Abstract
The biokinetics of inhaled nanoparticles (NP) is more complex than that of larger particles since NP may NP deposited on the nasal mucosa of the upper respiratory tract (URT) may translocate to the olfactory bulb of the brain and also via the trigeminus (URT neuronal route); and (b) NP deposited in the lower respiratory tract (LRT) may cross the ABB into blood and enter the brain across the blood-brain-barrier (BBB) or take a neuronal route from enervated tracheo-bronchial epithelia via the vagus nerve.
Translocation from both - the URT and the LRT - are quantified during the first 24 h after a 1-hour aerosol inhalation of 20 nm-sized, 192Ir radiolabeled iridium NP by healthy adult rats using differential exposures: (I) nose-only exposure of the entire respiratory tract or (II) intratracheal (IT) inhalation of intubated and ventilated rats, thereby bypassing the URT and extrathoracic nasal passages. After nose-only exposure brain accumulation (BrAcc) is significantly nine-fold higher than after IT inhalation since the former results from both pathways (a + b) while the latter exposure comes only from pathway (b). Interestingly, there are significantly more circulating NP in blood 24 h after nose-only inhalation than after IT inhalation. Distinguishing translocation from URT versus LRT estimated from the differential inhalation exposures, the former is significantly higher (8-fold) than from the LRT. Although the BrAcc fraction is rather low compared to total NP deposition after this short-term exposure, this study proofs that inhaled insoluble NP can accumulate in the brain from both – URT and LRT which may trigger and/or modulate adverse health effects in the central nervous system (CNS) during chronic exposure.
Keywords: Iridium nanoparticle, Nose-only and intratracheal inhalation, Brain accumulation, Neuronal pathway from upper respiratory tract, Systemic pathway from lower respiratory tract, Quantitative biokinetics
1. Introduction
As recently reviewed by Oberdörster et al. (2009, 2005) the translocation of nano-sized particles (NP) may occur in the upper respiratory tract (URT, extrathoracic airways) as well as in the lower respiratory tract (LRT, intrathoracic airways (or tracheobronchial tree) and alveolar region). In the URT direct neuronal pathways into the brain have been discussed in several papers over several decades and are listed in the review of Oberdörster et al. (2005). Besides the trigeminal pathway more studies describe the pathway from the nasal olfactory mucosa along olfactory nerves into the olfactory bulb of the brain. From the olfactory bulb NP may migrate into other parts of the brain (Oberdörster et al., 2004; Yu et al., 2007) eventually triggering and/or modulating pro-inflammatory reactions in the brain at various regions as a potential hazard of the central nervous system (CNS). Even though numerous reports describe this pathway into the brain after particle inhalation no report exists describing a similar pathway for inhaled larger sized sub-micron or micron-sized particles. Only one study reports on the trigeminal pathway after inhalation of manganese-oxide NP but results are not conclusive since no difference was found between unexposed control rats versus the exposed rats (Elder et al., 2006).
There also are possible pathways of NP from the LRT into the brain which may either occur by indirect neuronal pathways from intrathoracic airways via the vagus nerve or when NP cross the air-blood-barrier (ABB), and while circulating in blood eventually cross the brain-blood-barrier (BBB). To reach blood circulation, NP need to cross the ABB of the lungs first and enter into blood vessels directly or via lymphatic drainage. Depending on their physicochemical properties as well as their binding to plasma proteins or plasma biomolecules NP may cross the BBB. Earlier on the group of Kreuter found only intravenously injected albumin particles coated with Apo-lipoprotein E (Apo E) in the brain parenchyma which had crossed the BBB for crossing Zensi et al., 2009. Yet, the synthetized albumin particles studied were already without Apo-E coating 200 nm in size and therefore not really nano-sized. However, a more recent study by Sousa et al. (2010) provided clear evidence that intravenously injected, monodisperse 15 nm gold NP (surface linked with polyelectrolytes to firmly bind human albumin serum and fluorescently labeled with Cy5.5) were associated with the vascular endothelium 30 min after injection but crossed the murine BBB during the next 19 h to migrate deeper into the parenchyma and were found mainly in the hippocampus, thalamus, hypothalamus, and the cerebral cortex. These results were confirmed in a follow-up study on intravenously injected, monodisperse 15 nm gold NP (surface linked with polyelectrolytes to firmly bind either human albumin serum or Apo-E; both either fluorescently labeled with Cy5.5 or radio-labeled with 198Au) (Schäffler et al., 2014). Both radioactively labeled gold NP-protein conjugates showed strikingly enhanced accumulations in the brain when compared to radio-actively labeled citrate-coated gold NP. Conjugates were found in the left and right hemisphere, cerebellum and brainstem but much less in the olfactory bulb (private communication by Schäffler and Kreyling; manuscript in preparation). These studies emphasize the role of the molecular surface composition which was engineered prior to the intravenous injection of the NP conjugates.
