Abstract
Nanoparticle immunogenicity and antigenicity have been under investigation for many years. During the past decade, significant progress has been made in understanding what makes a nanoparticle immunogenic, how immune cells respond to nanoparticles, what consequences of nanoparticle-specific antibody formation exist and how they challenge the application of nanoparticles for drug delivery. Moreover, it has been recognized that accidental contamination of therapeutic protein formulations with nanosized particulate materials may contribute to the immunogenicity of this type of biotechnology products. While the immunological properties of engineered nanomaterials and their application as vaccine carriers and adjuvants have been given substantial consideration in the current literature, little attention has been paid to nanoparticle immuno- and antigenicity. To fill in this gap, we herein provide an overview of this subject to highlight the current state of the field, review past and present research, and discuss future research directions.
Keywords: Nanoparticles, Preclinical, Immunogenicity, Cytokines, Anaphylaxis, Phagocytosis, Antibody, Antigenicity
Introduction
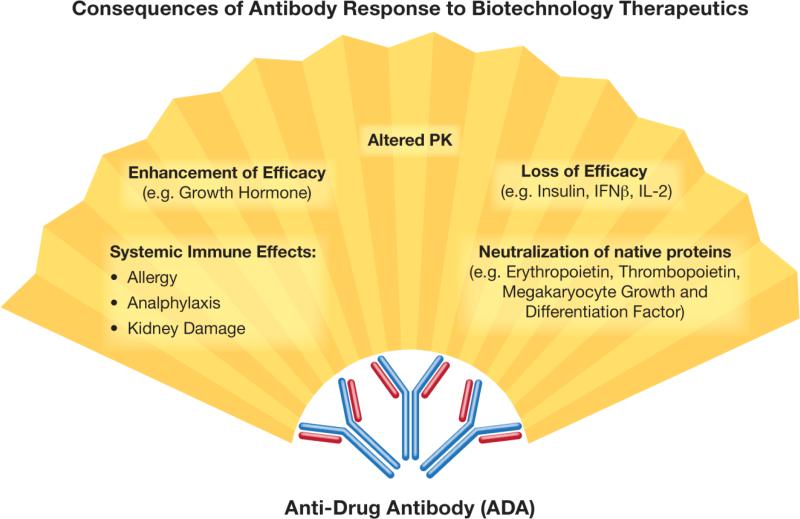
The immune system functions to protect the host from invading pathogens, abnormal self-antigens and the harm they cause. Fulfilling this function includes the rapid identification and elimination of harmful agents (e.g. bacteria, viruses, and transformed or otherwise damaged host cells). Antibodies, or immunoglobulins, are highly specialized proteins generated by a subset of terminally differentiated B-lymphocytes called plasma cells. There are two types of antibodies: those bound to the B-cell surface, also known as B-cell receptors (BCRs), and soluble immunoglobulins secreted by plasma cells. Binding of the soluble immunoglobulin to its respective antigen marks the antigen for uptake and elimination by the phagocytic cells and may also induce complement activation. The generation of an antibody response is typically initiated by pathogens; however, host molecules (e.g. DNA, lipids, and proteins) and certain types of pharmaceutical products (e.g. therapeutic proteins and antibodies) may also cause the formation of antibodies. The consequences of forming antibodies against pharmaceutical products vary, depending on the type of antibody and the function of the protein, and may even become detrimental to the host (Fig. 1). Generating antibodies to a pharmaceutical product can cause rapid clearance of the product. Furthermore, if the product-specific antibody is neutralizing, and cross-reacts with the host's native protein, its presence can result in neutralization of the endogenous protein. The consequences of this neutralization depend on the abundance of the host protein, its function, and the presence or absence of other proteins that perform the same function. If a therapeutic protein's immunogenicity leads to the formation of antibodies against a non-redundant host protein that performs a critical function, this is detrimental to the host. For example, antibodies formed in response to recombinant erythropoietin (Eprex®) neutralized both the recombinant product and endogenous erythropoietin, resulting in pure red-cell aplasia. Moreover, these antibodies were also neutralizing to other erythropoietin formulations, such as Epogen®, NeoRecormon®, and Aranesp®, rendering the treated patients transfusion dependent (Gershon et al., 2002; Chamberlain and Mire-Sluis, 2003; Hermeling et al., 2003). While the immunogenicity of therapeutic proteins have been extensively studied—with well-understood mechanisms and more-or-less established approaches for avoidance—less is known about the immunogenicity and antigenicity of rapidly evolving nanomaterials. Despite being used interchangeably, the terms immunogenicity and antigenicity have distinct meaning. The term immunogenicity refers to the ability of a substance to induce cellular and humoral immune response, while antigenicity is the ability to be specifically recognized by the antibodies generated as a result of the immune response to the given substance. While all immunogenic substances are antigenic, not all antigenic substances are immunogenic.
