Abstract
The delivery of drugs, antigens, and imaging agents benefits from using nanotechnology-based carriers. The successful translation of nanoformulations to the clinic involves thorough assessment of their safety profiles, which, among other endpoints, includes evaluation of immunotoxicity. The past decade of research focusing on nanoparticle interaction with the immune system has been fruitful in terms of understanding the basics of nanoparticle immunocompatibility, developing a bioanalytical infrastructure to screen for nanoparticle-mediated immune reactions, beginning to uncover the mechanisms of nanoparticle immunotoxicity, and utilizing current knowledge about the structure–activity relationship between nanoparticles’ physicochemical properties and their effects on the immune system to guide safe drug delivery. In the present review, we focus on the most prominent pieces of the nanoparticle–immune system puzzle and discuss the achievements, disappointments, and lessons learned over the past 15 years of research on the immunotoxicity of engineered nanomaterials.
Keywords: Nanoparticles, Preclinical, Immunotoxicity, Immunology, Drug delivery
Graphical Abstract
API- active pharmaceutical ingredient; NP – Nanoparticles; PCP – Physicochemical properties, CARPA – Complement activation-related pseudoallergy, ICH – International Conference on Harmonization
1. INTRODUCTION
The immune system's function in the maintenance of tissue homeostasis is to protect the host from environmental agents such as microbes or chemicals, and thereby preserve the integrity of the body. This is done through effective surveillance and elimination of foreign and abnormal self cells and structures from the body. It is well known that certain environmental contaminants and xenobiotics, as well as other drugs, may alter the immune system's normal function. Therefore, screening for immunotoxicity is a generally accepted step in toxicological research related to both environmental factors and pharmaceutical products (Luebke, 2012).
The interactions between nanoparticles and various components of the immune system have become an active area of research in bio- and nanotechnology because the benefits of using nanotechnology in industry and medicine are often questioned over concerns regarding the safety of these novel materials. The past decade of research has shown that, while nanoparticles can be toxic, nanotechnology engineering can modify these materials to either avoid or specifically target the immune system. Avoiding interaction with the immune system is desirable when the nanoparticles are being used for medical applications not intended to stimulate or inhibit the immune system, as well as when they are used for industrial and environmental applications. Specific targeting of the immune system, on the other hand, provides an attractive option for vaccine delivery, as well as for improving the quality of anti-inflammatory, anticancer, and antiviral therapies (Mallipeddi and Rohan, 2010; Gonzalez-Aramundizet al., 2012; Zaman et al., 2013; Tran and Amiji, 2015). Moreover, nanotechnology-based carriers can be used to reduce the immunotoxicity of traditional drugs (Libutti et al., 2010).
Some nanomaterials, metal colloids and liposomes, for example, were in use more than a decade ago (Gregoriadis et al., 1974), yet most active research in this field began in early 2000, fueled by the attention paid by regulatory agencies, such as the United States Environmental Protection Agency (EPA) and the U.S. Food and Drug Administration (FDA), to the rapidly growing number of applications containing various types of engineered nanomaterials. The increase in submissions was expected since innovative research in this area had been progressing for years, culminated by the establishment of several breakthrough technologies that led to the discovery of fullerenes (Benning et al., 1992), carbon nanotubes (Ramirez et al., 1994), dendritic polymers (Tomalia, 1991; Newkome et al., 2002), and quantum dots (Takagahara, 1987). In 2005–2006, many worldwide initiatives were launched to improve the understanding of nanoparticle safety and included, among others, the establishment of the Nanotechnology Task Force by the FDA (http://www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/ucm2006658.htm), several nanotechnology research programs by the EPA (http://www2.epa.gov/chemical-research/research-evaluating-nanomaterials-chemical-safety), the E56 committee by the American Society for Testing and Materials (ASTM) International (http://www.astm.org/COMMITTEE/E56.htm), and the TC229 Nanotechnologies technical committee by the International Organization for Standardization (ISO) (http://www.iso.org/iso/iso_technical_committee?commid=381983). In addition to these efforts, the U.S. National Cancer Institute established the Nanotechnology Characterization Laboratory (NCL) to accelerate the translation of nanotechnology-based concepts intended for medical applications in the area of cancer diagnosis and therapy from bench to bedside (http://ncl.cancer.gov/). One of the initial goals of the NCL was to support the nanotechnology community by developing a so-called assay cascade that would include, among other tests, a battery of immunological assays. This assay cascade contributed to the initial understanding of the interactions between nanoparticles and the immune system and created a framework for stimulating discussions in the area of nano-immunotoxicology (Dobrovolskaia and McNeil, 2007; Marx, 2008; Dobrovolskaia et al., 2009a; Pantic, 2011; Smith et al., 2013). Recently, the European Commission has established the European Nanomedicine Characterization Laboratory (EU-NCL), which shares several objectives with those of the NCL (https://ec.europa.eu/jrc/en/news/eu-ncl-launched).
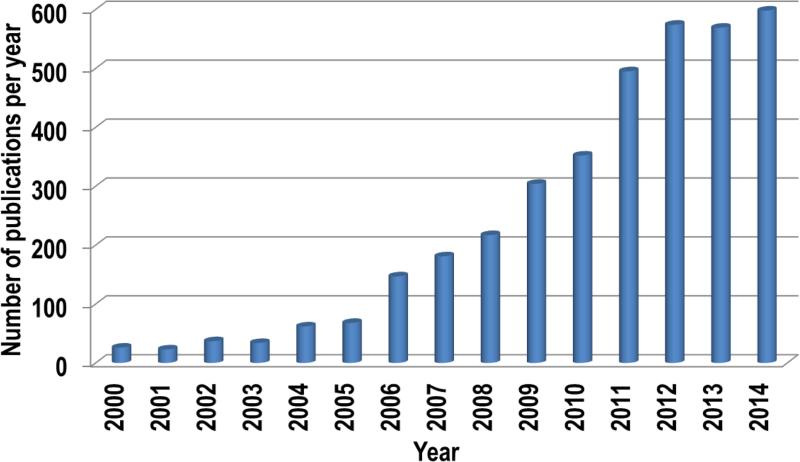
The rapid growth of this field becomes obvious when one compares the number of publications searchable in PubMed using the key words “nanoparticles” and “immune system” between years 2000 and 2015 (Fig. 1). Reviewing these data reveals many advances, as well as disappointments. Moreover, delving into the mechanisms of nanoparticle immunotoxicity uncovered many challenges in material characterization. Due to the wide variety of nanomaterials available, the characterization of their physicochemical properties is directed toward addressing parameters specific to certain type of particles (e.g. porosity is applicable to silicon nanoparticles, but is not informative for liposomes and dendrimers). The grand challenge in the particle characterization that precedes immunotoxicity studies relates to the estimation of immunoreactive contaminants, such as synthesis byproducts (e.g. iron catalysts in carbon nanotubes, cetyltrimethylammonium bromide [CTAB] in gold nanorods), and bacterial endotoxins, as well as excipients (e.g. Cremophor EL, polysorbate 80), and linkers (e.g. certain linkers used to attach poly(ethylene glycol) [PEG] to the nanoparticle surface) (Crist et al., 2013).
Fig. 1.
Publications statistics. The PubMed data base was searched using the keywords “nanoparticles” and “immune system” for the years 2000–2015. The data for 2015 were excluded from the analysis because the publication year was incomplete at the time of the search. Each bar shows the total publication number per year.
The challenges related to the physicochemical characterization (Clogston and Patri, 2013) and estimation of endotoxin contamination have been recently reviewed elsewhere (Crist et al., 2013; Dobrovolskaia, 2015).
The immunotoxicity of environmental materials has also been reviewed elsewhere (Kagan et al., 2010b).
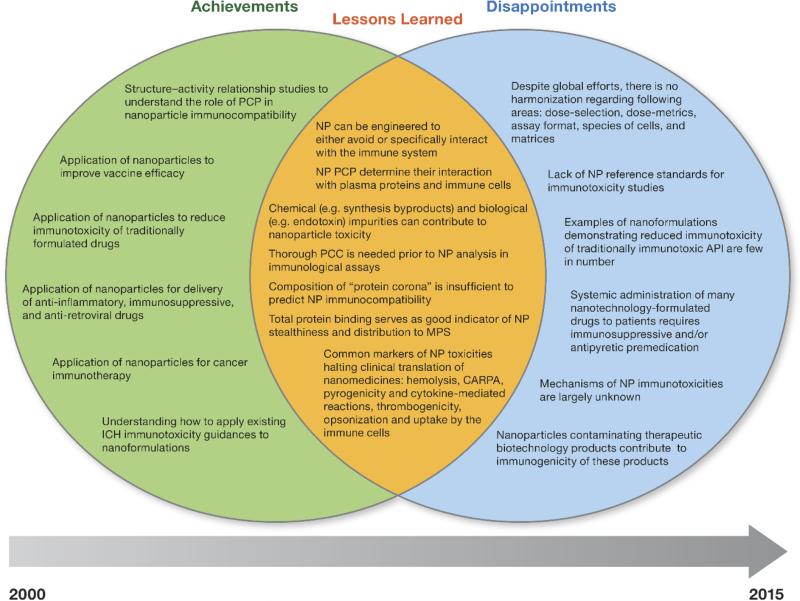
Herein, we focus on the most prominent pieces of the nanoparticle–immune system puzzle, discussing what worked, what didn't, and what has been learned over the past 15 years of research on nanomaterials engineered for biomedical applications. A summary of achievements, disappointments, and lessons learned is presented in Fig. 2, and is further discussed below.
Fig. 2.
Achievements, disappointments, and lessons learned from the characterization of engineered nanomaterials over the past decade. This diagram outlines achievements (left circle) and disappointments (right circle) based on the studies dedicated to investigating nanoparticle immunotoxicity over the past decade. The overlapping area shows the lessons learned from these studies. API- active pharmaceutical ingredient; NP – Nanoparticles; PCP – Physicochemical properties, CARPA – Complement activation-related pseudoallergy, ICH – International Conference on Harmonization.
2. ACHIEVEMENTS
2.1. Structure–activity relationship
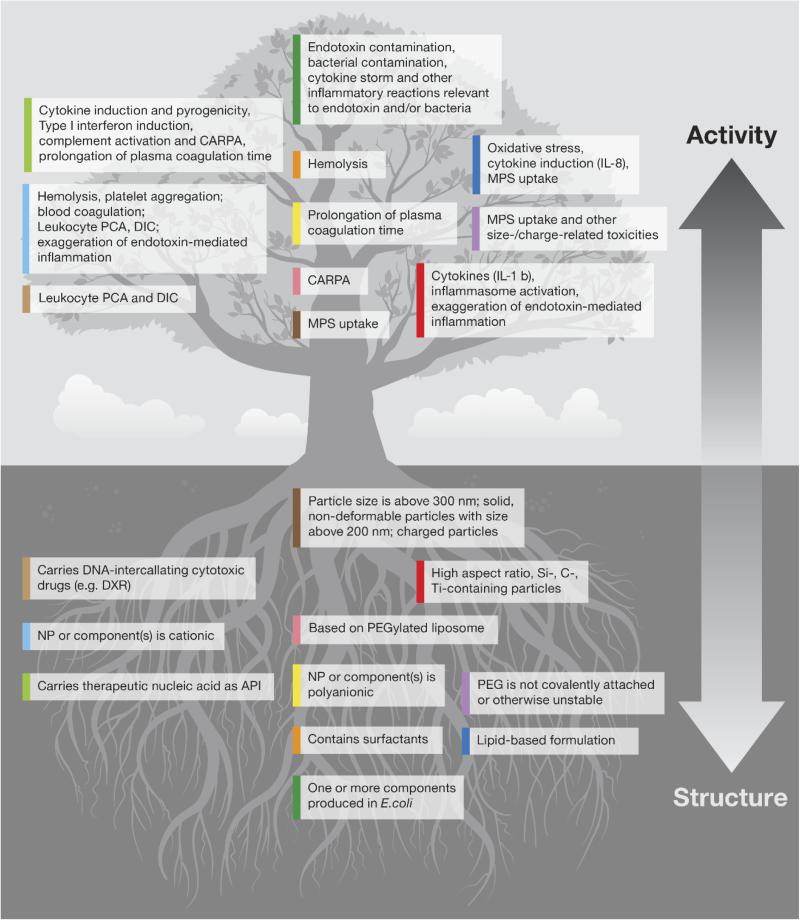
The physicochemical properties of nanoparticles determine their interactions with proteins in biological matrices (e.g. blood plasma and alveolar fluid) and with the immune cells. The structure–activity relationships between the most prominent physicochemical properties of nanoparticles and their effects on the immune system that lead to the most common types of immunotoxicity are summarized in Fig. 3. Below, we review several examples.