Hence, there is evidence for NP pathways into the brain – either from the URL via direct neuronal routes versus from the LRT via the “blood route” across the ABB of the lungs and across the BBB and may be via indirect neuronal pathways from the intrathoracic airways via the vagus.
Recently we had developed two sets of inhalation exposure equipment in which rats were either exposed to freshly generated, 20 nm iridium (IrNP) aerosols during spontaneous nose-only breathing or during enforced ventilation of the same aerosol while being intratracheally intubated under light anesthesia – sometimes called intratracheal inhalation. IrNP aerosols radio-labeled with 192Ir were generated by spark ignition between two 192Ir-labeled iridium electrodes as described previously (Kreyling et al., 2002). Hence, the 192IrNP formed by iridium evaporation and condensation to be inhaled within 5 s by the rats were not surface modified and consisted only of metallic Ir and eventually Ir-oxides. After deposition in the respiratory tract 192IrNP will interact with proteins and biomolecules of the epithelial lining fluid forming an in-vivo surface modification (sometimes called “protein corona”) which will modulate the biokinetic fate of the 192IrNP including organ membrane crossing. Since albumin is the most abundant protein in body fluids, it appears plausible that albumin coated 192IrNP circulating in blood will be able to cross the BBB according to the above mentioned studies.
The 192IrNP biokinetics after a 1-hour 192IrNP aerosol inhalation exposure by intubated and ventilated rats have been presented previously (Kreyling et al., 2002; Semmler et al., 2004; Semmler-Behnke et al., 2007). These data showed that 192IrNP had crossed the ABB and accumulated not only in major secondary organs like liver, spleen, kidneys, but also in soft tissue and skeleton. In addition, 192IrNP deposition in the lungs of different aged rats had been studied which spontaneously inhaled the 192IrNP aerosol via nose-only exposure (Semmler-Behnke et al., 2012).
Applying the nose-only inhalation exposure, the entire respiratory tract will be exposed from the tip of the nose to the alveolar region including the airways in the head (extrathoracic). Applying the intratracheal inhalation (ventilation – intubation exposure), only the thoracic part of the respiratory tract, i.e. the lungs, from the distal tip of the endotracheal tube to the alveolar region will be exposed. Therefore, during intratracheal inhalation the airways of the head including the nose will be bypassed and 192IrNP will only deposit in the lungs. Performing differential inhalation exposure studies in two groups of rats will allow determination of either 192IrNP translocation from both – URT and LRT after nose-only inhalation exposure, while after intratracheal inhalation 192IrNP accumulation in the brain will occur only from the LRT.
With this design the current study is not able to distinguish between the neuronal routes from the URT into the brain nor the “blood route” from the indirect neuronal route of the LRT; but it allows for the first time to quantitatively distinguish between translocation inhaled NP from the URT versus LRT towards the brain.
2. Materials and methods
Generation and characterization of the 20 nm iridium (IrNP) aerosol and the two sets of inhalation exposure equipment have been described in (Kreyling, submitted) and also previously: IT inhalation of intratracheally intubated and ventilated rats (Kreyling et al., 2002; Semmler et al., 2004; Semmler-Behnke et al., 2007) and nose-only inhalation exposure of spontaneously breathing rats (Semmler-Behnke et al., 2012). More experimental details are given in (Kreyling, submitted). In the previous reports we describe animal maintenance and care in accordance with German ethics rules and specific approvals of the rat experiments by the Government of the district of Upper Bavaria (approval No. 211-2531-108/99). In the study reported here we used an additional set of 16, adult, female Wystar Kyoto rats and challenged the question whether nose-only inhalation to the entire respiratory tract versus intratracheal inhalation to its thoracic part only will cause a difference in brain accumulation.
