance. Thirteen dogs were able to complete footprint collection on day 3, 21 dogs were able to complete footprint collection on day 10, and 28 dogs were able to complete footprint collection on day 30. The median total number of steps recorded per individual dog at each time point after surgery was 140 (range, 81-213). The median percentage of steps excluded from analysis for an individual dog at each time point after surgery was 12% (range, 0-46).
SL in normal dogs
The mean SL ± SD in normal dogs for the first testing session were as follows: 43.69 cm ± 8.19 (LTL), 43.68 ± 8.08 (RTL), 43.43 ± 7.19 (LPL), and 43.54 ± 7.50 (RPL). The mean SL (cm) and COV-SL of all four limbs in normal dogs for all testing sessions are reported in Table 1. Mean SL of each limb was compared across sessions and a significant difference in SL was not observed between sessions in normal dogs (LTL P=0.45; RTL P=0.46; LPL P= 0.48; RPL P=0.55). The same comparison was made for COV SL, with no differences observed (LTL P=0.22; RTL P=0.35; LPL P=0.56; RPL P=0.55).
Table 1.
Mean (cm) and coefficient of variance (COV) of stride lengths for each limb across three testing sessions in normal dogs (n=20)
| Dog | Stride length LTL | Stride length RTL | Stride length LPL | Stride length RPL | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 1 | Session 2 | Session 3 | Session 1 | Session 2 | Session 3 | Session 1 | Session 2 | Session 3 | |||||||||||||
| Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | |
| 1 | 42.75 | 0.13 | 42.84 | 0.07 | 41.51 | 0.12 | 40.72 | 0.09 | 42.16 | 0.08 | 40.47 | 0.12 | 41.28 | 0.13 | 41.96 | 0.07 | 41.19 | 0.12 | 40.80 | 0.12 | 41.60 | 0.08 | 41.38 | 0.12 |
| 2 | 46.03 | 0.16 | 54.74 | 0.05 | 51.43 | 0.17 | 44.99 | 0.14 | 54.48 | 0.05 | 47.64 | 0.19 | 45.89 | 0.16 | 54.20 | 0.05 | 48.97 | 0.18 | 47.00 | 0.16 | 53.92 | 0.05 | 47.62 | 0.18 |
| 3 | 50.69 | 0.20 | 45.19 | 0.07 | 52.68 | 0.09 | 50.58 | 0.19 | 45.01 | 0.08 | 52.78 | 0.08 | 52.02 | 0.16 | 44.17 | 0.09 | 53.60 | 0.06 | 51.97 | 0.17 | 43.39 | 0.09 | 53.79 | 0.06 |
| 4 | 46.66 | 0.08 | 49.70 | 0.06 | 49.13 | 0.06 | 47.38 | 0.07 | 49.86 | 0.05 | 49.71 | 0.06 | 45.10 | 0.12 | 49.20 | 0.07 | 48.96 | 0.06 | 46.66 | 0.09 | 49.10 | 0.07 | 48.50 | 0.09 |
| 5 | 36.32 | 0.22 | 43.43 | 0.11 | 36.80 | 0.09 | 36.25 | 0.21 | 44.07 | 0.11 | 36.45 | 0.11 | 40.23 | 0.11 | 43.22 | 0.12 | 36.83 | 0.10 | 38.87 | 0.13 | 43.47 | 0.12 | 36.24 | 0.12 |
| 6 | 34.42 | 0.07 | 27.67 | 0.16 | 24.24 | 0.10 | 34.08 | 0.08 | 28.95 | 0.17 | 24.10 | 0.09 | 34.34 | 0.07 | 27.05 | 0.16 | 24.21 | 0.10 | 34.03 | 0.08 | 27.57 | 0.16 | 24.21 | 0.10 |
| 7 | 33.07 | 0.06 | 32.50 | 0.10 | 37.48 | 0.10 | 33.08 | 0.07 | 32.40 | 0.09 | 36.64 | 0.11 | 32.33 | 0.08 | 32.13 | 0.10 | 37.12 | 0.10 | 32.77 | 0.06 | 32.34 | 0.11 | 36.83 | 0.11 |
| 8 | 35.64 | 0.10 | 40.51 | 0.11 | 41.40 | 0.13 | 34.77 | 0.14 | 40.63 | 0.12 | 41.63 | 0.11 | 35.27 | 0.10 | 40.40 | 0.11 | 42.63 | 0.09 | 34.99 | 0.14 | 40.01 | 0.12 | 42.46 | 0.10 |
| 9 | 44.73 | 0.17 | 57.85 | 0.12 | 50.30 | 0.15 | 47.08 | 0.20 | 55.92 | 0.16 | 49.56 | 0.16 | 43.22 | 0.21 | 51.56 | 0.16 | 47.95 | 0.17 | 44.05 | 0.22 | 53.19 | 0.16 | 47.70 | 0.19 |