Figure 3.
Structure–activity relationship summary. Shown are the structure–activity relationships between nanoparticles and their effects on the immune system. Each block listed in the bottom (structure) part of the figure is color-coded. To find what toxicity is related to the given structure block, please find the block in the top (activity) part of the figure marked with the color matching that of the structure block. PCA – Procoagulant activity, DIC – Disseminated intravascular coagulation, CARPA – Complement activation-related pseudoallergy, MPS – Mononuclear phagocytic system, IL – Interleukin, PEG – Polyethylene glycol, NP – Nanoparticle, DXR – Doxorubicin, API – Active pharmaceutical ingredient, DNA – Deoxyribonucleic acid.
Nanoparticles with cationic surfaces, or those that carry cationic ligands, interact with biological membranes electrostatically. This leads to cellular damage, which triggers hemolysis, platelet activation, and aggregation, and to the induction of leukocyte procoagulant activity (PCA) and disseminated intravascular coagulation (DIC) (Greish et al., 2012; Jones et al., 2012a; Jones et al., 2012b; Ziemba et al., 2012). For example, cationic dendrimers of different architecture and size (generation five [G5] and generation four [G4] poly (propylene imine) [PPI] dendrimers [Bhadra et al., 2005; Agashe et al., 2006], G4 polyamidoamine [PAMAM] dendrimers [Bhadra et al., 2003; Asthana et al., 2005], generation three [G3] PAMAM and G3 PPI dendrimers [Malik et al., 2000], as well as G4 poly-L-lysine [PLL] dendrimers [Agrawal et al., 2007]) were shown to be hemolytic both in vitro and in vivo. The in vitro percent hemolysis varied from 14 to 86% in whole blood from human donors and various animal species, and was dependent on the density of the surface groups. Likewise, cationic PAMAM dendrimers, but not their anionic and neutral counterparts, altered key platelet functions and perturbed plasma coagulation, which culminated with DIC (Greish et al., 2012; Jones et al., 2012a; Jones et al., 2012b). The particle size, surface charge, and conformation of the polymer coating are important determinants of particle clearance by the mononuclear phagocytic system (MPS) in that smaller particles (100–200 nm) with unprotected surfaces and surfaces coated with a hydrophilic polymer in a “mushroom” configuration are primarily cleared by Kupffer cells in the liver; larger particles are eliminated by red pulp macrophages in the spleen. The addition of a hydrophilic polymer coating in a “brush” configuration protects particles from immune recognition, while increasing the particle size above 300 nm provides no protection, regardless of the polymer conformation (Gbadamosi et al., 2002). Exposure to high aspect ratio particles (e.g. carbon nanotubes, titanium nanobelts, cellulose nanofibers), as well as certain metallic particles (e.g. Si), results in inflammasome activation and the induction of proinflammatory cytokine interleukin (IL)-1β. These particles, as well as certain cationic and carbon-based particles, can exaggerate endotoxin-mediated inflammation (Baron et al., 2015).
The immunotoxicity of a nanoparticle is also influenced by the therapeutic payload it carries. For example, the induction of cytokines and type I interferons, the inflammatory reaction, the prolongation of plasma coagulation time, and complement activation are common dose-limiting toxicities of therapeutic nucleic acids (Levin, 1999). These toxicities are also commonly observed with nanoformulated nucleic acids, and this limits their translation into clinical use (Dobrovolskaia and McNeil, 2015a; Dobrovolskaia and McNeil, 2015b). Cytotoxic DNA-intercalating drugs used to treat cancer (e.g. doxorubicin, daunorubicin, and vincristine) are known to induce PCA and DIC (Wheeler and Geczy, 1990; Swystun et al., 2009; Kim et al., 2011). Formulating these drugs using nanotechnology carriers may help avoiding the toxicity. However, if overcoming these toxicities is not considered during the design and optimization of nanoformulated versions of these drugs, both PCA and DIC may not be resolved.
2.2. Application of nanoparticles to improve vaccine efficacy
The efficacy of nanoparticle-based vaccines depends on the interactions between the particles and the target cells, and is determined by the physicochemical properties of the particles (size, shape, and surface functionalities) because these properties play a key role in particle recognition by the antigen-presenting cells (APCs). The type of nanocarrier is generally selected based on the type of immune response desired from the vaccine. Nanoparticles have been shown to provide a wide variety of advantages over conventional adjuvants. They can improve the solubility of hydrophobic antigens; provide controlled and sustainable release of antigens, therefore reducing the number of required immunizations; target antigens to specific cells and tissues, thus reducing side effects; prevent antigen degradation and deliver multiple antigens concurrently (reviewed in [Xiang et al., 2008; Xiang et al., 2010]). As of today, a wide variety of engineered nanomaterials from different classes (polymeric, chitosanic, magnetic, latex, gold, silica, and polystyrene) have been used successfully as antigen carriers and vaccine adjuvants (Tighe et al., 1998; Pavelic et al., 2001; Weiss et al., 2002; Walsh et al., 2003; Fifis et al., 2004a; Fifis et al., 2004b; Minigo et al., 2007; Mottram et al., 2007).
Depending on their size, particles are internalized by APCs via different pathways, including both pino- and phagocytosis (O'Hagan et al., 2001; Fifis et al., 2004a). Moreover, macrophages utilize multiple routes to take up the same types of nanoparticles (Franca et al., 2011). Several studies reported that smaller particles (20–200 nm) elicit stronger immune responses than their larger counterparts (O'Hagan et al., 2001; Fifis et al., 2004a; Fifis et al., 2004b; Minigo et al., 2007; Mottram et al., 2007; Manolova et al., 2008). For example, Plebanski and her group conducted a series of studies to demonstrate that 40–50 nm polystyrene particles induce potent CD4+ and CD8+ T-cell responses and do so more efficiently than their larger (> 500 nm) counterparts. In contrast, particles > 500 nm in size were more active in inducing interferon (IFN)-γ and antibody responses (Fifis et al., 2004a; Fifis et al., 2004b; Minigoet al., 2007; Mottram et al., 2007). Several studies demonstrated that small (< 100 nm) nanoparticles quickly travel to the draining lymph nodes (LNs) after intradermal injection and effectively target LN-resident dendritic cells (DCs), B-cells, and macrophages (Manolova et al., 2008),(Reddy et al., 2007b). These data suggested that large particles depend on interaction with and uptake by tissue-resident APCs, while smaller particles utilize both cell-associated migration and lymphatic drainage, thus providing better antigen presentation (Manolova et al., 2008). Manipulation of nanoparticle size, shape, surface chemistry, and charge is generally employed to maximize antigen delivery to DCs. For example, small (< 100 nm) nanoparticles were shown to be taken up more efficiently by DCs, while large (1 μm) particles were preferentially internalized by macrophages (Fifis et al., 2004a). Several other studies have also reported that ~ 50 nm is the optimal nanoparticle size for uptake by DCs (Aoyama et al., 2003; Nakai et al., 2003; Wang et al., 2011).
Particle size was also reported as a primary factor in determining the immunostimulatory profiles of vaccine formulations in that smaller particles (~ 220nm) were more potent in inducing IFN-α responses, while their larger counterparts (~1200 nm) induced tumor necrosis factor (TNF)-α (Rettig et al., 2010). This difference was attributed to the type of the cells that internalized these particles: plasmacytoid DCs engulfed smaller particles, while macrophages preferred larger particles. Moreover, particle size was also suggested to be a key factor in determining the type of immunity induced. For example 40-nm nanoparticles promoted Th1 and CD8+ T-cell responses, while 100-nm particles induced Th2 responses (Fifis et al., 2004a; Fifis et al., 2004b; Mottram et al., 2007).
Tuning particle zeta potential is another approach that has been explored in vaccine design. For example, positively charged particles demonstrate greater uptake by DCs (Thiele et al., 2003; Foged et al., 2005; Villanueva et al., 2009) and induction of DC maturation (Thiele et al., 2001; Jilek et al., 2004; Little et al., 2004; Jilek et al., 2005; Reddy et al., 2007a). Cationic poly(D,L-lactic-co-glycolic) (PLG) particles improved the delivery of DNA adsorbed on the particle surface to APCs and induced greater cytotoxic T-lymphocyte responses compared to plain DNA antigen (Singh et al., 2000). More comprehensive coverage of this subject is available elsewhere (Fesenkova, 2013; Xiang et al., 2013).
2.3. Application of nanoparticles for delivery of antiretroviral, immunosuppressive and anti-inflammatory drugs
2.3.1 Antiretroviral
Antiretroviral drug delivery has many assorted challenges, some of which are being effectively overcome using nanotechnology-based carriers. In addition to improving the solubility of antiretroviral drugs, nanoparticles are considered a means to improve drug delivery to tissues and cells serving as viral reservoirs. When antiretroviral drugs are administered using conventional routes and formulations, the concentrations of these drugs in the plasma are usually higher than the concentrations found in the lymphoid tissue, which serves as a major depot for the virus (Fletcher et al., 2014). HIV can replicate in the lymphatic tissue even when the viral load in the peripheral blood is low; therefore, the need to enhance drug delivery into lymphoid tissue is recognized by many as an effective way of targeting HIV both in systemic circulation and at its depot sites (Fletcher et al., 2014).
The physicochemical properties of nanoparticles that influence lymphatic delivery are: size, charge, molecular weight, lipophilicity, and surface ligands (surfactants, PEG, hyaluronic acid, biotin, peptides, antigens, and lectins) (Cho and Lee, 2014; Singh et al., 2014). Several antiretroviral drugs have been formulated using a wet-bead milling process and showed good stability and the desired tissue distribution. These examples include rilpivirine solid drug nanoparticles (Verloes, 2008; Baert et al., 2009) and a cabotegravir (S/GSK1265744) nanocrystal formulation (Spreen et al., 2013). Other approaches focusing on the use of nanoformulations for oral delivery of these drugs are in progress in order to address another problem related to poor patient adherence to antiretroviral therapy (Prinapori and Di Biagio, 2015). A more comprehensive review of this subject is available in a recent review by Liptrott et al. (Liptrott et al., in press).
2.3.2 Immunosuppressive and anti-inflammatory
Nanoparticles can be immunosuppressive per se or used to deliver immunosuppressive drugs. For example, inhaling carbon nanotubes was shown to suppress humoral immune response via a mechanism involving the production of transforming growth factor beta (TGF-β) by alveolar macrophages and subsequent prostaglandin production by spleenocytes leading to the systemic immunosuppression (Mitchell et al., 2009). Other examples of nanoparticles displaying immunosuppressive activities without bearing a therapeutic payload include the imaging agent Resovist, a single intravenous administration of which resulted in suppression of the antibody response to the model antigen (Shen et al., 2011). The water-soluble fullerene derivative polyhydroxy C60 was shown to inhibit type I hypersensitivity reactions to allergens, both in vitro and in vivo (Ryan et al., 2007). Likewise, allergen-loaded poly(D,L-lactic-co-glycolic) acid (PLGA) particles, chitosan, poly(lactic acid) (PLA), poly[methyl vinyl ether-co-maleic anhydride) nanoparticles, and dendrosomes were used to suppress type I and type II reactions to environmental and food allergens (Royet al., 1999; Scholl et al., 2004; Balenga et al., 2006; Gomez et al., 2007; Gomez et al., 2008), while synthetic peptide dendrimers were reported to block experimental allergic encephalomyelitis (Wegmann et al., 2008).