After the inhalation exposure rats were kept individually in metabolism cages to collect feces and urine quantitative and separate from each other. Twenty-four hours after inhalation, rats were anesthetized and exsanguinated prior to organ and tissue sampling as described in Supplementary Material.
2.1. Quantitative biokinetics analysis
Quantitative biokinetics after administration of 192IrNP was applied as we have recently reviewed (Geiser and Kreyling, 2010; Kreyling et al., 2013) and described in Kreyling (submitted) and the above mentioned papers. Briefly, besides all organs and tissues of interest as well as exsanguinated blood, the entire remaining carcass and the entire excretion of each individual rat was collected during 24 h, for the complete list see Table DB1, (Kreyling, submitted). Hence, without any further chemical treatment all samples of a given rat the 192Ir radioactivity of the 192IrNP in each sample was analyzed gamma-spectrometrically (fixed 192Ir radioactivity per IrNP mass at a given reference time). The sum of all sample 192Ir activities represents 100% of the received 192IrNP dose by the animal. More details of the radio-analytical methodology are given in Supplementary Material. For each inhalation exposure we studied eight female WKY rats (age 8–10 weeks, weight 270–300 g) and calculated the fractions for each organ or tissue relative to the sum of 192Ir activities of each rat (excluding those of the skin, the gastro-intestinal-tract and feces since fast cleared 192IrNP will negligibly contribute to the translocation pathways). Note, however, that for the calculation of 192IrNP deposition in the conducting airways versus alveolar deposition organs and tissue fractions were normalized to the sum of all 192Ir activities of each rat. This latter normalization to the total sum was also applied to the analysis of skin. All organ or tissue fractions were averaged over eight rats per exposure group and given as means ± SD.
In order to estimate only the parenchymal content of the organs or the remaining carcass, the residual blood was calculated based on the paper of Oeff and Konig (1955) and the 192Ir NP content of the residual blood was estimated using the measured specific 192Ir radioactivity (Bq/g) of the collected blood sample and subtracted from the measured 192IrNP content of each organ or the carcass. Since cross-contamination during exsanguination, skinning and dissection is extremely critical when determining the rather small IrNP accumulation in the brain a standard operational procedure (SOP) was developed and evaluated applying rigid measures to exclude cross-contaminations between different organs and tissues of each rat and among rats. This is described in detail in Supplementary Material. For a most realistic SOP evaluation, dead bodies of rats were exposed in a closed box for one hour while the freshly generated 192IrNP aerosol flew through the box allowing for diffusional deposition on the rats’ furs without any organ or tissue deposition other than the fur. After the exposure the dead bodies were studied according to the SOP. The results proofed satisfying: no detectable 192Ir cross-contamination over a dynamic range of more than six orders of magnitude; see Supplementary Material. The complete set of fractional biokinetics data for each dead rat is given in Table DB2 of Supplementary Material.
2.2. Estimated translocation from URT versus LRT to the brain
Hence, the 24-hour retained fraction of 192IrNP in the brain after spontaneous nose-only inhalation can be described by:
| (1) |
where TURT (t) is the translocated 192IrNP fraction deposited on the nasal mucosa which crossed the nasal epithelium via neuronal routes into the olfactory bulb or other compartments of the brain; TLRT (t) is the translocated fraction of 192IrNP deposited in the LRT which first crossed the ABB into circulation and then entered the brain across the BBB via brain endothelial cells (systemic route) or which translocated via neuronal routes from enervated airway epithelia (LRT neuronal route).
Twenty-four hours after the intratracheal inhalation exposure the retained fraction of 192IrNP in the brain resulted only from the translocated 192IrNP via thee systemic and/or neuronal LRT route:
| (2) |
The difference between retained brain fractions of both exposure groups at each time point represents the translocation via the neuronal route of the URT. All data were statistically analyzed using a student t-test as well as a non-parametric Mann-Whitney test and significance was noted when the p-value < 0.05.