| 10 | 40.23 | 0.18 | 37.57 | 0.13 | 42.07 | 0.21 | 41.24 | 0.17 | 37.98 | 0.12 | 46.26 | 0.16 | 39.12 | 0.19 | 38.40 | 0.13 | 43.70 | 0.25 | 39.60 | 0.18 | 37.09 | 0.13 | 42.48 | 0.21 |
| 11 | 44.23 | 0.05 | 40.85 | 0.09 | 42.18 | 0.05 | 44.62 | 0.04 | 40.67 | 0.12 | 42.09 | 0.06 | 44.37 | 0.04 | 39.67 | 0.10 | 40.09 | 0.08 | 43.68 | 0.05 | 40.26 | 0.11 | 40.31 | 0.06 |
| 12 | 46.06 | 0.17 | 60.61 | 0.05 | 56.64 | 0.11 | 46.45 | 0.16 | 61.24 | 0.05 | 57.49 | 0.10 | 47.63 | 0.21 | 60.30 | 0.06 | 54.96 | 0.12 | 49.25 | 0.20 | 60.78 | 0.07 | 56.03 | 0.10 |
| 13 | 38.50 | 0.14 | 37.71 | 0.08 | 38.95 | 0.10 | 38.71 | 0.11 | 38.37 | 0.07 | 38.47 | 0.11 | 39.00 | 0.12 | 37.86 | 0.09 | 38.88 | 0.11 | 38.50 | 0.12 | 37.58 | 0.11 | 38.54 | 0.10 |
| 14 | 44.60 | 0.10 | 45.95 | 0.16 | 46.21 | 0.06 | 46.22 | 0.09 | 44.43 | 0.18 | 46.32 | 0.07 | 45.02 | 0.10 | 45.09 | 0.18 | 45.91 | 0.06 | 45.40 | 0.10 | 45.82 | 0.18 | 46.10 | 0.07 |
| 15 | 47.99 | 0.05 | 48.39 | 0.07 | 47.66 | 0.07 | 47.58 | 0.05 | 48.54 | 0.07 | 46.87 | 0.09 | 47.74 | 0.06 | 47.09 | 0.09 | 47.48 | 0.07 | 47.41 | 0.06 | 47.93 | 0.08 | 46.67 | 0.12 |
| 16 | 40.83 | 0.19 | 44.55 | 0.15 | 51.74 | 0.09 | 40.97 | 0.23 | 46.22 | 0.12 | 50.96 | 0.10 | 41.58 | 0.17 | 44.24 | 0.14 | 50.98 | 0.06 | 41.94 | 0.18 | 44.83 | 0.14 | 51.40 | 0.06 |
| 17 | 50.00 | 0.09 | 44.76 | 0.09 | 54.68 | 0.09 | 49.53 | 0.09 | 44.42 | 0.09 | 55.14 | 0.06 | 49.25 | 0.08 | 43.73 | 0.08 | 54.23 | 0.09 | 48.78 | 0.08 | 43.45 | 0.09 | 55.47 | 0.07 |
| 18 | 28.43 | 0.25 | 30.60 | 0.21 | 33.33 | 0.29 | 28.33 | 0.27 | 28.38 | 0.30 | 33.91 | 0.33 | 30.73 | 0.13 | 30.50 | 0.25 | 33.90 | 0.21 | 29.51 | 0.21 | 31.20 | 0.20 | 34.30 | 0.25 |
| 19 | 55.82 | 0.09 | 57.94 | 0.08 | 56.25 | 0.12 | 55.74 | 0.11 | 57.94 | 0.07 | 57.38 | 0.13 | 51.64 | 0.15 | 53.87 | 0.13 | 56.88 | 0.11 | 52.58 | 0.13 | 54.18 | 0.15 | 56.28 | 0.14 |
| 20 | 66.81 | 0.10 | 62.59 | 0.11 | 61.73 | 0.08 | 65.23 | 0.11 | 62.79 | 0.11 | 60.99 | 0.06 | 62.75 | 0.11 | 57.22 | 0.16 | 59.56 | 0.12 | 63.02 | 0.15 | 55.52 | 0.16 | 57.58 | 0.11 |
| Mean | 43.69 | 0.13 | 45.30 | 0.10 | 45.82 | 0.11 | 43.68 | 0.13 | 45.22 | 0.11 | 45.74 | 0.12 | 43.43 | 0.13 | 44.09 | 0.12 | 45.40 | 0.11 | 43.54 | 0.13 | 44.16 | 0.12 | 45.19 | 0.12 |
LTL, left thoracic limb; RTL, right thoracic limb; LPL, left pelvic limb; RPL, right pelvic limb
SL in SCI-affected dogs
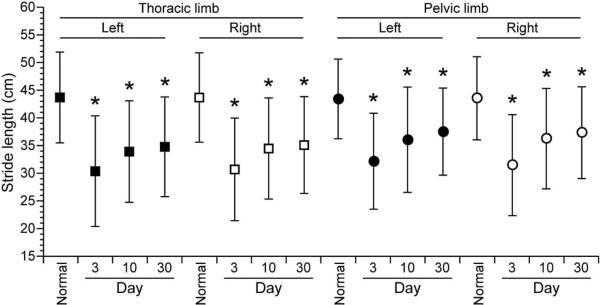
The mean SL ± SD for SCI-affected dogs on day 3 was 30.37 cm ± 10.0 (LTL), 30.69 cm ± 9.27 (RTL), 32.17 cm ± 8.67 (LPL), 31.46 cm ± 9.12 (RPL). The mean SL and COV-SL of all four limbs in SCI-affected dogs at days 3, 10 and 30 after surgery are reported in Table 2. SL in all four limbs of SCI-affected dogs was shorter than controls at all three time points after surgery and the difference in SL between normal and SCI-affected dogs decreased at each time point (Fig. 2). For dogs for which SL was measured at both day 10 and day 30 (n=21), there was an improvement toward normal SL between the two sessions in all four limbs (LTL, P=0.004; RTL, P=0.005; LPL, P=0.049; RPL, P=0.067). In SCI-affected dogs for which measurements could be obtained at all three time points (n=13), trend/slope analysis also indicated a significant increase in SL over the 30 day recovery period (LFL, P=0.027; RFL, P=0.021; LHL, P=0.041; RHL, P=0.034).
Table 2.