Other studies have shown the benefits of using nanoparticles for the targeted delivery of immunosuppressive and anti-inflammatory drugs, and to prevent the undesirable immunosuppression of small-molecule drugs (Stinchcombe et al., 2007). For example, using PLGA nanoparticles formulated to deliver glucocorticoids to inflamed joints in the mouse model of arthritis resulted in complete remission of the inflammatory response. The improved efficacy was due to the targeted and controlled release of steroids from the nanocarrier (Higaki et al., 2005). Liposomes loaded with clodronate were used to specifically eliminate macrophages in a swine model to protect animals from endotoxin-mediated lung injury (Gaca et al., 2003). Liposomal and polymeric nanoparticle reformulation of cyclosporine was reported to reduce off-target side effects (e.g. nephrotoxicity) (Freise et al., 1994; Italia et al., 2007). Tacrolimus delivery using lipid nanoparticles resulted in improved skin penetration and tissue deposition, as well as a reduction in side effects (Pople and Singh, 2012). Polylactide nanoparticles were used for ex vivo delivery of cyclosporine A into DCc (Azzi et al., 2010). Reinjection of these drug-loaded DCs into the footpads of mice improved drug delivery to the lymph nodes, where released cyclosporine suppressed T-cell proliferation (Azzi et al., 2010). Delivery of rapamycin by elastin-like polymeric nanoparticles resulted in reduced nephrotoxicity and injection site reactions, while demonstrating comparable efficacy (Shah et al., 2013).
The activity of liposomal formulations of glucocorticoids provides another example of how nanoparticles can alter a drug's tissue distribution so that it provides additional beneficial effects. In this example, the free drug affects T-lymphocytes, while its liposomal counterpart targets macrophages and induces an alternatively activated M2 phenotype, leading to the expression of anti-inflammatory cytokines and, consequently, reduced inflammation (Schweingruber et al., 2011). Nanotechnology is used not only to deliver single drugs, but also to co-deliver anti-inflammatory agents with different mechanisms of action. For example, dexamethasone-loaded PLGA nanoparticles can be combined with siRNA-targeting COX-2 to suppress inflammatory responses (Park et al., 2012). More examples illustrating the benefits of delivering immunosuppressive and anti-inflammatory drugs using nanoparticles have been recently reviewed elsewhere (Ilinskaya and Dobrovolskaia, 2014).
2.4. Application of nanoparticles for cancer immunotherapy
Cancer immunotherapy is another rapidly growing branch of nanomedicine. Drugs used for cancer immunotherapy vary both in structure and by mechanism of action. For example, Iipilimumab (anti-CTLA4) directly interacts with and activates immune cells by removing co-inhibitory signal, while GVAX (GM-CSF tumor vaccine) improves tumor recognition by making the tumor more immunostimulatory (Ali and Lee, 2015; Lipson et al., 2015). A recent study by Fiering et al. demonstrated the use of iron oxide nanoparticles and an alternating magnetic field to induce local hyperthermia in melanoma. Interestingly, besides heat-mediated tumor ablation, this treatment resulted in a potent CD8+ T-cell–dependent response against the tumor, preventing the recurrence of tumor growth (Toraya-Brown et al., 2014). Another study demonstrated that tumor-associated myeloid-derived suppressor cells (MDSCs) characterized by high levels of oxidative reactions may be responsible for the degradation of chemotherapeutic drugs in the tumor environment, and that this degradation could be significantly reduced by drug loading onto functionalized carbon nanotubes (Seo et al., 2015). This example offers an effective way to prolong drug function in the tumor environment by using a nanodelivery approach. Other recent examples include using poly (ethyleneimine) nanoparticles for the delivery of antiPD-1 siRNA (Teo et al., 2015), branched polyethyleneimine-superparamagnetic iron oxide nanoparticles for the enhancement of Th1 cell polarization of DCs (Hoang et al., 2015), 6-thioguanine-loaded polymeric micelles for the depletion of MDSCs (Jeanbart et al., 2015), and a CD4-targeted, oil-in-water emulsion for the co-delivery of IL-2 and TGF-β (McHugh et al., 2015).
2.5. Understanding the role of the immune system in nanoparticle biodegradation
The therapeutic use of biodegradable nanoparticles is accompanied by fewer safety concerns than those of durable nanomaterials. Not surprisingly, the majority of currently marketed nanomedicines are represented by biodegradable, lipid-based materials (Etheridge et al., 2013). Macrophages are known to play an active role in internalizing and degrading these nanocarriers (Song et al., 2012).
The use of nonbiodegradable nanoparticles is often associated with concern regarding their bioaccumulation and long-term toxicity. In this context, recent studies demonstrating the unique role of activated neutrophils in the enzymatic digestion of durable nanoparticles are very encouraging. Specifically, activated neutrophils were reported to participate in the biodegradation of carbon nanotubes. Myeloperoxidase (MPO)-reactive intermediates and hypochlorous acid (HClO) generated by MPO are believed to contribute to the biodegradation process (Kagan et al., 2010a; Bianco et al., 2011; Shvedova et al., 2012), and the existence of this mechanism was confirmed in vivo using MPO-deficient mice (Shvedova et al., 2012). MPO was also found to have a role in the oxidative biodegradation of single-wall carbon nanotubes activated human neutrophils (Kagan et al., 2010a). Moreover, additional research suggests that peroxynitrite (ONOO−)-driven oxidation, resulting from the enzymatic activities of NADPH-oxidase/inducible nitric oxide synthase (iNOS), functions as another carbon nanotube biodegradation pathway in activated macrophages (Vlasova et al., 2012; Bhattacharya et al., 2014; Farrera et al., 2014; Kagan et al., 2014). Recent data demonstrated that tumor-associated MDSCs expressing high levels of arginase, iNOS, NADPH oxidase, and MPO were also able to biodegrade carbon nanotubes via oxidative pathways. These MDSC properties were recently utilized for an interesting nanodelivery approach in which nitrogen-doped carbon nanotube cups (NCNCs) were loaded with therapeutic cargo (paclitaxel), sealed via conjugation with gold nanoparticles (GNPs), and opened by enzymatic oxidative processes within, or in the immediate proximity of, MDSCs. This mechanism was proposed to enhance antitumor immune responses by targeting and inactivating MDSCs using their own highly expressed oxidative enzymatic machinery (Zhao et al., 2015).
2.6. Application of nanoparticles to reducing the immunotoxicity of traditionally formulated drugs
In addition to their use as carriers of novel drugs, engineered nanomaterials are also increasingly being used to reformulate traditional drugs (low-molecular-weight drugs, therapeutic proteins, therapeutic antibodies, and nucleic acids). Reformulation of traditional drugs using nanotechnology has been shown to improve drug solubility and pharmacokinetics, as well as to reduce undesirable side effects. Below, we review several examples demonstrating how reformulation of traditional drugs using nanotechnology resulted in reduced immunotoxicity.
The traditional formulation of the cytotoxic oncology drug paclitaxel relies on the polyethoxylated castor oil excipient Cremophor-EL ®, which is known to induce anaphylaxis in sensitive individuals. The anaphylactic reaction to Cremophor-EL is mediated by its ability to activate the complement system (Weiszhar et al., 2012). Due to this side effect, the Cremophor-EL formulation of paclitaxel (Taxol ®) has to be administered via slow infusion, and after patient premedication with immunosuppressive drugs. In contrast to Taxol, the nanoalbumin formulation of paclitaxel (Abraxane ®) is administered via push injection, and does not require premedication (Gradishar et al., 2005). Likewise, TNF-α failed in clinical trials because it induces systemic immunostimulation, resulting in fever and hypotension. However, TNF-α immobilized on the surface of PEGylated colloidal gold nanoparticles (Cyt6091) has successfully passed a Phase I trial (Libutti et al., 2010). Therapeutic protein immunogenicity, which leads to the formation of antibodies that neutralize the drug and, in some cases, endogenous proteins, is a common reason for the discontinuation of such drugs (Rosenberg et al., 2012). Liposomes were shown to reduce the antigenicity of recombinant coagulation factor FVIII and to protect encapsulated protein from the antibodies formed in response to the traditional formulation of this therapeutic protein (Ramani et al., 2008). Other success stories demonstrating the reduction of immunotoxicity by reformulation of a traditional drug using nanotechnology-based carriers include the encapsulation of therapeutic antisense oligonucleotides into liposomes to prevent activation of the complement system (Klimuk et al., 2000) and to reduce cytokine-mediated toxicities (Yu et al., 2013), as well as the reformulation of the small-molecule oncology drug 5-fluorouracil, using chitosan nanoparticles to decrease its hematotoxicity (Giacalone et al., 2013; Cheng et al., 2014).
2.7. Understanding the applicability of the existing regulatory framework for immunotoxicity analysis of nanoformulations
Several nanotechnology-formulated drugs have already reached the market. Examples include solid lipid nanoparticles (e.g. Leunesse®), liposomes (e.g. Doxil®), protein-based nanoparticles (e.g. Abraxane), as well as nanocrystals (e.g. Emend®). Regulatory experience gained from working with these formulations, along with data from preclinical reports, has helped to develop a better understanding of how to apply the regulatory framework established and used for small-molecule and macromolecular therapeutics to complex nanotechnology formulations (Tyner and Sadrieh, 2011; Bancos et al., 2013; Cruz et al., 2013; Tyner et al., 2015). The properties shared by nanotechnology-based carriers that have already reached the market are biodegradability (e.g. emulsions and liposomes) and the ability to quickly dissolve in the body (e.g. nanocrystals). Durable nanoparticles (e.g. metals and metal colloids) are expected to accumulate in the body and, as such, raise immunological safety concerns. These concerns stem from the notion that durable materials tend to distribute to the MPS (Sadauskas et al., 2009; Di Gioacchino et al., 2011; Moon et al., 2011; Umbreit et al., 2012). Accumulation of these materials in the MPS may affect the normal function of this system, and therefore this concern should not be ignored.
The FDA applies the same regulatory framework established for all small-molecule and macromolecular therapeutics to the regulation of complex nanotechnology products (Bancos et al., 2013). The manufacture, characterization, and non-clinical safety evaluation of combination products containing nanotechnology platforms have also been covered in several recent regulatory documents, which recommend conducting studies relevant to each component of the complex formulation (CDER, 2006; CDER, 2008). For example, the International Conference on Harmonization (ICH) S8 and S6 guidelines are consulted to estimate the immunotoxicity of small-molecule and macromolecular components, respectively, of any nanotechnology-formulated combination product (CDER, 2001; Bancos et al., 2013).
3. DISAPPOINTMENTS
3.1. Harmonization of testing is still in progress
Ten years ago, international standards development organizations ASTM International and ISO established the E56 and TC229 committees, respectively, to lead the development of standard test methods for nanomaterials. This effort resulted in the development of three standardized test methods—ASTM E56-2524-08(2013), ASTM E56-2525-08(2013), and ISO 29701:210—for the analysis of the hemolytic properties of nanoparticles, the effects of nanoparticulate materials on the formation of mouse colony-forming unit granulocyte/macrophage colonies, and of potential endotoxin contamination in nanomaterials, respectively (http://www.astm.org/COMMIT/SUBCOMMIT/E5603.htm; http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_tc_browse.htm?commid=381983). While these harmonized methods are important to support immunotoxicity studies, they are obviously insufficient to address the broad spectrum of end-points that are indicative of nanoparticle immunotoxicity. Despite global efforts, there is no harmonization in the following areas: dose selection, dose metrics, assay format, cell species, and matrices. Recently, an integrated approach was proposed to estimate relevant in vitro doses of tested nanomaterials in terms of total mass, surface area, and particle number (Cohen et al., 2014). While the results of this study are encouraging, adaptation of this approach by other laboratories and harmonization efforts are needed.