2.3. Biokinetics of 192Ir ions released from 192IrNP
Because of the rather low fractions found in brain, the potential disintegration of the 192IrNP by ionic 192Ir release need to be carefully estimated. The derivation is provided in Supplementary Material (Kreyling (submitted) and is based on a previous study (Kreyling et al., 2002) in which the biokinetics of soluble 192IrCl3 was studied after intratracheal instillation in the same strain of rats. In addition, it is assumed that no 192IrNP are excreted in urine but only non-particulate 192Ir which have been released from the 192IrNP. Under this conservative assumption an upper estimate of non-particulate 192Ir in the brain is derived based on the ratios of measured urine and brain fraction either after intratracheal instillation of soluble 192IrCl3 or after 192IrNP inhalation; see Eq. DB1 of Supplementary Material.
2.4. Modelling of NP deposition probability
NP deposition fractions of a same sized NP aerosol like the 20 nm 192IrNP aerosol used in this study were calculated in the three regions of the entire respiratory tract of a rat (head, trachea-bronchial tree and alveolar region) using the Multiple Path Particle Deposition (MPPD) software (version 3.0) for both types of inhalation exposures (A. R. A., 2009; Anjilvel and Asgharian, 1995; Asgharian et al., 2001). Details of the parameters used for the modelling are given in Supplementary Material.
3. Results
Median diameters of all 192IrNP aerosols were 20 nm with a geometric standard deviation of 1.6. Deposited 192IrNP mass and the deposited 192Ir radioactivity are given in Table 1 as well as fractional 24 h lung retention – assigned as alveolar deposition – and the fast cleared 192IrNP fraction – assigned to represent the sum of deposited fractions in extra- + intrathoracic airways in case of the nose-only inhalation and the intrathoracic airway deposition only in case of the intratracheal inhalation.
Table 1.
192IrNP deposition data of rats 24-h after nose-only inhalation or intratracheal inhalation exposure. Mean ± SD of eight rats per each group are given. At 24 h most 192IrNP deposited in extrathoracic and intrathoracic airways were cleared into the gastro-intestinal tract and fecal excretion as measured. No 192IrNP fraction in the head (without brain and skin) was determined. Deposition of the alveolar region was determined from 192IrNP fraction retained in the lungs after 24 h.
| Nose-only inhalation
|
Intratracheal inhalation
|
|
|---|---|---|
| mean ± STD | mean ± STD | |
| Deposited 192Ir act. (kBq) | 44.5 ± 5.85 | 192.2 ± 51.5 |
| Deposited IrNP mass (μg) | 0.89 ± 0.11 | 3.84 ± 1.03 |
| Sum of deposited fraction of extra- + intrathoracic airways | 0.549 ± 0.054 | 0.257 ± 0.131 |
| Alveolar deposition fraction* | 0.451 ± 0.055 | 0.742 ± 0.131 |
p < 0.05.
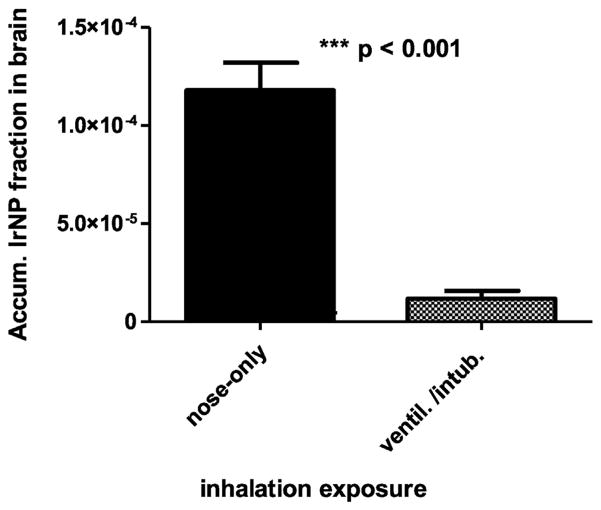
Retained 192IrNP fractions in the brains of both groups of rats are shown in Fig. 1. After nose-only exposure the 192IrNP fraction is significantly higher than after intratracheal inhalation (intubation and ventilation exposure).
Fig. 1.
Retained 192IrNP fractions in the brain 24 h after either nose-only inhalation or intratracheal inhalation exposure. Mean ± SD are given (n = 8 rats for each group).
To determine the fraction of non-particulate 192Ir which may have been released from the 192IrNP calculations (according to Materials and Methods and Supplementary Material) yielded that non-particulate 192Ir fractions in brain are 3.67·10−8 and 0.46·10−8 for nose-only and IT inhalation, respectively. These values are three orders and four orders of magnitude lower than the measured 192Ir activity fractions in brain (Fig. 1 and Table 2) after either inhalation clearly indicating that the measured data represent 192IrNP retention.