Mean (cm) and coefficient of variance (COV) of stride lengths for each limb in dogs with thoracolumbar spinal cord injury (SCI) due to acute intervertebral disc extrusion at day 3, 10 and 30 following decompressive surgery (n=27 dogs, 28 episodes of SCI)
| Doga | Stride length LTL | Stride length RTL | Stride length LPL | Stride length RPL | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 10 | Day 30 | Day 3 | Day 10 | Day 30 | Day 3 | Day 10 | Day 30 | Day 3 | Day 10 | Day 30 | |||||||||||||
| Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | |
| 1 | 36.09 | 0.11 | 36.23 | 0.16 | 36.17 | 0.13 | 35.94 | 0.11 | 37.31 | 0.16 | 37.93 | 0.14 | 35.43 | 0.14 | 38.28 | 0.12 | 40.67 | 0.09 | 36.76 | 0.15 | 38.38 | 0.13 | 39.51 | 0.16 |
| 2 | 34.8 | 0.2 | 45.72 | 0.08 | 42.5 | 0.08 | 34.48 | 0.19 | 45.2 | 0.09 | 42.22 | 0.07 | 34.4 | 0.2 | 45.03 | 0.1 | 41.17 | 0.09 | 34.09 | 0.22 | 44.08 | 0.13 | 41 | 0.1 |
| 3 | 27.05 | 0.18 | 32.43 | 0.18 | 30.87 | 0.15 | 26.82 | 0.17 | 32.43 | 0.18 | 31.1 | 0.14 | 26.38 | 0.16 | 32.98 | 0.19 | 30.74 | 0.15 | 26.84 | 0.15 | 32.68 | 0.19 | 30.59 | 0.16 |
| 4 | 17.79 | 0.23 | 34.92 | 0.09 | 33.4 | 0.06 | 18.43 | 0.22 | 35.22 | 0.09 | 33.32 | 0.08 | 27.69 | 0.39 | 35.47 | 0.07 | 32.86 | 0.11 | 18.58 | 0.19 | 35.12 | 0.07 | 33.13 | 0.08 |
| 5 | 34.56 | 0.2 | 44.57 | 0.05 | 38.58 | 0.07 | 35.29 | 0.17 | 45.04 | 0.05 | 38.5 | 0.07 | 34.91 | 0.18 | 44.93 | 0.05 | 38.39 | 0.05 | 35.75 | 0.15 | 44.47 | 0.07 | 37.92 | 0.06 |
| 6 | 46.9 | 0.07 | 41.8 | 0.14 | 47.36 | 0.14 | 45.86 | 0.06 | 42.36 | 0.15 | 47.58 | 0.14 | 47.27 | 0.06 | 41.16 | 0.14 | 48.18 | 0.14 | 45.27 | 0.04 | 41.83 | 0.18 | 46.45 | 0.14 |
| 7 | 41.98 | 0.16 | 45.42 | 0.12 | 51.02 | 0.09 | 40.91 | 0.19 | 44.49 | 0.13 | 51.48 | 0.08 | 44.59 | 0.15 | 45.44 | 0.13 | 51.02 | 0.06 | 42.41 | 0.2 | 45.11 | 0.15 | 51.55 | 0.04 |
| 8 | 41.7 | 0.19 | 39.02 | 0.12 | 38.64 | 0.15 | 40.44 | 0.15 | 39.57 | 0.13 | 39.53 | 0.15 | 41.08 | 0.08 | 45.43 | 0.08 | 48.2 | 0.1 | 42 | 0.07 | 46.49 | 0.08 | 47.47 | 0.11 |
| 9 | 14.58 | 0.37 | 22.4 | 0.17 | 24.96 | 0.29 | 15.94 | 0.27 | 22.38 | 0.16 | 25.23 | 0.27 | 19.42 | 0.15 | 23.06 | 0.15 | 27.11 | 0.17 | 17.34 | 0.25 | 22.38 | 0.17 | 25.12 | 0.27 |
| 10 | 24.44 | 0.4 | 23.64 | 0.47 | 32.92 | 0.31 | 29.49 | 0.24 | 30.69 | 0.25 | 37.3 | 0.25 | 28.16 | 0.24 | 29 | 0.3 | 35.94 | 0.23 | 29.61 | 0.23 | 31.49 | 0.23 | 37.29 | 0.24 |
| 11 | 22.36 | 0.27 | 35.42 | 0.07 | 36.6 | 0.09 | 22.43 | 0.25 | 35.83 | 0.05 | 37.09 | 0.07 | 23.1 | 0.22 | 35.57 | 0.08 | 35.68 | 0.17 | 23.35 | 0.25 | 35.61 | 0.09 | 34.17 | 0.24 |
| 12 | 27.88 | 0.22 | 29.41 | 0.13 | 37.58 | 0.16 | 29.08 | 0.18 | 29.54 | 0.11 | 36.74 | 0.16 | 29.91 | 0.16 | 29.65 | 0.12 | 36.01 | 0.13 | 30.28 | 0.16 | 29.81 | 0.12 | 35.8 | 0.16 |
| 13 | 24.65 | 0.28 | 25.77 | 0.2 | 35.33 | 0.15 | 23.85 | 0.32 | 26.16 | 0.21 | 35.58 | 0.15 | 25.81 | 0.22 | 26.11 | 0.19 | 36.36 | 0.13 | 26.69 | 0.21 | 26.32 | 0.16 | 36.29 | 0.12 |
| 14 | 49.41 | 0.1 | 51.22 | 0.07 | 50.24 | 0.08 | 50.44 | 0.1 | 59.14 | 0.15 | 51.72 | 0.06 | 58.41 | 0.12 | 52.23 | 0.07 | ||||||||
| 15 | 21.86 | 0.17 | 26.21 | 0.17 | 22.14 | 0.2 | 27.14 | 0.14 | 24.54 | 0.28 | 34.14 | 0.21 | 27.71 | 0.39 | 31.91 | 0.16 | ||||||||
| 16 | 43.94 | 0.19 | 52.42 | 0.08 | 45.5 | 0.15 | 50.51 | 0.15 | 42.91 | 0.22 | 51.26 | 0.12 | 42.9 | 0.2 | 53.43 | 0.12 | ||||||||
| 17 | 34.02 | 0.15 | 36.52 | 0.18 | 34.2 | 0.13 | 37.11 | 0.16 | 35.41 | 0.27 | 35.23 | 0.17 | 33.95 | 0.12 | 36.11 | 0.16 | ||||||||
| 18 | 21.51 | 0.3 | 22.03 | 0.34 | 20.91 | 0.29 | 21.13 | 0.35 | 27.76 | 0.2 | 24.51 | 0.25 | 24.89 | 0.17 | 23.77 | 0.24 | ||||||||
| 19 | 19.54 | 0.25 | 27.02 | 0.13 | 19.92 | 0.23 | 27.7 | 0.11 | 24.91 | 0.39 | 30.04 | 0.24 | 26.31 | 0.23 | 28.57 | 0.23 | ||||||||
| 20 | 25.77 | 0.14 | 33.13 | 0.08 | 24.95 | 0.17 | 34 | 0.08 | 25.97 | 0.13 | 33.62 | 0.1 | 30.47 | 0.4 | 35.21 | 0.12 | ||||||||
| 21 | 39.35 | 0.09 | 43.63 | 0.12 | 39.72 | 0.07 | 43.35 | 0.13 | 44.33 | 0.12 | 45.21 | 0.07 | 42.84 | 0.12 | 44.99 | 0.07 | ||||||||
| 22 | 38.91 | 0.13 | 38.65 | 0.16 | 40.44 | 0.11 | 40.51 | 0.07 | ||||||||||||||||
| 23 | 22.19 | 0.21 | 23.49 | 0.19 | 28.71 | 0.18 | 30.39 | 0.18 | ||||||||||||||||
| 24 | 29.13 | 0.12 | 29.58 | 0.13 | 30.59 | 0.11 | 30.99 | 0.13 | ||||||||||||||||
| 25 | 22.8 | 0.17 | 22.95 | 0.17 | 33.39 | 0.11 | 31.62 | 0.19 | ||||||||||||||||
| 26 | 25.07 | 0.21 | 25.07 | 0.2 | 27.1 | 0.21 | 28.52 | 0.16 | ||||||||||||||||
| 27 | 34.09 | 0.21 | 33.56 | 0.25 | 44.41 | 0.36 | 47.75 | 0.35 | ||||||||||||||||
| 28 | 23.49 | 0.21 | 24.22 | 0.14 | 37.85 | 0.24 | 32.65 | 0.24 | ||||||||||||||||
| Mean | 30.37 | 0.22 | 33.91 | 0.16 | 34.78 | 0.15 | 30.69 | 0.20 | 34.47 | 0.15 | 35.09 | 0.15 | 32.17 | 0.18 | 36.05 | 0.17 | 37.52 | 0.15 | 31.46 | 0.18 | 36.25 | 0.17 | 37.32 | 0.16 |
LTL, left thoracic limb; RTL, right thoracic limb; LPL, left pelvic limb; RPL, right pelvic limb
Missing values represent dogs unable to walk unsupported in the pelvic limbs at time of testing
Fig. 2.