Fig. 1.
Consequences of antibody response to biotechnology-based therapeutics. Antidrug antibodies (ADA) have a broad spectrum of effects, which may lead to changes in protein efficacy, possibly resulting in undesirable toxicity and clearance of the biotechnology-based product. PK – Pharmacokinetics, IFN – Interferon, IL – Interleukin.
Nanoparticle physicochemical properties determine their interaction with the immune system (Dobrovolskaia and McNeil, 2007; Dobrovolskaia et al., 2008; Aggarwal et al., 2009). Nanoparticles with surfaces unprotected by polyethylene glycol (PEG) or other polymers interact with plasma proteins, rendering these particles ready for quick uptake by the phagocytic cells (Owens and Peppas, 2006; Monopoli et al., 2011). It has also been established that some nanoparticles can be immunogenic, serve as adjuvants to increase the immunogenicity of weak antigens and benefit vaccine development (Fifis et al., 2004; Reddy et al., 2007; Smith et al., 2013). Furthermore, manipulating their size, surface charge and route of administration allows efficient lymphatic delivery and antigen presentation to dendritic cells (DCs) (Fifis et al., 2004; Reddy et al., 2007; Smith et al., 2013). In addition to vaccine applications, in which stimulation of the immune response is desirable, many other nanotechnology-based platforms are used to carry proteins, peptides, lipids, and nucleic acids, either as targeting moieties or as active pharmaceutical ingredients (APIs). When nanoparticles are used as drug carriers, stimulation of the immune response and antigenicity of both the therapeutic payload and the nanotechnology-based carrier are undesirable. Several studies have demonstrated that nanoparticles may become immunogenic after binding to protein carriers or loading with toll-like receptor (TLR) ligands (Banerji et al., 1982; Alving et al., 1996; Chen et al., 1998, Braden, 2000 #19; Alving, 1996 #25; Braden et al., 2000). Moreover, certain nanosized particulates found as accidental contaminants in therapeutic protein formulations have been demonstrated to enhance the immunogenicity of the therapeutic proteins (Carpenter et al., 2010). Altogether, these findings have raised attention to the problem of nanomaterial immuno- and antigenicity and emphasized the need for a better understanding of this subject and the potential safety concerns that undesirable nanoparticle immuno- and antigenicity may cause. Desirable nanoparticle immunogenicity and the use of nanoparticles to deliver antigens and serve as adjuvants have been reviewed elsewhere (Xiang et al., 2010; Smith et al., 2013)). Herein, we will focus on reviewing the literature regarding the immuno- and antigenicity of nanoparticle-based drug carriers and their payloads, as well as discuss the contribution of accidentally introduced nanosized particulates to the immunogenicity of therapeutic proteins. The aim of our review is to show the importance of distinguishing drug-delivery nanocarriers from accidental contaminants when discussing antigenicity and its potential safety concerns.
Principles of and factors responsible for immunogenicity
According to the clonal selection theory of Macfarlane Burnet, B-cells with specificity to a particular antigen preexist in an organism, even before they encounter this antigen. However, not every antigen is able to trigger the immune response. Antigen-intrinsic features such as origin, composition, size, and the presence of repetitive epitopes define the immune system's reaction to the antigen. Frequently, the simple presence of the antigen is insufficient to successfully generate antibodies, and additional stimulation is required. Activation of the innate immunity by microbial patterns through their respective receptors significantly amplifies the immune response. B-cell activation via binding with its cognate antigen results in clonal expansion, culminated by differentiation into antibody-producing plasma cells (Saadati et al., 2013).
There are two mechanisms of antibody induction: thymus-dependent (TD) and thymus-independent (TI). The TD mechanism is usually triggered by proteins, and begins with antigen uptake by and subsequent activation of DCs. DCs produce cytokines that activate T-helper cells capable of recognizing antigen in the context of major histocompatibility complex class II (MHC II) on the surface of antigen-presenting cells (APCs) such as DCs. The next step involves the interaction of the activated T-cells with B-cells, which present the cognate antigen in the context of MHC II. Interaction between T-helper and B-cells results in B-cell proliferation and differentiation into plasma cells. The TD pathway is characterized by isotype switching, the generation of high-affinity antibodies, and the formation of immunological memory (Sauerborn et al., 2010). Biotechnology-derived therapeutics bearing foreign or unknown peptide epitopes usually act through the TD mechanism. Examples of this antigen type include botulinum toxin (used to treat dystonia), streptokinase (used to dissolve blood clots), and coagulation factor VIII (FVIII) (used to treat hemophilia). In the case of the TI mechanism, B-cell activation is triggered by repetitive elements in the antigen and occurs without T-cell involvement (Bachmann et al., 1993; De Groot and Scott, 2007; Sauerborn et al., 2010). This mechanism results in formation of IgM. Two types of TI antigens have been described: TI-1 and TI-2. A TI-1–type response is elicited when additional B-cell activation, through TLR receptors, for instance, is involved. When present at high concentrations, TI-1 antigens stimulate the polyclonal activation of B-cells. TI-2 antigens are characterized by the presence of highly repetitive structures that are recognized by BCRs, and no additional activation signal is required. TI-2 antigens primarily activate marginal zone B-cells, which represent a non-circulating pool of mature B-cells located at the border of the splenic white pulp (Murphy, 2012). This mechanism results in the generation of antibodies in response to polysaccharides and lipids and occurs through the breaking of B-cell tolerance, which occurs when previously non-antigenic epitopes aggregate and form a repetitive pattern (Sauerborn et al., 2010).