3.2. Lack of nanoparticle reference standards for immunotoxicity studies
Reference standards are well-characterized materials with known properties. These materials can be used to validate toxicology protocols and ensure the quality of measurements specific to a given protocol measuring given end points. It is generally acknowledged that the lack of nanoparticle reference standards limits the validation of instruments, protocols, and materials used to assess exposure to nanomaterials and understand their biocompatibility. While many types of nanomaterials have been linked to certain types of immunotoxicity (e.g. cationic dendrimers are thrombogenic; PEGylated liposomes induce anaphylaxis), there are no standard reference materials to use for these and other types of immunotoxicity studies. Stefaniak et al. conducted a literature review and identified 25 nanomaterials that were considered to be candidate nanoparticle reference materials by standards development organizations worldwide (Stefaniak et al., 2013). Interestingly, this study found a limited consensus regarding the types of candidate nanoparticles between various organizations involved in the development of reference materials: the U.S. National Metrology Institute, the U.S. National Institute of Standards and Technology, the REFNANO project funded by the UK government, the Organization for Economic Cooperation and Development, and several NanoImpactNet projects (NanoImpactNet, NanoSustain, and NanoValid) funded by the European Commission (Stefaniak et al., 2013).
In the absence of consensus on and availability of relevant, well-characterized reference materials, immunotoxicity studies are conducted using traditional positive controls, such as lipopolysaccharide for cytokine induction, phytohemagglutinin for leukocyte proliferation, Triton X-100 for hemolysis, and cobra venom factor for complement activation (Dobrovolskaia, 2015). Preclinical studies often rely on nanomedicines with known clinical immunotoxicities (e.g. Doxil or Taxol for complement activation and anaphylaxis tests) (Dobrovolskaia, 2015). Despite the thorough characterization of their physicochemical properties, the main limitation in using these products as reference materials is their expense and limited accessibility to the nanotechnology research community.
3.3. Examples of nanoformulations demonstrating reduced immunotoxicity of traditionally immunotoxic APIs are fewer than expected
Despite clear examples demonstrating the potential of nanotechnology for reducing the immunotoxicity of traditional active pharmaceutical ingredients (APIs), the systemic administration of many nanoformulations to patients still requires immunosuppressive and/or antipyretic premedication. For example, the complexing of therapeutic nucleic acids with a nanocarrier is commonly based on electrostatic interactions. As such, the lipid and polymeric carriers used to formulate therapeutic nucleic acids tend to be cationic. As discussed earlier, cationic particles are known to be cytotoxic to a variety of immune cells, induce cytokine secretion, exaggerate endotoxin-mediated toxicities, activate complement, bind plasma proteins, trigger pro-coagulant activity, and may also affect protein conformation and function (Pantic, 2011; Boraschi et al., 2012; Dobrovolskaia and McNeil, 2013a). These data raise safety concerns. Indeed, several studies have demonstrated that base modifications were insufficient to reduce the immunostimulatory activity of certain nucleic acids (e.g. siRNA). Furthermore, lipid nanocarriers were shown to contribute to the drug's immunostimulation (Abrams et al., 2010). Examples of immunostimulatory lipids used to prepare nanocarriers for therapeutic nucleic acids include 2-(4-[(3β)-cholest-5-en-3-yloxy]butoxy)-N,N-dimethyl-3-[(9Z,12Z)-octadeca-9,12-dien-1-yloxy]propan-1-amine (CLinDMA) and PEG-dimyristoylglycerol (Abrams et al., 2010), protamine (Li et al., 1999), trimethyl ammonium propane-cholesterol (Kim et al., 2007), and 1,3-dioleoyl-3-trimethylammonium propane (DOTAP) (Li et al., 1999; Vasievich et al., 2011). Clinical studies investigating the safety of such formulations are often designed to prevent adverse reactions by premedicating patients with immunosuppressive cocktails containing immunosuppressive agents (e.g dexamethasone), antipyretic agents (e.g acetaminophen), histamine H1 receptor blockers (e.g. diphenhydramine), and histamine H2 receptor blockers (e.g. ranitidine) (Coelho et al., 2013). It is clear that the advantages of tuning the physicochemical properties of nanoparticles for the purpose of reducing drug immunotoxicity have not yet been fully explored. The continuing evolution of the framework for evaluating nanoparticle immunotoxicity is the likely reason for this observation.
3.4. The mechanisms of nanoparticle immunotoxicity are largely unknown
The majority of studies reported in the past decade were focused on understanding the structure–activity relationship between the physicochemical properties nanoparticles and immunotoxicity. Uncovering the mechanisms of nanoparticle immunotoxicity is still a work in progress. Several interesting mechanisms have been recently described. For example, the inhibition of phosphoinositol-3-kinase (PI3K) by cationic PAMAM dendrimers has been suggested to contribute to the exaggeration of lipopolysaccharide (LPS)-induced leukocyte PCA in human peripheral blood mononuclear cells by these particles (Ilinskaya et al., 2014). The activation of mitogen-activated protein kinase (MAPK) p38 by gold nanoparticles functionalized with α-lipoyl-ω–methoxy poly(ethylene glycol) was proposed as a mechanism for exaggeration of LPS-triggered nitric oxide and IL-6 secretion in murine macrophages (Liu et al., 2012). The binding of colloidal gold nanoparticles to high-mobility group B-1 was implicated in the attenuation of nuclear factor-kappaB (NF-kB) signaling, the phosphorylation of JNK, and the secretion of TNF-α triggered by TLR9 activation by CpG oligonucleotides (Tsai et al., 2012).
The induction of oxidative and nitrosative stress by zinc oxide nanoparticles was described as a mechanism through which these particles activate redox-sensitive NF-κB and MAPK signaling pathways, leading to an inflammatory response in human monocytes (Senapati et al., 2015). The oxidative stress induced by the nanoemulsion Cremophor-EL was also suggested to trigger IL-8 production by human monocytes via a mechanism that bypasses gene expression (Ilinskaya et al., 2015).
It is also important to note that the effects of nanomaterials on immune cells may result in both suppression of the immune effector cells and activation of the immune regulatory (immunosuppressive) cells. Therefore, any discussion of nanoparticle immunotoxicity should consider specific immune cell subsets. For instance, airborne carbon nanotubes have recently been reported to induce rapid accumulation of pulmonary MDSCs in mice, which was associated with the accelerated growth of lung carcinomas in vivo (Shvedova et al., 2013). Further analysis of the mechanism revealed that carbon nanotubes may presensitize MDSCs to produce the immunosuppressive cytokine TGF-β, which contributes to the observed immunosuppression and, as a consequence, tumor growth (Shvedova et al., 2013). Thus, both uncovering the immunomodulatory properties of nanomaterials and understanding the molecular and cellular mechanisms of their activities are important for the clinical translation of these materials and potential minimization of any undesirable immunoreactivity.
Further research is clearly needed to uncover additional mechanisms and to link them to the physicochemical properties of nanoparticles.
3.5. Accidental nanoparticulate contaminants contribute to the immunogenicity of therapeutic proteins
The observation that particulate materials between 0.1 and 10 μm in size can contaminate recombinant protein therapeutics and contribute to their immunogenicity has generated increasing levels of concern (Carpenter et al., 2010). The mechanism by which this occurs has not been fully investigated; however, several factors, such as nanoparticle-triggered protein aggregation (Mire-Sluis et al., 2011; Van Beers et al., 2012); the adsorption of proteins on the particle surface, leading to the formation of highly immunogenic repeated-protein structures (Mire-Sluis et al., 2011; Van Beers et al., 2012); and the exaggeration of inflammatory responses triggered by trace amounts of endotoxin (Dobrovolskaia and McNeil, in press) may play a role. Tungsten microparticles originating from the tungsten pins used in the manufacturing process were shown to induce protein aggregation and increase the immunogenicity of a recombinant protein product (Liu et al., 2010).
Another study suggested that hydrophobic metal, glass, and polystyrene particulates adsorb protein on the particle surface and contribute to protein aggregation and immunogenicity (Van Beers et al., 2012). Several other materials were named among common particulates found to contaminate therapeutic proteins, including cellulose and glass fibers, silicon oil, rubber, stainless steel, fluoropolymers, and plastics (Carpenteret al., 2010; Liu et al., 2010; Van Beers et al., 2012). Cellulose fibers were shown to exaggerate the production of endotoxin-induced pro-inflammatory cytokines (Dobrovolskaia and McNeil, in press). Gowns and other materials used in clean rooms during the manufacturing of therapeutic proteins—closures, filling pumps, containers, vial stoppers, etc.—serve as sources of these particulate contaminants (Carpenter et al., 2010). These data led regulatory agencies to require the detection and characterization of particulate materials that can contaminate therapeutic proteins; however, there is no general agreement as to what methods should be used (Carpenteret al., 2010). The major challenge in understanding the mechanism of therapeutic protein immunogenicity in the presence of contaminating particles is the limited quantities of these particulate materials. Even when a particulate contaminant is detected and isolated from the protein product, the quantity of the isolate is usually insufficient to conduct a follow-up mechanistic study (Carpenter et al., 2010). It is obvious that more research is needed to address this important issue.
4. LESSONS LEARNED
4.1. Nanoparticles’ physicochemical properties are the keys to determining particle interaction with the immune system
It is now well established that nanoparticles can be engineered to either avoid or specifically interact with the immune system. The tuning of nanoparticles to attain desirable attributes can be achieved by manipulating the physicochemical properties (size, charge, hydrophobicity, shape) of the particle that determine its interaction with plasma proteins and immune cells. This subject has been extensively reviewed elsewhere (Smith et al., 2013). We have also discussed many examples underlining this point in the achievement section of this paper.
4.2. Chemical (e.g. synthesis byproducts) and biological (e.g. endotoxin) impurities can contribute to nanoparticle toxicity
It is very important to distinguish nanoparticle-mediated immunotoxicities from those triggered by chemical and biological impurities. The presence of traces of CTAB used as a stabilizing agent in the synthesis of gold nanorods was implicated in the cytotoxicity of these particles (Leonov et al., 2008). Iron and nickel used to catalyze reactions involved in the synthesis of carbon nanotubes were shown to trigger inflammatory reactions in response to nanotube exposure (Madani et al., 2013). Bacterial endotoxin is a common biological impurity affecting over 30% of preclinical-grade nanomaterials (Crist et al., 2013; Dobrovolskaia and McNeil, 2013b). If not properly identified in and eliminated from nanoformulations, endotoxin can confound the results of both nanoparticle immunotoxicity and efficacy studies. The elimination of endotoxin from nanoparticles was shown to reduce their immunotoxicity (Vallhov et al., 2006). Moreover, some nanomaterials, while not inflammatory themselves, were able to potentiate endotoxin-mediated inflammation. Silica- and carbon-based nanomaterials, as well as some metal oxides, have been shown to exaggerate endotoxin-mediated inflammation in the lungs (Inoue et al., 2006; Inoue et al., 2007; Shi et al., 2010; Inoue, 2011; Inoue and Takano, 2011), while cationic PAMAM dendrimers were reported to exaggerate endotoxin-induced leukocyte PCA (Dobrovolskaia et al., 2012; Ilinskaya et al., 2014). Strategies for endotoxin detection have been discussed elsewhere (Dobrovolskaia, 2015).