Mean stride length (SL) of each limb in normal dogs was compared to spinal cord injury (SCI) affected dogs at day 3, 10 and 30 following decompressive surgery. Whiskers represent ± standard deviation. Asterisk denotes statistically significant differences from normal dogs (P<0.05). Mean SL was significantly lower in all limbs at all time points in SCI-affected dogs compared to normal dogs. A gradually increasing trend in mean SL is seen in all limbs of SCI-affected dogs with post-operative recovery.
Comparisons of the COV-SL for each limb were made between normal dogs at the first testing session and SCI-affected dogs at all three time points. COV-SL of the LTL (P=0.006), RTL (P=0.013), LPL (P=0.035), and RPL (P=0.047) were significantly higher in SCI-affected dogs at day 3 after surgery, but differences in COV-SL at days 10 and 30 were not significant for any limb.
Mean and COV of BS of normal dogs
The mean BS ± SD for normal dogs during the first testing session was 6.28 cm ± 1.63 (TL) and 8.18 cm ± 2.92 (PL). BS and COV-BS for TL and PL in normal dogs across three testing sessions are reported in Table 3. Comparisons of mean BS-TL and BS-PL between sessions did not reveal significant differences across testing sessions in normal dogs (BS-TL, P=0.78; BS-PL, P=0.69). The same comparison revealed no difference in COV-BS across testing sessions in normal dogs (BS-TL, P=0.73; BS-PL, P=0.61).
Table 3.
Mean (cm) and coefficient of variance (COV) of base of support in the thoracic limbs (TL) and pelvic limbs (PL) across three testing sessions in normal dogs (n=20)
| Dog | Base of support TL | Base of support PL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 1 | Session 2 | Session 3 | |||||||
| Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | |
| 1 | 6.66 | 0.48 | 6.44 | 0.41 | 6.13 | 0.44 | 7.30 | 0.34 | 6.80 | 0.34 | 7.07 | 0.41 |
| 2 | 7.77 | 0.54 | 7.41 | 0.47 | 9.73 | 0.54 | 8.99 | 0.53 | 6.19 | 0.64 | 8.17 | 0.59 |
| 3 | 10.30 | 0.34 | 8.22 | 0.29 | 9.43 | 0.31 | 11.18 | 0.37 | 12.88 | 0.28 | 13.04 | 0.24 |
| 4 | 8.64 | 0.31 | 7.54 | 0.34 | 8.27 | 0.40 | 9.25 | 0.22 | 7.77 | 0.19 | 8.24 | 0.36 |
| 5 | 4.94 | 0.44 | 5.02 | 0.48 | 4.99 | 0.46 | 4.26 | 0.60 | 4.26 | 0.60 | 5.48 | 0.37 |
| 6 | 3.77 | 0.55 | 3.64 | 0.59 | 5.32 | 0.40 | 6.47 | 0.35 | 6.85 | 0.32 | 6.20 | 0.25 |
| 7 | 6.39 | 0.20 | 5.70 | 0.34 | 6.49 | 0.34 | 7.07 | 0.23 | 7.28 | 0.22 | 6.33 | 0.32 |
| 8 | 4.82 | 0.49 | 4.73 | 0.32 | 4.24 | 0.47 | 8.96 | 0.31 | 8.85 | 0.29 | 8.66 | 0.21 |
| 9 | 6.06 | 0.54 | 4.74 | 0.85 | 5.63 | 0.80 | 10.33 | 0.36 | 9.04 | 0.37 | 9.05 | 0.47 |
| 10 | 4.91 | 0.68 | 5.84 | 0.43 | 3.83 | 0.73 | 8.20 | 0.53 | 8.29 | 0.37 | 8.00 | 0.41 |
| 11 | 6.82 | 0.41 | 7.73 | 0.36 | 7.26 | 0.40 | 5.32 | 0.47 | 5.05 | 0.67 | 5.02 | 0.50 |
| 12 | 7.53 | 0.49 | 7.74 | 0.43 | 7.60 | 0.27 | 15.49 | 0.34 | 13.00 | 0.24 | 17.23 | 0.17 |
| 13 | 4.12 | 0.57 | 3.27 | 0.61 | 3.38 | 0.52 | 7.07 | 0.33 | 7.80 | 0.27 | 6.96 | 0.30 |
| 14 | 5.85 | 0.45 | 5.92 | 0.36 | 5.09 | 0.50 | 6.67 | 0.39 | 7.77 | 0.24 | 7.44 | 0.28 |
| 15 | 5.80 | 0.27 | 5.80 | 0.40 | 6.65 | 0.35 | 9.61 | 0.17 | 8.72 | 0.24 | 9.47 | 0.28 |
| 16 | 7.23 | 0.52 | 7.18 | 0.50 | 6.42 | 0.45 | 12.90 | 0.31 | 11.14 | 0.28 | 10.82 | 0.19 |
| 17 | 6.30 | 0.40 | 5.68 | 0.46 | 4.83 | 0.44 | 4.02 | 0.66 | 5.96 | 0.43 | 2.93 | 0.66 |
| 18 | 7.93 | 0.64 | 7.58 | 0.35 | 7.80 | 0.55 | 10.03 | 0.35 | 9.97 | 0.30 | 10.98 | 0.28 |
| 19 | 3.81 | 0.79 | 5.88 | 0.61 | 4.21 | 0.65 | 3.37 | 0.75 | 3.38 | 0.64 | 2.56 | 0.73 |
| 20 | 6.00 | 0.48 | 4.92 | 0.66 | 6.30 | 0.54 | 7.15 | 0.60 | 5.96 | 0.55 | 4.11 | 0.70 |
| Mean | 6.28 | 0.48 | 6.05 | 0.46 | 6.18 | 0.48 | 8.18 | 0.41 | 7.85 | 0.37 | 7.89 | 0.39 |
Mean and COV BS of SCI-affected dogs
The mean BS ± SD for SCI-affected dogs on day 3 after surgery was 8.41 cm ± 2.21 (TL) and 7.9 cm ± 2.71 (PL). The mean BS and COV-BS for all limbs of SCI-affected dogs is reported in Table 4. The mean BS-TL and BS-PL were compared between the first testing session in normal dogs and SCI-affected dogs at days 3, 10 and 30 following surgery. BS-TL was wider in SCI-affected dogs at all three time points: day 3 (P=0.004), day 10 (P=0.046), and day 30 (P=0.014; Fig. 3). BS-PL and COV-BS did not differ between normal and SCI-affected dogs at any time point after injury.