Table 2.
Biodistribution of 192IrNP 24 h after nose-only inhalation or intratracheal inhalation. Fractional and 192IrNP mass (ng per organ or tissue) data are presented as mean ± SD, n = 8 rats of each group. Data are normalized to the initial peripheral lung deposit (excluding skin and fast cleared 192IrNP found in GIT and feces) and corrected for 192IrNP content in the residual blood volume of each organ or tissue. Additionally, the entire 192IrNP translocation from the respiratory tract into blood and 2nd organs, blood + carcass is given. The gamma-spectrometric detection limit is <0.8 Bq as describe in (Kreyling, submitted). Additionally urinary excretion is also provided used for an upper estimate of 192Ir ions released from 192IrNP; note for conservative reasons we assume that only 192Ir ions released from 192IrNP are excreted in urine, therefore no 192IrNP mass is given for urine (n.d. ≡ not determined). Furthermore skin fractions are shown.
| Organ/tissue | Nose-only inhalation 24 h retention
|
Nose-only inhalation 24 h retention
|
Intratracheal inhalation 24 h retention
|
Intratracheal inhalation 24 h retention
|
|---|---|---|---|---|
| 192Ir fraction | 192IrNP mass (ng) | 192Ir fraction | 192IrNP mass (ng) | |
| Lungs | (9.81 ± 0.10) (·10−1) | (3.65 ± 0.75) (·102) | (9.43 ± 0.24) (·10−1)*** | (3.63 ± 0.96) (·102) |
| Liver | (5.54 ± 3.24) (·10−4) | (1.58 ± 1.40) (·10−1) | (2.99 ± 2.20) (·10−4) | (1.10 ± 0.82) (·10−1) |
| Spleen | (3.31 ± 1.68) (·10−5) | (7.27 ± 4.87) (·10−3) | (2.76 ± 1.54) (·10−5) | (1.04 ± 0.57) (·10−2) |
| Kidneys | <1.6 · 10−5 | <0.8 Bq | (3.11 ± 0.75) (·10−5) | (1.19 ± 0.48) (·10−2) |
| Heart | (3.14 ± 1.71) (•10−5) | (6.55 ± 6.25) (·10−3) | (9.08 ± 8.51) (·10−6) | (3.49 ± 3.60) (·10−2) |
| Brain | (11.8 ± 4.03) (·10−5) | (4,15 ± 1.24) (·10−2) | (1.35 ± 1.10) (·10−5)*** | (4,16 ± 3.87) (·10−2) |
| Carcass | (14.9 ± 9.66) (·10−3) | (5.31 ± 2.91) (·100) | (1.48 ± 0.88) (·10−3)** | (5.87 ± 4.11) (·100) |
| Total blood | (12.3 ± 7.74) (·10−4) | (1.09 ± 0.63) (·100) | (0.35 ± 0.16) (·10−4)*** | (1.31 ± 0.69) (·10−1) |
| Translocation | (1.89 ± 0.95) (·10−3) | (7.92 ± 3.75) (·101) | (1.69 ± 0.97) (·10−2)*** | (1.84 ± 1.59) (·101) |
| Skina | 8.21 ± 3.65 (·10−2) | (7.36 ± 3.54) (·101) | 0.13 ± 0.13 (·10−2) | (9.11 ± 11.6) (·100) |
| Urine | 1.11 ± 0.49 (·10−3) | n.d. | 0.33 ± 0.095 (·10−3) | n.d. |
Significant differences between both inhalation exposures are indicated by:
p < 0.01,
p < 0.001.
Note, in contrast to all other fractions, skin fractions are normalized to the sum of all fractions including skin and fast cleared 192IrNP found in GIT and feces.