Generating antibodies to engineered nanomaterials
The concept of a versatile immune system implies that it is able to recognize a diverse variety of epitopes, including inorganic compounds. Antibodies with affinities for gallium arsenide (Artzy Schnirman et al., 2006), magnetite (Barbas et al., 1993), and gold (Watanabe et al., 2008) have been described. Some of them, antibodies specific for gold surfaces (A14P-b2), for example, could also bind to the surfaces of gold nanoparticles without cross-reacting with other noble metal surfaces made of platinum, palladium, or silver (Watanabe et al., 2008). To our knowledge, this is the only report describing the isolation of a naturally occurring antibody specific for the surface of a colloidal metal nanoparticle.
As discussed earlier, the presence of (and interaction with) specific antibodies does not necessarily result in the development of an immune response and expansion of the specific B-cells. Due to their small size, most nanoparticles are poorly immunogenic or not immunogenic at all. This is why immunizing animals with pristine nanoparticles, even in the presence of strong adjuvants, is frequently reported as unsuccessful in terms of generating nanoparticle-specific antibodies. For example, immunizing rabbits with C60 fullerene derivatives in the presence of Freund's adjuvant did not result in the generation of fullerene-specific antibodies (Andreev et al., 2000a). However, when immunization was performed using the same procedure and the fullerene derivatives were conjugated to a protein carrier (thyroglobulin), antibodies specific for the C60 fullerene were generated (Chen et al., 1998; Braden et al., 2000; Hendrickson et al., 2012). Further research demonstrated that this anti-fullerene antibody interacted with the core components of the C60 fullerene, but not with the functional groups present on the fullerene surface (Hendrickson et al., 2012). This finding was subsequently confirmed by the observation that this antibody also reacted with C70 fullerenes and single-wall carbon nanotubes (Chen et al., 1998; Erlanger et al., 2001). Likewise, polyamidoamine (PAMAM) dendrimers were not immunogenic per se, in that administering these particles did not result in the formation of dendrimer-specific antibodies, even in the presence of Freud's adjuvant (Roberts et al., 1996; Lee et al., 2001a). However, conjugating them to a protein carrier (bovine serum albumin to generation zero [G0] dendrimers and interleukin-3 [IL-3] to generation five [G5] dendrimers) resulted in the formation of antibodies specific for the amine, oxyamine, or sulfhydryl dendrimer surface functional groups (Lee et al., 2001a). Antibodies specific for the oxyamine groups did not cross-react with the sulfhydryl terminal groups, even when these groups were exposed on PAMAM dendrimers of equivalent size (G5) (Lee et al., 2001a). Antibodies raised against amine-terminated G0 PAMAM dendrimers were specific for the primary amine terminal groups and recognized amine-terminated PAMAM dendrimers of different generations, but failed to recognize primary amines on the surfaces of poly(triethylenemine) (POPAM) dendrimers with higher terminal group density, emphasizing the importance of the spatial separation between the terminal groups. These studies illustrate the requirement for nanoparticle conjugation to a protein carrier for successful antibody induction. Zolnik et al. suggested that this response resembles the T-cell–dependent pathway and proposed the following mechanism: (1) B-cells bearing BCRs capable of recognizing structural elements in nanoparticles (surface groups or core components) take up the nanoparticle–protein complex; (2) the protein carrier is then processed inside the B-cells, and the resulting peptides are presented to T-cells in the context of MHCII on the B-cell surface; (3) this event promotes interaction between B- and T-cells; and (4) both cells become activated, resulting in the expansion of B-cells producing antibodies specific for the nanoparticle surface (Zolnik et al., 2010). In this mechanism, nanoparticles behave as haptens.