Table 4.
Mean (cm) and coefficient of variance of base of support (cm) for the thoracic (TL) and pelvic (PL) limbs in dogs with thoracolumbar spinal cord injury due to acute intervertebral disc extrusion at day 3, 10 and 30 following decompressive surgery (n=27 dogs, 28 episodes of SCI).
| Doga | Base of support TL | Base of support PL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day3 | Day 10 | Day 30 | Day 3 | Day 10 | Day 30 | |||||||
| Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | Mean | COV | |
| 1 | 3.95 | 0.57 | 4.48 | 0.73 | 4.02 | 0.73 | 9.12 | 0.44 | 10.74 | 0.40 | 11.27 | 0.31 |
| 2 | 6.60 | 0.40 | 5.67 | 0.43 | 5.74 | 0.31 | 7.10 | 0.40 | 6.38 | 0.35 | 8.06 | 0.36 |
| 3 | 7.06 | 0.29 | 6.59 | 0.30 | 5.91 | 0.28 | 4.18 | 0.40 | 5.70 | 0.31 | 6.46 | 0.31 |
| 4 | 7.34 | 0.32 | 7.43 | 0.26 | 8.71 | 0.31 | 7.23 | 0.89 | 5.81 | 0.28 | 6.53 | 0.31 |
| 5 | 10.30 | 0.19 | 10.30 | 0.17 | 10.12 | 0.15 | 10.36 | 0.24 | 10.12 | 0.17 | 10.58 | 0.23 |
| 6 | 12.05 | 0.15 | 9.99 | 0.48 | 10.02 | 0.33 | 11.75 | 0.32 | 16.74 | 0.35 | 15.95 | 0.13 |
| 7 | 10.07 | 0.43 | 8.01 | 0.40 | 6.56 | 0.48 | 4.89 | 0.77 | 4.98 | 0.58 | 5.79 | 0.51 |
| 8 | 9.58 | 0.38 | 8.37 | 0.31 | 10.15 | 0.33 | 10.64 | 0.27 | 10.95 | 0.24 | 8.76 | 0.36 |
| 9 | 9.00 | 0.35 | 6.99 | 0.26 | 7.17 | 0.34 | 6.71 | 0.49 | 6.20 | 0.31 | 7.52 | 0.30 |
| 10 | 11.24 | 0.39 | 14.46 | 0.36 | 11.15 | 0.36 | 12.31 | 0.46 | 14.23 | 0.28 | 13.31 | 0.28 |
| 11 | 9.26 | 0.43 | 6.20 | 0.55 | 6.67 | 0.34 | 6.17 | 0.65 | 5.21 | 0.45 | 5.19 | 0.71 |
| 12 | 6.73 | 0.58 | 4.52 | 0.61 | 5.91 | 0.46 | 8.51 | 0.41 | 7.64 | 0.36 | 3.79 | 0.49 |
| 13 | 6.16 | 0.36 | 6.14 | 0.27 | 6.09 | 0.37 | 3.68 | 0.63 | 6.15 | 0.48 | 6.69 | 0.33 |
| 14 | 9.33 | 0.43 | 11.26 | 0.30 | 6.86 | 0.84 | 9.74 | 0.31 | ||||
| 15 | 5.54 | 0.47 | 6.33 | 0.33 | 4.65 | 0.66 | 2.44 | 0.65 | ||||
| 16 | 8.93 | 0.54 | 8.81 | 0.52 | 8.31 | 0.72 | 8.21 | 0.44 | ||||
| 17 | 6.18 | 0.42 | 4.80 | 0.54 | 4.52 | 0.50 | 5.87 | 0.27 | ||||
| 18 | 5.02 | 0.43 | 6.28 | 0.45 | 8.46 | 0.44 | 8.05 | 0.45 | ||||
| 19 | 7.53 | 0.73 | 5.60 | 0.53 | 7.62 | 0.77 | 7.22 | 0.38 | ||||
| 20 | 8.08 | 0.27 | 7.18 | 0.28 | 7.60 | 0.35 | 7.23 | 0.29 | ||||
| 21 | 10.06 | 0.28 | 8.85 | 0.35 | 3.85 | 0.84 | 6.64 | 0.32 | ||||
| 22 | 6.86 | 0.32 | 2.05 | 1.05 | ||||||||
| 23 | 6.49 | 0.33 | 6.88 | 0.45 | ||||||||
| 24 | 9.45 | 0.23 | 9.86 | 0.22 | ||||||||
| 25 | 5.09 | 0.46 | 6.42 | 0.49 | ||||||||
| 26 | 12.49 | 0.28 | 12.31 | 0.24 | ||||||||
| 27 | 12.24 | 0.32 | 10.10 | 0.71 | ||||||||
| 28 | 10.66 | 0.30 | 12.29 | 0.53 | ||||||||
| Mean | 8.41 | 0.37 | 7.61 | 0.41 | 7.88 | 0.37 | 7.90 | 0.49 | 7.75 | 0.46 | 8.04 | 0.41 |
Missing values represent dogs unable to walk unsupported in the pelvic limbs at time of testing.