When the mean value of the translocated 192IrNP fraction into the brain obtained after intratracheal inhalation exposure T̄LRT (24 h) is subtracted from the individual values of those obtained during nose-only inhalation exposure, then – according to Eq. (1) - the 192IrNP fraction transported via the neuronal route from the URT to the brain can be estimated for each rat which was nose-only exposed as noted in the following Eq. (3).
| (3) |
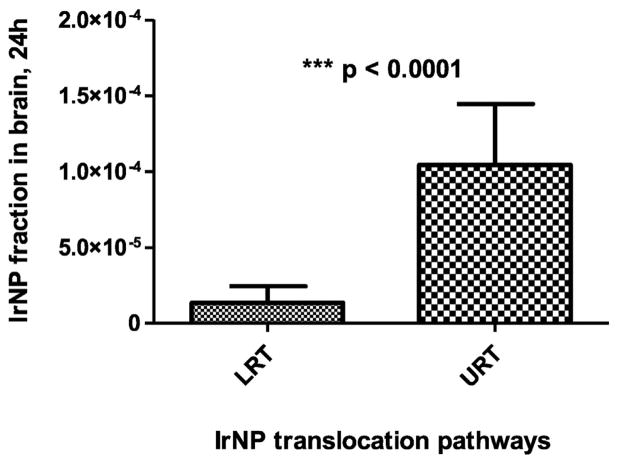
Thereby, 192IrNP fractions of both NP translocation routes can be distinguished: the neuronal route from the URT versus the systemic and neuronal route from the LRT. This is shown in Fig. 2.
Fig. 2.
Contribution of 192IrNP translocation from the URT versus translocation from LRT according to Eqs. (2) and (3). About a factor of 9 more 192IrNP were translocated from URT than from LRT.
In Table 2 the 192IrNP biodistribution 24 h after both inhalation exposures are given for the lungs, secondary organs (liver, spleen, kidneys, heart, brain), the remaining carcass and total blood as described in the Materials and Methods section. There are highly significant differences for the lungs between the different inhalation exposures. No significant differences for liver, spleen and kidneys but high significant differences not only for the brain as shown above but also for carcass and blood. The significant differences observed in blood after 24 h are quite surprising and require further investigations. Since carcass consists predominantly of muscle tissue the significant differences suggest enhanced 192IrNP accumulation in soft tissue likely modulated by the increased 192IrNP concentration in blood still after 24 h. Note, that 192IrNP in the residual blood volume of the carcass was corrected as explained above. When considering the total translocation after 24 h the significant difference results mainly from the significant differences in the carcass since 192IrNP retention in this tissue dominates the estimate of translocation. In Table 2 urinary excretion is also provided; note, however, that it is assumed that no 192IrNP excreted in urine but only 192Ir released as ions from 192IrNP is excreted. Under this conservative assumption it is possible to estimate the ionic 192Ir fraction which is released from 192IrNP and accumulate in the brain; for details see Supplementary Material. Furthermore, skin fractions are provided also in Table 2 showing that both inhalation exposures led to fur contamination since neither the nose-only exposure nor the IT inhalation proofed completely leak tight. However, the SOP evaluation experiment exposing dead rat bodies to the aerosol as described in Supplementary Material, our SOP proofed satisfying that at a 10-fold higher fur contamination cross-contaminations in organs stayed below the detection limit; see Table DB2 in Supplementary Material.
4. Discussion
Fig. 2 demonstrates that significantly more 192IrNP reach the brain via neuronal pathways from the URT (factor 9) than from LRT. The latter includes crossings of two organ membrane barriers: ABB and BBB as well as a neuronal pathway from enervated airway epithelia. Yet, for the latter no quantitative data exist particularly not after NP inhalation. Therefore, this indirect neuronal pathway via the vagus cannot be excluded seems to be more like an option rather than an important translocation pathway. On the other hand systemic translocation across the ABB has been described in many papers as discussed by Balasubramanian et al. (2013) and Oberdörster et al. (2009). In addition, crossing the BBB was only recently demonstrated (Schäffler et al., 2014; Sousa et al., 2010). These results need to be considered relative to the 192IrNP fractions deposited on the nasal versus the alveolar epithelium during inhalation. In Table 3 regional deposition fractions of an equally distributed NP aerosol compared to our 20-nm sized 192IrNP aerosol are given as calculated by the MPPD Software. Regions are: the head (extrathoracic), the tracheo-bronchial airways (intrathoracic) and the alveolar region for both inhalation exposures; breathing parameters and other model parameters are given in Table DB3 of Supplementary Material. Fractions are either normalized to the inhaled aerosol (left columns) or to the deposited NP (right columns). In order to compare with the results from the 192IrNP aerosol where only fractions relative to the deposited 192IrNP are provided, the left columns of Table 3 need to be considered. According to the model data for nose-only inhalation about a factor of 4 less NP are deposited in the extrathoracic airways when compared to the lungs, i.e. sum of intrathoracic airways and alveolar region. Hence, the probability of 192IrNP deposited in the nose reaching the brain via the neuronal route is a factor of 45 (=4 × 9, see Fig. 2) higher compared to that of NP deposited in the lungs.