4.3. Thorough physicochemical characterization is needed prior to nanoparticle analysis in immunological assays
Since the physicochemical properties of nanoparticles are the keys to determining particle interaction with the immune system, thorough particle characterization is needed prior to immunotoxicity studies (Clogston and Patri, 2013). Several examples have shown that instability of particle surface coatings results in inflammatory reactions, yet partial loss of surface coating is undetectable by dynamic light scattering and zeta potential analysis, traditionally used for particle characterization (Clogston and Patri, 2013; Crist et al., 2013). Certain processes and reagents commonly involved in nanoparticle research (e.g. sterilization procedures and inhibitors used for signal transduction studies) may also affect nanoparticle integrity (Zheng et al., 2011; Dobrovolskaia and McNeil, in press). Missing such details may lead to misinterpretation of the study results and faulty conclusions. Another important lesson has been learned from using nanoparticles for drug delivery: Drug conjugation to a nanotechnology-based carrier may change the drug's original properties. For example, celastrol conjugated to a dendrimer carrier retained its ability to suppress LPS-induced nitric oxide release, but lost its ability to inhibit production of pro-inflammatory cytokines (Boridy et al., 2012).
4.4. Total protein binding serves as good indicator of nanoparticle stealthiness and its distribution to the MPS
Proteins bind to a nanoparticle surface instantaneously upon entry of the particle into the bloodstream. Some of these proteins stay on the surface as long as the particles circulate in the bloodstream, while others dissociate from the particle surface or get replaced by proteins with a higher binding affinity. Protein binding was shown to affect nanoparticle hydrodynamic size and charge (Dobrovolskaia et al., 2009b), and was also suggested to influence the way cells and tissues interact with and process the particles, ultimately guiding cellular uptake, clearance route, and tissue distribution (Goppert and Muller, 2005; Michaelis et al., 2006; Nagayama et al., 2007; Zensi et al., 2009). It is now well established that total protein binding can serve as an indicator of particle “stealthiness.” Stealthy particles tend to stay in circulation longer. In contrast, particles with proteins bound to their surface are cleared by the cells of the MPS.
4.5. Composition of protein corona is insufficient to predict nanoparticle immunocompatibility
In contrast to total protein binding, the composition of the protein corona has less predictive value. Complement and fibrinogen are abundant plasma proteins, and they have been reported as part of the so-called “protein corona” for many engineered nanomaterials. However, the presence of these proteins on the particle surface per se does not mean that the proteins will be activated (Salvador-Morales et al., 2006; Salvador-Morales et al., 2008; Deng et al., 2009; Deng et al., 2011; Deng et al., 2012a; Deng et al., 2012b). The particle concentration needed to deplete these proteins to a level that would affect their function must be very high. Achieving such concentration in vitro is possible for some, but not all, nanoparticles. Moreover, protein levels in vivo vary from donor to donor, and even within the same donor, due to homeostasis. Therefore, the identification of the composition of a nanoparticle's protein corona cannot reliably serve as a predictor of particle immunotoxicity (Salvador-Morales et al., 2006; Salvador-Morales et al., 2008; Deng et al., 2009; Deng et al., 2011; Deng et al., 2012a; Deng et al., 2012b; Dobrovolskaia et al., 2014).
4.6. Common markers of nanoparticle immunotoxicities halting clinical translation of nanomedicines
The toxicities that represent the most common safety concerns and reasons for nanoparticle failure in the preclinical stage include erythrocyte damage, thrombogenicity (platelet aggregation, plasma coagulation, DIC, and leukocyte PCA), cytokine-mediated inflammation and cytokine storming, pyrogenicity (mainly due to bacterial endotoxin contamination), and anaphylaxis and other complement activation-mediated reactions, as well as recognition and uptake by the cells of the MPS (Dobrovolskaia, 2015). As such, screening for these toxicities early in preclinical development helps in eliminating potentially toxic candidates. Fig. 3 can be consulted as a guide to selecting the most appropriate screening method based on the immunotoxicity endpoint, which can be suggested by nanoparticle physicochemical properties.
5. CONCLUSION
The past decade has been full of exciting discoveries: unraveling trends in nanoparticle immunocompatibility, understanding the role of particle characterization, and finding therapeutic applications for variety of nanocarriers. Since the mechanisms of nanoparticle immunotoxicity are not completely understood, the next decade of research should focus on identifying mechanisms and mapping them to the physicochemical properties of nanoparticles.
Highlights.
Achievements, disappointments and lessons learned over past decade are reviewed
Areas in focus include characterization, immunotoxicity and utility in drug delivery
Future direction focusing on mechanistic immunotoxicity studies is proposed
Acknowledgments
M.D.'s work has been funded with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The content and conclusions of this publication are those of the authors and do not necessarily reflect the views or policies of the Department of Health and Human Services, National Institute for Occupational Safety and Health, Center for Disease Control, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
The authors have no financial information to disclose related to this study.
References
- Abrams MT, Koser ML, Seitzer J, Williams SC, DiPietro MA, Wang W, Shaw AW, Mao X, Jadhav V, Davide JP, Burke PA, Sachs AB, Stirdivant SM, Sepp-Lorenzino L. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Mol Ther. 2010;18:171–180. doi: 10.1038/mt.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agashe HB, Dutta T, Garg M, Jain NK. Investigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimer. J Pharm Pharmacol. 2006;58:1491–1498. doi: 10.1211/jpp.58.11.0010. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Gupta U, Jain NK. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials. 2007;28:3349–3359. doi: 10.1016/j.biomaterials.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ali S, Lee SK. Ipilimumab Therapy for Melanoma: A Mimic of Leptomeningeal Metastases. AJNR Am J Neuroradiol. 2015 doi: 10.3174/ajnr.A4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama Y, Kanamori T, Nakai T, Sasaki T, Horiuchi S, Sando S, Niidome T. Artificial Viruses and Their Application to Gene Delivery. Size-Controlled Gene Coating with Glycocluster Nanoparticles. Journal of the American Chemical Society. 2003;125:3455–3457. doi: 10.1021/ja029608t. [DOI] [PubMed] [Google Scholar]
- Asthana A, Chauhan AS, Diwan PV, Jain NK. Poly(amidoamine) (PAMAM) dendritic nanostructures for controlled site-specific delivery of acidic anti-inflammatory active ingredient. AAPS PharmSciTech. 2005;6:E536–542. doi: 10.1208/pt060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, Mfarrej B, Yang S, Jurewicz M, Ichimura T, Lindeman N, Cheng J, Abdi R. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB J. 2010;24:3927–3938. doi: 10.1096/fj.10-154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert L, van 't Klooster G, Dries W, Francois M, Wouters A, Basstanie E, Iterbeke K, Stappers F, Stevens P, Schueller L, Van Remoortere P, Kraus G, Wigerinck P, Rosier J. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2009;72:502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Balenga NA, Zahedifard F, Weiss R, Sarbolouki MN, Thalhamer J, Rafati S. Protective efficiency of dendrosomes as novel nano-sized adjuvants for DNA vaccination against birch pollen allergy. J Biotechnol. 2006;124:602–614. doi: 10.1016/j.jbiotec.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Bancos S, Tyner KM, Weaver JL. Immunotoxicity testing of drug-nanoparticle conjugates: regulatory considerations. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterials. World Scientific Publishing Ltd; Singapore: 2013. pp. 671–685. [Google Scholar]
- Baron L, Gombault A, Fanny M, Villeret B, Savigny F, Guillou N, Panek C, Le Bert M, Lagente V, Rassendren F, Riteau N, Couillin I. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015;6:e1629. doi: 10.1038/cddis.2014.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning PJ, Poirier DM, Ohno TR, Chen Y, Jost MB, Stepniak F, Kroll GH, Weaver JH, Fure J, Smalley RE. C60 and C70 fullerenes and potassium fullerides. Phys Rev B Condens Matter. 1992;45:6899–6913. doi: 10.1103/physrevb.45.6899. [DOI] [PubMed] [Google Scholar]
- Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257:111–124. doi: 10.1016/s0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- Bhadra D, Yadav AK, Bhadra S, Jain NK. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int J Pharm. 2005;295:221–233. doi: 10.1016/j.ijpharm.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Bhattacharya K, Sacchetti C, El-Sayed R, Fornara A, Kotchey GP, Gaugler JA, Star A, Bottini M, Fadeel B. Enzymatic ‘stripping’ and degradation of PEGylated carbon nanotubes. Nanoscale. 2014;6:14686–14690. doi: 10.1039/c4nr03604b. [DOI] [PubMed] [Google Scholar]
- Bianco A, Kostarelos K, Prato M. Making carbon nanotubes biocompatible and biodegradable. Chem Commun (Camb) 2011;47:10182–10188. doi: 10.1039/c1cc13011k. [DOI] [PubMed] [Google Scholar]
- Boraschi D, Costantino L, Italiani P. Interaction of nanoparticles with immunocompetent cells: nanosafety considerations. Nanomedicine (Lond) 2012;7:121–131. doi: 10.2217/nnm.11.169. [DOI] [PubMed] [Google Scholar]
- Boridy S, Soliman GM, Maysinger D. Modulation of inflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers. Nanomedicine (Lond) 2012;7:1149–1165. doi: 10.2217/nnm.12.16. [DOI] [PubMed] [Google Scholar]
- Carpenter J, Cherney B, Lubinecki A, Ma S, Marszal E, Mire-Sluis A, Nikolai T, Novak J, Ragheb J, Simak J. Meeting report on protein particles and immunogenicity of therapeutic proteins: filling in the gaps in risk evaluation and mitigation. Biologicals. 2010;38:602–611. doi: 10.1016/j.biologicals.2010.07.002. [DOI] [PubMed] [Google Scholar]
- CDER HHS, editor. Guidance for Industry: immunotoxicology evaluation of investigational new drugs. 2001 [Google Scholar]
- CDER U.S. Department of Health and Human Services, F.a.D.A., editor. Guidance for Industry: non-clinical safety evaluation of drug or biologic combinations. 2006 [Google Scholar]
- CDER Services, U.D.o.H.a.H., editor. Guidance for Industry and Review Staff: non-clinical safety evaluation of reformulated drug products and products intended for administration by alternative route. 2008 [Google Scholar]
- Cheng M, Chen H, Wang Y, Xu H, He B, Han J, Zhang Z. Optimized synthesis of glycyrrhetinic acid-modified chitosan 5-fluorouracil nanoparticles and their characteristics. Int J Nanomedicine. 2014;9:695–710. doi: 10.2147/IJN.S55255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Lee YB. Nano-sized drug delivery systems for lymphatic delivery. J Nanosci Nanotechnol. 2014;14:868–880. doi: 10.1166/jnn.2014.9122. [DOI] [PubMed] [Google Scholar]
- Clogston JD, Patri AK. Importance of physicochemical characterization prior to immunological studies. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterials. World Scientific Publishing Ltd; Singapore: 2013. [Google Scholar]
- Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- Cohen JM, Teeguarden JG, Demokritou P. An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part Fibre Toxicol. 2014;11:20. doi: 10.1186/1743-8977-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RM, Grossman JH, Patri AK, Stern ST, Dobrovolskaia MA, Adiseshaiah PP, Clogston JD, McNeil SE. Common pitfalls in nanotechnology: lessons learned from NCI's Nanotechnology Characterization Laboratory. Integr Biol (Camb) 2013;5:66–73. doi: 10.1039/c2ib20117h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CN, Tyner KM, Velazquez L, Hyams KC, Jacobs A, Shaw AB, Jiang W, Lionberger R, Hinderling P, Kong Y, Brown PC, Ghosh T, Strasinger C, Suarez-Sharp S, Henry D, Van Uitert M, Sadrieh N, Morefield E. CDER risk assessment exercise to evaluate potential risks from the use of nanomaterials in drug products. AAPS J. 2013;15:623–628. doi: 10.1208/s12248-013-9466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- Deng ZJ, Liang M, Toth I, Monteiro M, Minchin RF. Plasma protein binding of positively and negatively charged polymer-coated gold nanoparticles elicits different biological responses. Nanotoxicology. 2012a doi: 10.3109/17435390.2012.655342. [DOI] [PubMed] [Google Scholar]
- Deng ZJ, Liang M, Toth I, Monteiro MJ, Minchin RF. Molecular interaction of poly(acrylic acid) gold nanoparticles with human fibrinogen. ACS Nano. 2012b;6:8962–8969. doi: 10.1021/nn3029953. [DOI] [PubMed] [Google Scholar]
- Deng ZJ, Mortimer G, Schiller T, Musumeci A, Martin D, Minchin RF. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. 2009;20:455101. doi: 10.1088/0957-4484/20/45/455101. [DOI] [PubMed] [Google Scholar]
- Di Gioacchino M, Petrarca C, Lazzarin F, Di Giampaolo L, Sabbioni E, Boscolo P, Mariani-Costantini R, Bernardini G. Immunotoxicity of nanoparticles. Int J Immunopathol Pharmacol. 2011;24:65S–71S. [PubMed] [Google Scholar]
- Dobrovolskaia MA. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy. J Control Release. 2015 doi: 10.1016/j.jconrel.2015.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Germolec DR, Weaver JL. Evaluation of nanoparticle immunotoxicity. Nat Nanotechnol. 2009a;4:411–414. doi: 10.1038/nnano.2009.175. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials: an introduction. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterials. World Scientific publishing Co. Pte. Ltd.; Singapore: 2013a. pp. 1–25. [Google Scholar]
- Dobrovolskaia MA, McNeil SE. Nanoparticles and Endotoxin. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaperials. World Scientific Publishing Ltd; Singapore: 2013b. pp. 77–115. [Google Scholar]
- Dobrovolskaia MA, McNeil SE. Immunological and hematological toxicities challenging clinical translation of nucleic acid-based therapeutics. Expert Opin Biol Ther. 2015a;15:1023–1048. doi: 10.1517/14712598.2015.1014794. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, McNeil SE. Strategy for selecting nanotechnology carriers to overcome immunological and hematological toxicities challenging clinical translation of nucleic acid-based therapeutics. Expert Opin Drug Deliv. 2015b;12:1163–1175. doi: 10.1517/17425247.2015.1042857. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, McNeil SE. Nanoparticles and Endotoxin. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaperials. World Scientific Publishing Ltd; Singapore: in press. [Google Scholar]
- Dobrovolskaia MA, Neun BW, Man S, Ye X, Hansen M, Patri AK, Crist RM, McNeil SE. Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles. Nanomedicine. 2014;10:1453–1463. doi: 10.1016/j.nano.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Patri AK, Potter TM, Rodriguez JC, Hall JB, McNeil SE. Dendrimer-induced leukocyte procoagulant activity depends on particle size and surface charge. Nanomedicine (Lond) 2012;7:245–256. doi: 10.2217/nnm.11.105. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Patri AK, Zheng J, Clogston JD, Ayub N, Aggarwal P, Neun BW, Hall JB, McNeil SE. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. 2009b;5:106–117. doi: 10.1016/j.nano.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 2013;9:1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrera C, Bhattacharya K, Lazzaretto B, Andon FT, Hultenby K, Kotchey GP, Star A, Fadeel B. Extracellular entrapment and degradation of single-walled carbon nanotubes. Nanoscale. 2014;6:6974–6983. doi: 10.1039/c3nr06047k. [DOI] [PubMed] [Google Scholar]
- Fesenkova V. Nanoparticles and dendritic cells. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterials. World Scientific Publishing Ltd; Singapore: 2013. [Google Scholar]
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nanovaccines against tumors. J Immunol. 2004a;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- Fifis T, Mottram P, Bogdanoska V, Hanley J, Plebanski M. Short peptide sequences containing MHC class I and/or class II epitopes linked to nano-beads induce strong immunity and inhibition of growth of antigen-specific tumour challenge in mice. Vaccine. 2004b;23:258–266. doi: 10.1016/j.vaccine.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, Anderson J, Perkey K, Stevenson M, Perelson AS, Douek DC, Haase AT, Schacker TW. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. International Journal of Pharmaceutics. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Franca A, Aggarwal P, Barsov EV, Kozlov SV, Dobrovolskaia MA, Gonzalez-Fernandez A. Macrophage scavenger receptor A mediates the uptake of gold colloids by macrophages in vitro. Nanomedicine (Lond) 2011;6:1175–1188. doi: 10.2217/nnm.11.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freise CE, Liu T, Hong K, Osorio RW, Papahadjopoulos D, Ferrell L, Ascher NL, Roberts JP. The increased efficacy and decreased nephrotoxicity of a cyclosporine liposome. Transplantation. 1994;57:928–932. doi: 10.1097/00007890-199403270-00027. [DOI] [PubMed] [Google Scholar]
- Gaca JG, Palestrant D, Lukes DJ, Olausson M, Parker W, Davis RD., Jr. Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate. J Surg Res. 2003;112:19–25. doi: 10.1016/s0022-4804(03)00142-2. [DOI] [PubMed] [Google Scholar]
- Gbadamosi JK, Hunter AC, Moghimi SM. PEGylation of microspheres generates a heterogeneous population of particles with differential surface characteristics and biological performance. FEBS Lett. 2002;532:338–344. doi: 10.1016/s0014-5793(02)03710-9. [DOI] [PubMed] [Google Scholar]
- Giacalone G, Bochot A, Fattal E, Hillaireau H. Drug-induced nanocarrier assembly as a strategy for the cellular delivery of nucleotides and nucleotide analogues. Biomacromolecules. 2013;14:737–742. doi: 10.1021/bm301832v. [DOI] [PubMed] [Google Scholar]
- Gomez S, Gamazo C, Roman BS, Ferrer M, Sanz ML, Irache JM. Gantrez AN nanoparticles as an adjuvant for oral immunotherapy with allergens. Vaccine. 2007;25:5263–5271. doi: 10.1016/j.vaccine.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Gomez S, Gamazo C, San Roman B, Ferrer M, Sanz ML, Espuelas S, Irache JM. Allergen immunotherapy with nanoparticles containing lipopolysaccharide from Brucella ovis. Eur J Pharm Biopharm. 2008;70:711–717. doi: 10.1016/j.ejpb.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aramundiz JV, Cordeiro AS, Csaba N, de la Fuente M, Alonso MJ. Nanovaccines : nanocarriers for antigen delivery. Biol Aujourdhui. 2012;206:249–261. doi: 10.1051/jbio/2012027. [DOI] [PubMed] [Google Scholar]
- Goppert TM, Muller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. J Drug Target. 2005;13:179–187. doi: 10.1080/10611860500071292. [DOI] [PubMed] [Google Scholar]
- Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O'Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G, Wills EJ, Swain CP, Tavill AS. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet. 1974;1:1313–1316. doi: 10.1016/s0140-6736(74)90682-5. [DOI] [PubMed] [Google Scholar]
- Greish K, Thiagarajan G, Herd H, Price R, Bauer H, Hubbard D, Burckle A, Sadekar S, Yu T, Anwar A, Ray A, Ghandehari H. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology. 2012;6:713–723. doi: 10.3109/17435390.2011.604442. [DOI] [PubMed] [Google Scholar]
- Higaki M, Ishihara T, Izumo N, Takatsu M, Mizushima Y. Treatment of experimental arthritis with poly(D, L-lactic/glycolic acid) nanoparticles encapsulating betamethasone sodium phosphate. Ann Rheum Dis. 2005;64:1132–1136. doi: 10.1136/ard.2004.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang MD, Lee HJ, Jung SH, Choi NR, Vo MC, Nguyen-Pham TN, Kim HJ, Park IK, Lee JJ. Branched Polyethylenimine-Superparamagnetic Iron Oxide Nanoparticles (bPEI-SPIONs) Improve the Immunogenicity of Tumor Antigens and Enhance Th1 Polarization of Dendritic Cells. J Immunol Res. 2015;2015:706379. doi: 10.1155/2015/706379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilinskaya AN, Clogston JD, McNeil SE, Dobrovolskaia MA. Induction of oxidative stress by Taxol(R) vehicle Cremophor-EL triggers production of interleukin-8 by peripheral blood mononuclear cells through the mechanism not requiring de novo synthesis of mRNA. Nanomedicine. 2015 doi: 10.1016/j.nano.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilinskaya AN, Dobrovolskaia MA. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol. 2014;171:3988–4000. doi: 10.1111/bph.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilinskaya AN, Man S, Patri AK, Clogston JD, Crist RM, Cachau RE, McNeil SE, Dobrovolskaia MA. Inhibition of phosphoinositol 3 kinase contributes to nanoparticle-mediated exaggeration of endotoxin-induced leukocyte procoagulant activity. Nanomedicine (Lond) 2014;9:1311–1326. doi: 10.2217/nnm.13.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K. Promoting effects of nanoparticles/materials on sensitive lung inflammatory diseases. Environ Health Prev Med. 2011;16:139–143. doi: 10.1007/s12199-010-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Takano H. Aggravating impact of nanoparticles on immune-mediated pulmonary inflammation. ScientificWorldJournal. 2011;11:382–390. doi: 10.1100/tsw.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Hirano S, Kobayashi T, Fujitani Y, Shimada A, Yoshikawa T. Effects of inhaled nanoparticles on acute lung injury induced by lipopolysaccharide in mice. Toxicology. 2007;238:99–110. doi: 10.1016/j.tox.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Hirano S, Sakurai M, Shimada A, Yoshikawa T. Effects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in mice. Environ Health Perspect. 2006;114:1325–1330. doi: 10.1289/ehp.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italia JL, Bhatt DK, Bhardwaj V, Tikoo K, Kumar MN. PLGA nanoparticles for oral delivery of cyclosporine: nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral. J Control Release. 2007;119:197–206. doi: 10.1016/j.jconrel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Jeanbart L, Kourtis IC, van der Vlies AJ, Swartz MA, Hubbell JA. 6-Thioguanine-loaded polymeric micelles deplete myeloid-derived suppressor cells and enhance the efficacy of T cell immunotherapy in tumor-bearing mice. Cancer Immunol Immunother. 2015;64:1033–1046. doi: 10.1007/s00262-015-1702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek S, Merkle HP, Walter E. DNA-loaded biodegradable microparticles as vaccine delivery systems and their interaction with dendritic cells. Advanced Drug Delivery Reviews. 2005;57:377–390. doi: 10.1016/j.addr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Jilek S, Ulrich M, Merkle HP, Walter E. Composition and surface charge of DNA-loaded microparticles determine maturation and cytokine secretion in human dendritic cells. Pharmaceutical Research. 2004;21:1240–1247. doi: 10.1023/b:pham.0000033012.16152.5d. [DOI] [PubMed] [Google Scholar]