Fig. 3.
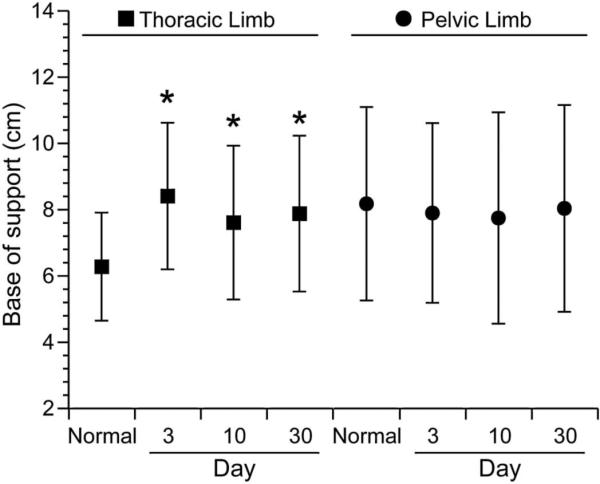
Mean base of support of the thoracic limbs and pelvic limbs in normal dogs was compared to dogs with spinal cord injury (SCI) dogs at day 3, 10 and 30 following decompressive surgery. Whiskers represent ± standard deviation. Asterisk denotes statistically significant differences from normal dogs (P<0.05). Mean base of support-thoracic limbs was higher in SCI-affected dogs at all three time points compared to normal dogs. Mean pelvic limb base of support in SCI-affected dogs was not significantly different from normal dogs at any time point.
Discussion
To our knowledge, this is the first study evaluating over-ground locomotion using a ‘finger paint’ technique to compare normal dogs to dogs with spontaneous acute SCI caused by IVDE. With this technique, we were able to reliably measure several useful footprint parameters such as SL and BS in normal dogs and to compare these parameters to those obtained from SCI-affected dogs. We demonstrated that dogs with thoracolumbar SCI have a measureable decrease in SL in all four limbs and a widened BS-TL during the first 30 days of post-operative recovery.
Our results are similar to previous rodent studies using commercially available methods of walking track analysis, showing that SL of PLs typically decreases following injury (Kunkel-Bagden and Bregman, 1990, Stokes and Reier et al., 1992; Kunkel-Bagden et al., 1993; Bregman et al., 1995; Keirstead et al., 2005; Hamers et al., 2006; McEwen and Springer, 2006; Plemel et al., 2008; Gordon-Evans et al., 2009). A decrease in SL of the PL is therefore an expected finding in SCI-affected dogs, and may be due to loss of supraspinal excitatory input to motor neurons innervating the pelvic limb extensor muscles. This leads to paraparesis, decreased weight support in the affected limbs, reduced limb propulsion, and decreased stance or swing duration (Hamers et al., 2001; McEwen and Springer, 2006; Collazos-Castro et al., 2006; Rangasamy, 2013).
Several studies have also demonstrated a decrease in SL of the TLs following thoracolumbar SCI (Hamers et al., 2001; McEwen and Springer, 2006; Plemel et al., 2008; Gordon-Evans et al., 2009). This finding likely reflects compensatory changes to gait and weight bearing which occur after SCI. After thoracolumbar SCI, dogs show increased weight bearing in the TL. This can result in decreased SL and increased vertical force to compensate for decreased PL function (Cheng et al., 1997; Hamers et al., 2001; McEwen and Springer, 2006; Gordon-Evans et al., 2009). While we are unable to measure more complex parameters such as vertical force and swing phase using the method we report, a simple method of documenting changes in SL after SCI is valuable, as this parameter is commonly used as an outcome measure to determine efficacy of interventional therapies for acute SCI (Stokes and Reier, 1992; Bregman et al., 1995; Keirstead et al., 2005; McEwen and Springer, 2006).
Our technique was also useful for evaluating BS, and revealed a significantly wider BS-TL in SCI-affected dogs compared to controls. Increased BS-TL in rats with thoracic SCI has been previously reported and may reflect attempts to stabilize the trunk cranial to the lesion using a wider center of gravity to compensate for instability and paresis of pelvic limbs (McEwen and Springer, 2006).
BS-PL as measured by our method did not differ between normal and SCI-affected dogs. Rodent studies have yielded mixed results regarding the effect of SCI on BS-PL. This measurement may increase after SCI, and then gradually decrease with neurologic recovery (Kunkel-Bagden and Bregman, 1990, Behrmann et al., 1992; Stokes and Reier, 1992, Kunkel-Bagden et al., 1993; Metz et al., 2000; Hamers et al., 2001 Keirstead et al., 2005). However, BS-PL also varies with lesion severity, such that animals with more severe lesions may have decreased BS-PL, while those with milder lesions have increased BS-PL (Cheng et al., 1997). Asymmetrical lesions could also confound BS-PL data.
In dogs with SCI, a measure similar to COV BS-PL has also been used previously to assess recovery after SCI (Hamilton et al., 2008; Jeffery et al., 2011; Granger et al., 2012). These studies assessed lateral stability of the PL, which is largely determined by descending supraspinal pathways from the brainstem (Hamilton et al., 2008). Dogs with thoracolumbar SCI have increased variability of lateral pelvic limb placement during treadmill walking, suggesting instability (Hamilton et al., 2008). The lack of observable increase in COV BS-PL in our cohort of dogs following SCI may reflect variable injury severity, lesion asymmetry, the fact that testing in other studies employed a treadmill, inclusion of only small breed dogs, or the fact that more severely affected animals could not be evaluated at early time points after injury using our technique.