Table 3.
Calculated deposition probability of a same sized NP aerosol like our 20 nm 192IrNP aerosol in the respiratory tract of rats according to the Multiple Path Particle Deposition (MPPD) software either during nose-only inhalation or during intratracheal inhalation. The modelling parameters used are given in Table DB3 of Supplementary Material for both inhalation exposures.
| Regions of respiratory tract
|
Nose-only inhalation
|
Intratracheal inhalation
|
Nose-only inhalation
|
Intratracheal inhalation
|
|---|---|---|---|---|
| Deposition relative to | Inhaled 192IrNP | Inhaled 192IrNP | Deposited 192IrNP | Deposited 192IrNP |
| Total | 0.439 | 0.666 | 1 | 1 |
| Head | 0.095 | – | 0.206 | – |
| Trachea-bronchial | 0.077 | 0.208 | 0.175 | 0.313 |
The higher probability of translocation from the URT is plausible not only by the fact that the distance from the head airways to the brain is much shorter but also by the fact that in the nasal mucosa a 192IrNP only needs to enter an axon of an olfactory sensory nerve by which it may translocate directly into the olfactory bulb of the brain. Although not well reported by inhalation studies a similar translocation via the trigeminus has to be assumed. In contrast, 192IrNP on the alveolar epithelium need (a) to be endocytosed by epithelial type I and/or type II cells, (b) exocytosed into the interstitial alveolar space and at least one more endo- and exocytosis step by endothelial vascular cells before they reach blood circulation. (Note, we cannot exclude paracellular transport through the alveolar epithelium but in contrast to the trans-cellular NP transport the paracellular pathway has not been validated experimentally under healthy conditions as reviewed by Oberdörster et al. (2005). Once 192IrNP are circulating in blood they need to cross the BBB via endothelial vascular cells in the brain (systemic route). For the sake of completeness the indirect neuronal pathway from the LRT has to be noted from enervated cells of the intrathoracic airways via the vagus nerve and eventually to the brain.
From our long-term study after intratracheal inhalation we know that the 192IrNP fraction in the brain increases during the next seven days (Kreyling et al., 2002; Semmler et al., 2004; Semmler-Behnke et al., 2007). Unfortunately, the corresponding data after nose-only inhalation are not available. A similar increase of 192IrNP fractions was also observed in liver, spleen, kidneys and heart during the next seven days indicating continued translocation via the “systemic route” towards all secondary organs and – as we show here – into the remaining carcass consisting of the skeleton and soft tissues. This clearly indicates a prominent role of the systemic translocation route from the LRT.
Comparing the olfactory mucosa of the rat with that of the human nose, the surface area of the rat olfactory mucosa normalized to entire nasal mucosa is about 50%, while it is only 5% in the human nose (Oberdörster et al., 2005). This may be the result of different evolution of the two species, since the smelling sensation of rats is much more efficiently developed than in the human nose. Therefore, it is likely that NP translocation via the olfactory route in the human nose is less probable than in the rat nose. However, a direct comparison is missing and, in addition, there are more biochemical processes involved which may mediate the olfactory NP translocation.
Similarly, there are no human data on the NP translocation across the human ABB and, hence, there are no data describing the NP biokinetics in blood circulation leading to NP accumulation in secondary organs like the brain. Those human biokinetics studies are of urgent interest in the next future.
5. Conclusion
Translocation of inhaled 192IrNP towards the brain was distinguished either from the URT or the LRT by the analysis of two different inhalation exposures – nose-only inhalation or intratracheal inhalation. Translocation from the URT is significantly higher (8-fold) than from the LRT. Although the accumulated 192IrNP fraction in the brain is rather low compared to total NP deposition after this short-term exposure, this study proofs that inhaled insoluble NP can accumulate in the brain from both – URT and LRT. When normalizing the translocation to the estimated 192IrNP deposition dose in the URT versus the LRT, the translocation from URT increases 45-fold compared to that from LRT emphasizing the importance of the neuronal pathways from the URT.