- Jones CF, Campbell RA, Brooks AE, Assemi S, Tadjiki S, Thiagarajan G, Mulcock C, Weyrich AS, Brooks BD, Ghandehari H, Grainger DW. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano. 2012a;6:9900–9910. doi: 10.1021/nn303472r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CF, Campbell RA, Franks Z, Gibson CC, Thiagarajan G, Vieira-de-Abreu A, Sukavaneshvar S, Mohammad SF, Li DY, Ghandehari H, Weyrich AS, Brooks BD, Grainger DW. Cationic PAMAM dendrimers disrupt key platelet functions. Mol Pharm. 2012b;9:1599–1611. doi: 10.1021/mp2006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Kapralov AA, St Croix CM, Watkins SC, Kisin ER, Kotchey GP, Balasubramanian K, Vlasova II, Yu J, Kim K, Seo W, Mallampalli RK, Star A, Shvedova AA. Lung macrophages “digest” carbon nanotubes using a superoxide/peroxynitrite oxidative pathway. ACS Nano. 2014;8:5610–5621. doi: 10.1021/nn406484b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi J, Kisin ER, Murray AR, Franks J, Stolz D, Gou P, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010a;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Shi J, Feng W, Shvedova AA, Fadeel B. Fantastic voyage and opportunities of engineered nanomaterials: what are the potential risks of occupational exposures? J Occup Environ Med. 2010b;52:943–946. doi: 10.1097/JOM.0b013e3181dc6c52. [DOI] [PubMed] [Google Scholar]
- Kim JY, Choung S, Lee EJ, Kim YJ, Choi YC. Immune activation by siRNA/liposome complexes in mice is sequence- independent: lack of a role for Toll-like receptor 3 signaling. Mol Cells. 2007;24:247–254. [PubMed] [Google Scholar]
- Kim SH, Lim KM, Noh JY, Kim K, Kang S, Chang YK, Shin S, Chung JH. Doxorubicin-induced platelet procoagulant activities: an important clue for chemotherapy-associated thrombosis. Toxicol Sci. 2011;124:215–224. doi: 10.1093/toxsci/kfr222. [DOI] [PubMed] [Google Scholar]
- Klimuk SK, Semple SC, Nahirney PN, Mullen MC, Bennett CF, Scherrer P, Hope MJ. Enhanced anti-inflammatory activity of a liposomal intercellular adhesion molecule-1 antisense oligodeoxynucleotide in an acute model of contact hypersensitivity. J Pharmacol Exp Ther. 2000;292:480–488. [PubMed] [Google Scholar]
- Leonov AP, Zheng J, Clogston JD, Stern ST, Patri AK, Wei A. Detoxification of gold nanorods by treatment with polystyrenesulfonate. ACS Nano. 2008;2:2481–2488. doi: 10.1021/nn800466c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AA. A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim Biophys Acta. 1999;1489:69–84. doi: 10.1016/s0167-4781(99)00140-2. [DOI] [PubMed] [Google Scholar]
- Li S, Wu SP, Whitmore M, Loeffert EJ, Wang L, Watkins SC, Pitt BR, Huang L. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am J Physiol. 1999;276:L796–804. doi: 10.1152/ajplung.1999.276.5.L796. [DOI] [PubMed] [Google Scholar]
- Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Jr., Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson EJ, Sharfman WH, Chen S, McMiller TL, Pritchard TS, Salas JT, Sartorius-Mergenthaler S, Freed I, Ravi S, Wang H, Luber B, Sproul JD, Taube JM, Pardoll DM, Topalian SL. Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting. J Transl Med. 2015;13:214. doi: 10.1186/s12967-015-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptrott N, Curley P, Tatham LM, Owen A. Opportunities and Challenges in Nanotechnology-enabled Antiretroviral Delivery. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterials. World Scientific Publishing Ltd; Singapore: in press. [Google Scholar]
- Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, Eisen HN, Langer R. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A. 2004;101:9534–9539. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Swift R, Torraca G, Nashed-Samuel Y, Wen ZQ, Jiang Y, Vance A, Mire-Sluis A, Freund E, Davis J, Narhi L. Root Cause Analysis of Tungsten-Induced Protein Aggregation in Pre-filled Syringes. PDA J Pharm Sci Technol. 2010;64:11–19. [PubMed] [Google Scholar]
- Liu Z, Li W, Wang F, Sun C, Wang L, Wang J, Sun F. Enhancement of lipopolysaccharide-induced nitric oxide and interleukin-6 production by PEGylated gold nanoparticles in RAW264.7 cells. Nanoscale. 2012;4:7135–7142. doi: 10.1039/c2nr31355c. [DOI] [PubMed] [Google Scholar]
- Luebke R. Immunotoxicant screening and prioritization in the twenty-first century. Toxicol Pathol. 2012;40:294–299. doi: 10.1177/0192623311427572. [DOI] [PubMed] [Google Scholar]
- Madani SY, Mandel A, Seifalian AM. A concise review of carbon nanotube's toxicology. Nano Rev. 2013;4 doi: 10.3402/nano.v4i0.21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- Mallipeddi R, Rohan LC. Progress in antiretroviral drug delivery using nanotechnology. Int J Nanomedicine. 2010;5:533–547. [PMC free article] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. European Journal of Immunology. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- Marx V. Poised to branch out. Nat Biotechnol. 2008;26:729–732. doi: 10.1038/nbt0708-729. [DOI] [PubMed] [Google Scholar]
- McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM. Paracrine co-delivery of TGF-beta and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials. 2015;59:172–181. doi: 10.1016/j.biomaterials.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, Kreuter J, Langer K. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–1253. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- Minigo G, Scholzen A, Tang CK, Hanley JC, Kalkanidis M, Pietersz GA, Apostolopoulos V, Plebanski M. Poly-L-lysine-coated nanoparticles: a potent delivery system to enhance DNA vaccine efficacy. Vaccine. 2007;25:1316–1327. doi: 10.1016/j.vaccine.2006.09.086. [DOI] [PubMed] [Google Scholar]
- Mire-Sluis A, Cherney B, Madsen R, Polozova A, Rosenberg A, Smith H, Arora T, Narhi L. Analysis and immunogenic potential of aggregates and particles: a practical approach, part 2. BioProcess International. 2011;9:38–43. [Google Scholar]
- Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EY, Yi GH, Kang JS, Lim JS, Kim HM, Pyo S. An increase in mouse tumor growth by an in vivo immunomodulating effect of titanium dioxide nanoparticles. J Immunotoxicol. 2011;8:56–67. doi: 10.3109/1547691X.2010.543995. [DOI] [PubMed] [Google Scholar]
- Mottram P, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M. Type 1 and Type 2 Immunity Following Vaccination Is Influenced by Nanoparticle Size: Formulation of a Model Vaccine for Respiratory Syncytial Virus. Molecular Pharmaceutics. 2007;4:73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Ogawara K, Minato K, Fukuoka Y, Takakura Y, Hashida M, Higaki K, Kimura T. Fetuin mediates hepatic uptake of negatively charged nanoparticles via scavenger receptor. Int J Pharm. 2007;329:192–198. doi: 10.1016/j.ijpharm.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Nakai T, Kanamori T, Sando S, Aoyama Y. Remarkably Size-Regulated Cell Invasion by Artificial Viruses. Saccharide-Dependent Self-Aggregation of Glycoviruses and Its Consequences in Glycoviral Gene Delivery. Journal of the American Chemical Society. 2003;125:8465–8475. doi: 10.1021/ja035636f. [DOI] [PubMed] [Google Scholar]
- Newkome GR, Mishra A, Moorefield CN. Improved synthesis of an ethereal tetraamine core for dendrimer construction. J Org Chem. 2002;67:3957–3960. doi: 10.1021/jo025625p. [DOI] [PubMed] [Google Scholar]
- O'Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng. 2001;18:69–85. doi: 10.1016/s1389-0344(01)00101-0. [DOI] [PubMed] [Google Scholar]
- Pantic I. Nanoparticles and modulation of immune responses. Sci Prog. 2011;94:97–107. doi: 10.3184/003685011X12979697342151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Yang HN, Jeon SY, Woo DG, Kim MS, Park KH. The use of anti-COX2 siRNA coated onto PLGA nanoparticles loading dexamethasone in the treatment of rheumatoid arthritis. Biomaterials. 2012;33:8600–8612. doi: 10.1016/j.biomaterials.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Pavelic K, Hadzija M, Bedrica L, Pavelic J, Dikic I, Katic M, Kralj M, Bosnar MH, Kapitanovic S, Poljak-Blazi M, Krizanac S, Stojkovic R, Jurin M, Subotic B, Colic M. Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. J Mol Med (Berl) 2001;78:708–720. doi: 10.1007/s001090000176. [DOI] [PubMed] [Google Scholar]
- Pople PV, Singh KK. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis-Part II: in vivo assessment of dermatopharmacokinetics, biodistribution and efficacy. Int J Pharm. 2012;434:70–79. doi: 10.1016/j.ijpharm.2012.04.051. [DOI] [PubMed] [Google Scholar]
- Prinapori R, Di Biagio A. Efficacy, safety, and patient acceptability of elvitegravir/cobicistat/emtricitabine/tenofovir in the treatment of HIV/AIDS. Patient Prefer Adherence. 2015;9:1213–1218. doi: 10.2147/PPA.S88490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verloes R, G.v.K., Baert L, van Velsen F, Bouche M-P, Spittaels K, Leempoels J, Williams P, Kraus G, Wigerinck P. TMC278 long acting - a parenteral nanosuspension formulation that provides sustained clinically relevant plasma concentrations in HIV-negative volunteers. 17th International AIDS Conference; Mexico City, Mexico City. 2008. [Google Scholar]
- Ramani K, Miclea RD, Purohit VS, Mager DE, Straubinger RM, Balu-Iyer SV. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J Pharm Sci. 2008;97:1386–1398. doi: 10.1002/jps.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AP, Haddon RC, Zhou O, Fleming RM, Zhang J, McClure SM, Smalley RE. Magnetic susceptibility of molecular carbon: nanotubes and fullerite. Science. 1994;265:84–86. doi: 10.1126/science.265.5168.84. [DOI] [PubMed] [Google Scholar]
- Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007a;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotech. 2007b;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- Rettig L, Haen SP, Bittermann AG, von Boehmer L, Curioni A, Krämer SD, Knuth A, Pascolo S. Particle size and activation threshold: a new dimension of danger signaling. Blood. 2010;115:4533–4541. doi: 10.1182/blood-2009-11-247817. [DOI] [PubMed] [Google Scholar]
- Rosenberg AS, Verthelyi D, Cherney BW. Managing uncertainty: a perspective on risk pertaining to product quality attributes as they bear on immunogenicity of therapeutic proteins. J Pharm Sci. 2012;101:3560–3567. doi: 10.1002/jps.23244. [DOI] [PubMed] [Google Scholar]
- Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Bateman HR, Stover A, Gomez G, Norton SK, Zhao W, Schwartz LB, Lenk R, Kepley CL. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179:665–672. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- Sadauskas E, Danscher G, Stoltenberg M, Vogel U, Larsen A, Wallin H. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine. 2009;5:162–169. doi: 10.1016/j.nano.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Salvador-Morales C, Basiuk EV, Basiuk VA, Green ML, Sim RB. Effects of covalent functionalization on the biocompatibility characteristics of multi-walled carbon nanotubes. J Nanosci Nanotechnol. 2008;8:2347–2356. doi: 10.1166/jnn.2008.090. [DOI] [PubMed] [Google Scholar]
- Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green ML, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Scholl I, Weissenbock A, Forster-Waldl E, Untersmayr E, Walter F, Willheim M, Boltz-Nitulescu G, Scheiner O, Gabor F, Jensen-Jarolim E. Allergen-loaded biodegradable poly(D,L-lactic-co-glycolic) acid nanoparticles down-regulate an ongoing Th2 response in the BALB/c mouse model. Clin Exp Allergy. 2004;34:315–321. doi: 10.1111/j.1365-2222.2004.01884.x. [DOI] [PubMed] [Google Scholar]
- Schweingruber N, Haine A, Tiede K, Karabinskaya A, van den Brandt J, Wust S, Metselaar JM, Gold R, Tuckermann JP, Reichardt HM, Luhder F. Liposomal encapsulation of glucocorticoids alters their mode of action in the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2011;187:4310–4318. doi: 10.4049/jimmunol.1101604. [DOI] [PubMed] [Google Scholar]