Of particular interest to us were the differences in TL gait parameters noted after SCI. Functional reorganization of the sensory and motor cortex occurs after SCI, allowing for increased representation of the trunk and thoracic limbs, and structural reorganization of damaged motor pathways in the form of increased collateral sprouting of the corticospinal pathway occurs to increase connections in the cervical spinal cord immediately and weeks after injury (Fouad et al., 2001; Bazley et al., 2014; Oza and Giszter, 2014; Yagüe et al., 2014). Furthermore, changes in thoracic limb and trunk activity in rodent models of thoracic SCI have been previously demonstrated, such as increased thoracic limb and back extensor muscle activity, increased stepping frequency of the thoracic limbs, increased weight bearing in the thoracic limbs, and increased peak vertical forces of the thoracic limbs (Webb and Muir, 2002; Ballermann et al., 2006). The changes in SL and BS detected in the TL of the SCI-affected dogs in our study underscore the importance of the adaptations of the trunk and TL in quadrupedal locomotor recovery from SCI.
In rats, strain- related differences in the amount of weight-bearing of the thoracic and pelvic limbs have been recognized (McEwen and Springer, 2006). Similar breed and conformation (i.e. chondrodystrophic vs. non-chondrodystrophic) differences in weight bearing, footprint parameters and adaptive TL gait parameters might also exist in dogs. Here we controlled for breed to minimize conformation-related differences in biomechanics; however, further studies are needed to determine the presence and significance of such breed-related differences as they relate to SCI and locomotor recovery in dogs.
A limitation of the current study is that stride length data was not normalized to limb length, as has been reported in a previous study (Hamilton et al., 2008). Hamilton et al. used tibial length to normalize data across a cohort of dogs with significant variability in size; however they showed that parameters such as BS were not dependent on tibial length. In the present study, we enrolled only small breed chondrodystrophic dogs so that variability in limb length and body conformation was controlled for between groups. Additionally, we evaluated COV of SL and BS. This value represents only the variability in the measurement of SL and BS and is independent of limb length.
There are some limitations to the use of footprint analysis in the method described herein as a sole outcome measure of SCI in dogs. The most important limitation was that dogs needed to be consistently ambulatory for footprints to be measurable. Therefore, footprint analysis could not be performed in dogs with more severe SCI in the early stages of recovery (i.e. there is non-random censoring of footprint analysis data at earlier time points after injury). This limitation resulted in a significant underestimation of the true differences between normal and SCI-affected groups, as more severe cases of SCI could not be tested during initial evaluation. Additionally, although significantly cheaper in equipment cost when compared to computerized gait analysis equipment, the current method proved time and labor intensive.
Conclusions
This study demonstrated that footprint analysis could be performed in both normal and SCI-affected dogs using a simple, ‘finger painting’ method of walking track analysis with affordable supplies. Significant differences in footprint parameters in both the PLs and TLs were found between normal and SCI-affected dogs. Our results support the use of footprint analysis by this technique as an objective outcome measure of SCI in dogs. Future studies are needed to fully document changes in gait parameters throughout the recovery process and to determine the degree of agreement between this technique and commercially available gait analysis equipment.
Highlights.
Stride length (SL) and base of support (BS) in normal and acute thoracolumbar spinal cord injury (SCI) affected dogs were compared.
SL and BS were consistent across three testing sessions in normal dogs.
SL was shorter in pelvic and thoracic limbs for SCI-affected dogs at 3, 10, and 30 days.
BS in the thoracic limbs was wider in SCI-affected dogs at 3 and 30 days.
Pelvic limb BS did not differ between normal and SCI-affected dogs at any time point.
Acknowledgements
This study was funded by the Morris Animal Foundation D13CA-024 and NIH CCTS UL1TR001070. The authors also gratefully acknowledge Mrs. Amanda Disher, Ms. Heather Myers, Ms. Tamra Mathie and, Ms. Annie Adrian for their assistance with data collection and Mr. Tim Vojt for his assistance with figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
References
- Aikawa T, Fujita H, Shibata M, Takahashi T. Recurrent thoracolumbar intervertebral disc extrusion after hemilaminectomy and concomitant prophylactic fenestration in 662 chondrodystrophic dogs. Veterinary Surgery. 2012;41:381–390. doi: 10.1111/j.1532-950X.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- Ballermann M, Tse ADY, Misiaszek JE, Fouad K. Adaptations in the walking pattern of spinal cord injured rats. Journal of Neurotrauma. 2006;23:897–907. doi: 10.1089/neu.2006.23.897. [DOI] [PubMed] [Google Scholar]
- Bazley FA, Maybhate A, Tan CS, Thakor NV, Kerr C, All AH. Enhancement of bilateral cortical somatosensory evoked potentials to intact forelimb stimulation following thoracic contusion spinal cord injury in rats. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2014;22:953–964. doi: 10.1109/TNSRE.2014.2319313. [DOI] [PubMed] [Google Scholar]
- Bergknut N, Egenvall A, Hagman R, Gustås P, Hazewinkel HA, Meij BP, Lagerstedt AS. Incidence of intervertebral disk degeneration-related diseases and associated mortality rates in dogs. Journal of the American Veterinary Medical Association. 2012;240:1300–1309. doi: 10.2460/javma.240.11.1300. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Toombs JP, Breur G, Widmer WR, Waters D, Harbath AM, March P, Adams LG. An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. Journal of Neurotrauma. 1999;16:639–657. doi: 10.1089/neu.1999.16.639. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antiboides to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Cheng H, Almström S, Giménex-Llort L, Chang R, Ove Ogren S, Hoffer B, Olson L. Gait Analysis of adult paraplegic rats after spinal cord repair. Experimental Neurology. 1997;148:544–557. doi: 10.1006/exnr.1997.6708. [DOI] [PubMed] [Google Scholar]