Supplementary Material
Acknowledgments
I like to express my sincere gratitude to Manuela Semmler-Behnke, DVM, and to Alexander Wenk for the jointly performed experimental work and radio-analytical data evaluation.
This work was partly supported by the NIH grant BRP (HL074022), EU FP6 project Particle Risk (012912 (NEST)) and the EU FP7 project NeuroNano (NMP4-SL-2008-214547).
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.taap.2016.02.004.
Footnotes
Conflict of interest
The author declares to have no conflicts of interest.
Transparency document
The Transparency document associated with this article can be found, in the online version.
References
- ARA . Multiple-path Particle Dosimetry Model (MPPD Version 3.0) 2009. [Google Scholar]
- Anjilvel S, Asgharian B. A multiple-path model of particle deposition in the rat lung. Fundam Appl Toxicol. 1995;28:41–50. doi: 10.1006/faat.1995.1144. [DOI] [PubMed] [Google Scholar]
- Asgharian B, Hofmann Werner, Bergmann Rudolf. Particle deposition in a multiple-path model of the human lung. Aerosol Sci Technol. 2001;34:332–339. [Google Scholar]
- Balasubramanian SK, Poh KW, Ong CN, Kreyling WG, Ong WY, Yu LE. The effect of primary particle size on biodistribution of inhaled gold nano-agglomerates. Biomaterials. 2013;34:5439–5452. doi: 10.1016/j.biomaterials.2013.03.080. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdorster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdörster G, Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health-Part A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler-Behnke M, Takenaka S, Moller W. Differences in the biokinetics of inhaled nano-versus micrometer-sized particles. Acc Chem Res. 2013;46:714–722. doi: 10.1021/ar300043r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for Concern? J Nanosci Nanotechnol. 2009;9:4996–5007. doi: 10.1166/jnn.2009.gr02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeff K, Konig A. Blood volume of rat organs and residual amount of blood after blood letting or irrigation; determination with radiophosphorus-labeled erythrocytes. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1955;226:98–102. [PubMed] [Google Scholar]
- Schäffler M, Sousa F, Wenk A, Sitia L, Hirn S, Schleh C, Haberl N, Violatto M, Canovi M, Andreozzi P, Salmona M, Bigini P, Kreyling WG, Krol S. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials. 2014;35:3455–3466. doi: 10.1016/j.biomaterials.2013.12.100. [DOI] [PubMed] [Google Scholar]
- Schäffler M, Kreyling WG. private communication.
- Semmler M, Seitz J, Erbe F, Mayer P, Heyder J, Oberdorster G, Kreyling WG. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal Toxicol. 2004;16:453–459. doi: 10.1080/08958370490439650. [DOI] [PubMed] [Google Scholar]
- Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, Oberdorster G, Kreyling WG. Efficient elimination of inhaled nanoparticles from the alveolar region: evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ Health Perspect. 2007;115:728–733. doi: 10.1289/ehp.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler-Behnke M, Kreyling W, Schulz H, Takenaka S, Butler J, Henry F, Tsuda A. Nanoparticle delivery in infant lungs. Proc Natl Acad Sci U S A. 2012;109:5092–5097. doi: 10.1073/pnas.1119339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa F, Mandal S, Garrovo C, Astolfo A, Bonifacio A, Latawiec D, Menk RH, Arfelli F, Huewel S, Legname G, Galla HJ, Krol S. Functionalized gold nanoparticles: a detailed in vivo multimodal microscopic brain distribution study. Nanoscale. 2010;2:2826–2834. doi: 10.1039/c0nr00345j. [DOI] [PubMed] [Google Scholar]
- Yu LE, Lanry Yung LY, Ong CN, Tan YL, Suresh Balasubramaniam K, Hartono D, Shui G, Wenk MR, Ong WY. Translocation and effects of gold nanoparticles after inhalation exposure in rats. Nanotoxicology. 2007;1:235–242. [Google Scholar]
- Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, Buchel C, Von Briesen H, Kreuter J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J Control Release. 2009;137:78–86. doi: 10.1016/j.jconrel.2009.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.