- Senapati VA, Kumar A, Gupta GS, Pandey AK, Dhawan A. ZnO nanoparticles induced inflammatory response and genotoxicity in human blood cells: A mechanistic approach. Food Chem Toxicol. 2015 doi: 10.1016/j.fct.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Seo W, Kapralov AA, Shurin GV, Shurin MR, Kagan VE, Star A. Payload drug vs. nanocarrier biodegradation by myeloperoxidase- and peroxynitrite-mediated oxidations: pharmacokinetic implications. Nanoscale. 2015;7:8689–8694. doi: 10.1039/c5nr00251f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Edman MC, Janga SR, Shi P, Dhandhukia J, Liu S, Louie SG, Rodgers K, Mackay JA, Hamm-Alvarez SF. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren's syndrome. J Control Release. 2013 doi: 10.1016/j.jconrel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CC, Wang CC, Liao MH, Jan TR. A single exposure to iron oxide nanoparticles attenuates antigen-specific antibody production and T-cell reactivity in ovalbumin-sensitized BALB/c mice. Int J Nanomedicine. 2011;6:1229–1235. doi: 10.2147/IJN.S21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yadav S, Wang F, Wang H. Endotoxin promotes adverse effects of amorphous silica nanoparticles on lung epithelial cells in vitro. J Toxicol Environ Health A. 2010;73:748–756. doi: 10.1080/15287391003614042. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kapralov AA, Feng WH, Kisin ER, Murray AR, Mercer RR, St Croix CM, Lang MA, Watkins SC, Konduru NV, Allen BL, Conroy J, Kotchey GP, Mohamed BM, Meade AD, Volkov Y, Star A, Fadeel B, Kagan VE. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS One. 2012;7:e30923. doi: 10.1371/journal.pone.0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Tkach AV, Kisin ER, Khaliullin T, Stanley S, Gutkin DW, Star A, Chen Y, Shurin GV, Kagan VE, Shurin MR. Carbon nanotubes enhance metastatic growth of lung carcinoma via up-regulation of myeloid-derived suppressor cells. Small. 2013;9:1691–1695. doi: 10.1002/smll.201201470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Swami R, Khan W, Sistla R. Lymphatic system: a prospective area for advanced targeting of particulate drug carriers. Expert Opin Drug Deliv. 2014;11:211–229. doi: 10.1517/17425247.2014.866088. [DOI] [PubMed] [Google Scholar]
- Singh M, Briones M, Ott G, O'Hagan D. Cationic microparticles: A potent delivery system for DNA vaccines. Proc Natl Acad Sci U S A. 2000;97:811–816. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Simon JK, Baker JR., Jr. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Wu H, Yoshino K, Zamboni WC. Factors affecting the pharmacokinetics and pharmacodynamics of liposomal drugs. J Liposome Res. 2012;22:177–192. doi: 10.3109/08982104.2012.655285. [DOI] [PubMed] [Google Scholar]
- Spreen WR, Margolis DA, Pottage JC., Jr. Long-acting injectable antiretrovirals for HIV treatment and prevention. Current opinion in HIV and AIDS. 2013;8:565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak AB, Hackley VA, Roebben G, Ehara K, Hankin S, Postek MT, Lynch I, Fu WE, Linsinger TP, Thunemann AF. Nanoscale reference materials for environmental, health and safety measurements: needs, gaps and opportunities. Nanotoxicology. 2013;7:1325–1337. doi: 10.3109/17435390.2012.739664. [DOI] [PubMed] [Google Scholar]
- Stinchcombe TE, Socinski MA, Walko CM, O'Neil BH, Collichio FA, Ivanova A, Mu H, Hawkins MJ, Goldberg RM, Lindley C, Claire Dees E. Phase I and pharmacokinetic trial of carboplatin and albumin-bound paclitaxel, ABI-007 (Abraxane) on three treatment schedules in patients with solid tumors. Cancer Chemother Pharmacol. 2007;60:759–766. doi: 10.1007/s00280-007-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swystun LL, Shin LY, Beaudin S, Liaw PC. Chemotherapeutic agents doxorubicin and epirubicin induce a procoagulant phenotype on endothelial cells and blood monocytes. J Thromb Haemost. 2009;7:619–626. doi: 10.1111/j.1538-7836.2009.03300.x. [DOI] [PubMed] [Google Scholar]
- Takagahara T. Excitonic optical nonlinearity and exciton dynamics in semiconductor quantum dots. Phys Rev B Condens Matter. 1987;36:9293–9296. doi: 10.1103/physrevb.36.9293. [DOI] [PubMed] [Google Scholar]
- Teo PY, Yang C, Whilding LM, Parente-Pereira AC, Maher J, George AJ, Hedrick JL, Yang YY, Ghaem-Maghami S. Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance T cell killing. Adv Healthc Mater. 2015;4:1180–1189. doi: 10.1002/adhm.201500089. [DOI] [PubMed] [Google Scholar]
- Thiele L, Merkle HP, Walter E. Phagocytosis and phagosomal fate of surface-modified microparticles in dendritic cells and macrophages. Pharm Res. 2003;20:221–228. doi: 10.1023/a:1022271020390. [DOI] [PubMed] [Google Scholar]
- Thiele L, Rothen-Rutishauser B, Jilek S, Wunderli-Allenspach H, Merkle HP, Walter E. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? J Control Release. 2001;76:59–71. doi: 10.1016/s0168-3659(01)00412-6. [DOI] [PubMed] [Google Scholar]
- Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunology Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- Tomalia DA. Dendrimer research. Science. 1991;252:1231. doi: 10.1126/science.252.5010.1231-b. [DOI] [PubMed] [Google Scholar]
- Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR, Fiering S. Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine. 2014;10:1273–1285. doi: 10.1016/j.nano.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Amiji MM. Targeted delivery systems for biological therapies of inflammatory diseases. Expert Opin Drug Deliv. 2015;12:393–414. doi: 10.1517/17425247.2015.972931. [DOI] [PubMed] [Google Scholar]
- Tsai CY, Lu SL, Hu CW, Yeh CS, Lee GB, Lei HY. Size-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophages. J Immunol. 2012;188:68–76. doi: 10.4049/jimmunol.1100344. [DOI] [PubMed] [Google Scholar]
- Tyner K, Sadrieh N. Considerations when submitting nanotherapeutics to FDA/CDER for regulatory review. Methods Mol Biol. 2011;697:17–31. doi: 10.1007/978-1-60327-198-1_3. [DOI] [PubMed] [Google Scholar]
- Tyner KM, Zou P, Yang X, Zhang H, Cruz CN, Lee SL. Product quality for nanomaterials: current U.S. experience and perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:640–654. doi: 10.1002/wnan.1338. [DOI] [PubMed] [Google Scholar]
- Umbreit TH, Francke-Carroll S, Weaver JL, Miller TJ, Goering PL, Sadrieh N, Stratmeyer ME. Tissue distribution and histopathological effects of titanium dioxide nanoparticles after intravenous or subcutaneous injection in mice. J Appl Toxicol. 2012;32:350–357. doi: 10.1002/jat.1700. [DOI] [PubMed] [Google Scholar]
- Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 2006;6:1682–1686. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
- Van Beers MM, Gilli F, Schellekens H, Randolph TW, Jiskoot W. Immunogenicity of recombinant human interferon beta interacting with particles of glass, metal, and polystyrene. J Pharm Sci. 2012;101:187–199. doi: 10.1002/jps.22744. [DOI] [PubMed] [Google Scholar]
- Vasievich EA, Chen W, Huang L. Enantiospecific adjuvant activity of cationic lipid DOTAP in cancer vaccine. Cancer Immunol Immunother. 2011;60:629–638. doi: 10.1007/s00262-011-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva A, Cañete M, Roca AG, Calero M, Veintemillas-Verdaguer S, Serna CJ, Morales M.d.P., Miranda R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology. 2009;20:115103. doi: 10.1088/0957-4484/20/11/115103. [DOI] [PubMed] [Google Scholar]
- Vlasova II, Vakhrusheva TV, Sokolov AV, Kostevich VA, Gusev AA, Gusev SA, Melnikova VI, Lobach AS. PEGylated single-walled carbon nanotubes activate neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Toxicol Appl Pharmacol. 2012;264:131–142. doi: 10.1016/j.taap.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Banas JA, Mudzinski SP, Preissler MT, Graziano RF, Gosselin EJ. A two-component modular approach for enhancing T-cell activation utilizing a unique anti-FcgammaRI-streptavidin construct and microspheres coated with biotinylated-antigen. Biomolecular engineering. 2003;20:21–33. doi: 10.1016/s1389-0344(02)00089-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Fu L, Gu F, Ma Y. Notch1 is involved in migration and invasion of human breast cancer cells. Oncol Rep. 2011 doi: 10.3892/or.2011.1399. [DOI] [PubMed] [Google Scholar]
- Wegmann KW, Wagner CR, Whitham RH, Hinrichs DJ. Synthetic Peptide dendrimers block the development and expression of experimental allergic encephalomyelitis. J Immunol. 2008;181:3301–3309. doi: 10.4049/jimmunol.181.5.3301. [DOI] [PubMed] [Google Scholar]
- Weiss R, Scheiblhofer S, Freund J, Ferreira F, Livey I, Thalhamer J. Gene gun bombardment with gold particles displays a particular Th2-promoting signal that over-rules the Th1-inducing effect of immunostimulatory CpG motifs in DNA vaccines. Vaccine. 2002;20:3148–3154. doi: 10.1016/s0264-410x(02)00250-5. [DOI] [PubMed] [Google Scholar]
- Weiszhar Z, Czucz J, Revesz C, Rosivall L, Szebeni J, Rozsnyay Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur J Pharm Sci. 2012;45:492–498. doi: 10.1016/j.ejps.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Wheeler HR, Geczy CL. Induction of macrophage procoagulant expression by cisplatin, daunorubicin and doxorubicin. Int J Cancer. 1990;46:626–632. doi: 10.1002/ijc.2910460413. [DOI] [PubMed] [Google Scholar]
- Xiang SD, Fuchsberger M, JKarlson TDL, Hardy CL, Selomulya C, Plebanski M. Nanoparticles, immunomodulation and vaccine delivery. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterials. World Scientific Publishing Ltd; Singapore: 2013. [Google Scholar]
- Xiang SD, Scalzo-Inguanti K, Minigo G, Park A, Hardy CL, Plebanski M. Promising particle-based vaccines in cancer therapy. Expert Rev Vaccines. 2008;7:1103–1119. doi: 10.1586/14760584.7.7.1103. [DOI] [PubMed] [Google Scholar]
- Xiang SD, Selomulya C, Ho J, Apostolopoulos V, Plebanski M. Delivery of DNA vaccines: an overview on the use of biodegradable polymeric and magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:205–218. doi: 10.1002/wnan.88. [DOI] [PubMed] [Google Scholar]
- Yu B, Mao Y, Bai LY, Herman SE, Wang X, Ramanunni A, Jin Y, Mo X, Cheney C, Chan KK, Jarjoura D, Marcucci G, Lee RJ, Byrd JC, Lee LJ, Muthusamy N. Targeted nanoparticle delivery overcomes off-target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. Blood. 2013;121:136–147. doi: 10.1182/blood-2012-01-407742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman M, Good MF, Toth I. Nanovaccines and their mode of action. Methods. 2013;60:226–231. doi: 10.1016/j.ymeth.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, Buchel C, von Briesen H, Kreuter J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J Control Release. 2009;137:78–86. doi: 10.1016/j.jconrel.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Burkert SC, Tang Y, Sorescu DC, Kapralov AA, Shurin GV, Shurin MR, Kagan VE, Star A. Nano-gold corking and enzymatic uncorking of carbon nanotube cups. J Am Chem Soc. 2015;137:675–684. doi: 10.1021/ja511843w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Clogston JD, Patri AK, Dobrovolskaia MA, McNeil SE. Sterilization of Silver Nanoparticles Using Standard Gamma Irradiation Procedure Affects Particle Integrity and Biocompatibility. J Nanomed Nanotechnol. 2011;2011:001. doi: 10.4172/2157-7439.S5-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba B, Halets I, Shcharbin D, Appelhans D, Voit B, Pieszynski I, Bryszewska M, Klajnert B. Influence of fourth generation poly(propyleneimine) dendrimers on blood cells. J Biomed Mater Res A. 2012;100:2870–2880. doi: 10.1002/jbm.a.34222. [DOI] [PubMed] [Google Scholar]