- Collazos-Castro JE, López-Dolado E, Nieto-Sampedro M. Locomotor deficits and adaptive mechanisms after thoracic spinal cord contusion in the adult rat. Journal of Neurotrauma. 2006;23:1–17. doi: 10.1089/neu.2006.23.1. [DOI] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt JR. An Index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experimental Neurology. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pedersen V, Schwab ME, Brösamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Current Biology. 2001;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- Gordon-Evans WJ, Evans RB, Conzemius MG. Accuracy of spatiotemporal variables in gait analysis of neurologic dogs. Journal of Neurotrauma. 2009;26:1055–1060. doi: 10.1089/neu.2008.0805. [DOI] [PubMed] [Google Scholar]
- Granger N, Blamires H, Franklin RJ, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135:3227–3237. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers FP, Lankhorst AJ, Van Laar TJ, Veldhuis WB, Gispen WH. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. Journal of Neurotrauma. 2001;18:187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- Hamers FP, Kiipmans GC, Joosten EAJ. Catwalk-assisted gait analysis in the assessment of spinal cord injury. Journal of Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- Hamilton L, Franklin RJ, Jeffery ND. Quantification of deficits in lateral paw positioning after spinal cord injury in dogs. BMC Veterinary Research. 2008;4:47. doi: 10.1186/1746-6148-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Matsunaga S, Jeffery ND, Sasaki N, Nishimura R, Mochizuki M, Kasahara M, Fujiwara R, Ogawa H. Prognostic value of magnetic resonance imaging in dogs with paraplegia caused by thoracolumbar intervertebral disk extrusion: 77 cases (2000-2003). Journal of American Veterinary Medical Association. 2005;227:1454–1460. doi: 10.2460/javma.2005.227.1454. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Smith PM, Lakatos A, Ibanez C, Ito D, Franklin RJ. Clinical canine spinal cord injury provides an opportunity to examine the issues in translating laboratory techniques into practical therapy. Spinal Cord. 2006;44:584–593. doi: 10.1038/sj.sc.3101912. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Hamilton L, Granger N. Designing clinical trials in canine spinal cord injury as a model to translate successful laboratory interventions into clinical practice. Veterinary Record. 2011;168:102–107. doi: 10.1136/vr.d475. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nitor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. Journal of Neuroscience. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapdor K, Dulfer BG, Hammann A, Van der Staay FJ. A low-cost method to analyse footprint patterns. Journal of Neuroscience Methods. 1997;75:49–54. doi: 10.1016/s0165-0270(97)00042-3. [DOI] [PubMed] [Google Scholar]
- Kunkel-Bagden E, Bregman BS. Spinal cord transplants enhance the recovery of locomotor function after spinal cord injury at birth. Experimental Brain Research. 1990;81:25–34. doi: 10.1007/BF00230097. [DOI] [PubMed] [Google Scholar]
- Kunkel-Bagden E, Dai HN, Bregman BS. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Experimental Neurology. 1993;119:153–164. doi: 10.1006/exnr.1993.1017. [DOI] [PubMed] [Google Scholar]
- Laverty PH, Leskovar A, Breur GJ, Coates JR, Bergman RL, Widmer WR, Toombs JP, Shapiro S, Borgens RB. A preliminary study of intravenous surfactants in paraplegic dogs: polymer therapy in canine clinical SCI. Journal of Neurotrauma. 2004;21:1767–1777. doi: 10.1089/neu.2004.21.1767. [DOI] [PubMed] [Google Scholar]
- Levine JM, Levine GJ, Porter BF, Topp K, Noble-Haeusslein LJ. Naturally occurring disk herniation in dogs: an opportunity for pre-clinical spinal cord injury research. Journal of Neurotrauma. 2011;28:675–688. doi: 10.1089/neu.2010.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Muguet-Chanoit AC, Smith DT, Laber E, Olby NJ. Potassium channel antagonists 4-aminopyridine and the T-butyl carbamate derivative of 4-aminopyridine improve hind limb function in chronically non-ambulatory dogs; a blinded, placebo-controlled trial. PLoS One. 2014;9:e116139. doi: 10.1371/journal.pone.0116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Research. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Springer JE. Quantification of locomotor recovery following spinal cord contusion in adult rats. Journal of Neurotrauma. 2006;23:1632–1653. doi: 10.1089/neu.2006.23.1632. [DOI] [PubMed] [Google Scholar]
- Olby N, Levine J, Harris T, Muñana K, Skeen T, Sharp N. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996-2001). Journal of American Veterinary Association. 2003;222:762–769. doi: 10.2460/javma.2003.222.762. [DOI] [PubMed] [Google Scholar]
- Olby N, Harris T, Burr J, Muñana K, Sharp N, Keene B. Recovery of pelvic limb function in dogs following acute intervertebral disc herniations. Journal of Neurotrauma. 2004;21:49–59. doi: 10.1089/089771504772695940. [DOI] [PubMed] [Google Scholar]
- Oza CS, Giszter SF. Plasticity and alterations of trunk motor cortex following spinal cord injury and non-stepping robot and treadmill training. Experimental Neurology. 2014;256:57–69. doi: 10.1016/j.expneurol.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RMA, Hendricks A, Volk HA, Shihab NK, Burn CC. How long and low can you go? Effect of conformation on the risk of thoracolumbar intervertebral disc extrusion in domestic dogs. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0069650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemel JR, Duncan G, Chen KW, Shannon C, Park S, Sparling JS, Tetzlaff W. A graded forceps crush spinal cord injury model in mice. Journal of Neurotrauma. 2008;25:350–370. doi: 10.1089/neu.2007.0426. [DOI] [PubMed] [Google Scholar]
- Rangasamy SB. Locomotor recovery after spinal cord hemisection/contusion injuries in Bonnet Monkeys: footprint testing-a minireview. Synapse. 2013;67:427–453. doi: 10.1002/syn.21645. [DOI] [PubMed] [Google Scholar]
- Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Preclinical Pain Consortium. Mogil JS, Stöhr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: A critical appraisal and call for uniform reporting standards. Pain. 2009;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Stokes BT, Reier PJ. Fetal grafts alter chronic behavioral outcome after contusion damage to the adult rat spinal cord. Experimental Neurology. 1992;116:1–12. doi: 10.1016/0014-4886(92)90171-l. [DOI] [PubMed] [Google Scholar]
- Varejao AS, Cabrita AM, Meek MF, Bulas-Cruz J, Melo-Pinto P, Raimondo S, Geuna S, Giacobini-Robecchi MG. Functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. Journal of Neurotrauma. 2004;21:1652–1670. doi: 10.1089/neu.2004.21.1652. [DOI] [PubMed] [Google Scholar]
- Webb AA, Muir GD. Compensatory locomotor adjustments of rats with cervical or thoracic spinal cord hemisections. Journal of Neurotrauma. 2002;19:239–256. doi: 10.1089/08977150252806983. [DOI] [PubMed] [Google Scholar]
- Yagüe JG, Humanes-Valera D, Aguilar J, Foffani G. Functional reorganization of the forepaw cortical representation immediately after thoracic spinal cord hemisection in rats. Experimental Neurology. 2014;257:19–24. doi: 10.1016/j.expneurol.2014.03.015. [DOI] [PubMed] [Google Scholar]