The generation of nanoparticle-specific antibodies via the TI mechanism is exemplified by those generated against liposomes. Similar to fullerenes and dendrimers, as discussed above, liposomes per se are poorly immunogenic. For example, the repeated administration of liposomes to rabbits did not result in antibody formation (Schuster et al., 1979). However, the presence of TLR-ligands, which provide additional B-cell stimulation, was shown to result in the expansion of B-cell clones producing IgM antibodies specific to the individual lipid constituents of the liposomes. For example, immunization with liposomes containing lipid A resulted in the generation of liposome-specific antibodies (Schuster et al., 1979; Alving, 1986; Alving et al., 1996). Another interesting study described the generation of antibodies that recognized liposomes of several compositions—dimyristoyl phosphatidylcholine/ cholesterol/dicetyl phosphate (DMPC/Chol/DCP), DMPC/Chol/DCP/cardiolipin (CL), DMPC/Chol/DCP/phosphatidylinositol (PI), and DMPC/Chol/DCP/ phosphatidylinositol phosphate (PIP)—when the rabbits were infected with Trypanosoma rhodesiense (Richards et al., 1983). Lipid composition analysis of T. rhodesiense revealed the presence of PC, PI, CL, and PIP. These lipids, though derived from different sources, are also commonly used to prepare liposomes. Liposome-specific antibodies were shown to be predominantly IgM and generated equally in both wild-type and athymic mice (Banerji et al., 1982). These findings further confirmed that these antibodies are generated via the TI-mechanism.
In 1984, Alving described naturally occurring human antibodies that could specifically bind to liposomes containing PC/Chol, PC/Chol/DCP, and PS/Chol from egg (Alving, 1984). Alving later suggested that these antibodies recognize phosphate and sulfate esters, which are also present on numerous biological substances, including other phospholipids, lipoteichoic acids, and DNA (Alving, 1986).
Antibody response to nanoparticle surface coatings
PEG is widely used to modify therapeutic proteins and nanoparticle surfaces to improve their hydrophilicity, extend their circulation time, and mask them from uptake by phagocytic cells (Abuchowski et al., 1977a; Abuchowski et al., 1977b) (Eggebeen, 2007; Macdougall et al., 2013). Although PEGylation is generally intended for the prevention of immune recognition, accelerated blood clearance (ABC) of PEGylated products upon repeated administration has been reported (Abu Lila et al., 2013; Verhoef et al., 2014). The precise mechanism of the ABC phenomenon is incompletely understood, but the possibility that it is based on the generation of an immune response against PEG coatings has been considered. For example, one study showed that ABC of PEGylated liposomes in rats and rhesus monkeys was mediated by heat-labile molecules, but they did not belong to an immunoglobulin class (Dams et al., 2000). In contrast to this study, however, Kiwada et al. (Ishida et al., 2004; Ishida et al., 2005; Ishida et al., 2006a; Ishida et al., 2006b; Ishida et al., 2006c; Ishida et al., 2007; Ishida et al., 2008; Ishida and Kiwada, 2008; Ishihara et al., 2010) and Kaminskas et al. (Kaminskas et al., 2011) suggested that anti-PEG IgM antibodies are responsible for the accelerated clearance of PEGylated nanomaterials. It has also been suggested that anti-PEG IgM binds to PEGylated liposomes, activating complement, which is then followed by opsonin generation, liposome opsonization, and increased uptake of opsonized nanoparticles by resident liver macrophages (Ishida et al., 2006c; Hashimoto et al., 2015). According to the study by Shimizu et al., conducted in Wistar rats, B-cells located in the splenic marginal zone are responsible for the generation of anti-PEG IgM because depletion of these cells significantly reduces antibody production (Shimizu et al., 2013). The absence of isotype switching, the lack of memory development, and the presence of repetitive structures on the surface of PEGylated liposomes suggest a TI mechanism of anti-PEG IgM production. Separately, some studies speculate that PEGylated liposomes act as TI-2 antigens due to the presence of repetitive structures that cross-link receptors on the surface on B-cells located in the marginal zone of spleen (Ichihara et al., 2011; Shimizu et al., 2013). Experiments in athymic mice confirmed the lack of T-cell involvement (Semple et al., 2005; Koide et al., 2010), while studies in SCID (Koide et al., 2010) or splenectomized mice (Ichihara et al., 2011) demonstrated the contribution of the B-cells in the development of the ABC phenomenon.
Many factors contribute to the development of ABC, including the animal species used; the dose; the interval between the first and second doses; the nanoparticle size, charge, and cargo; and the liposomal composition, as well as the density and length of the PEG coating. These factors, the controversies over different study results and conclusions, and possible explanations for the differences have been reviewed in detail elsewhere (Dobrovolskaia, 2013). The properties of PEGylated liposomes and micelles, and their contribution to the generation of PEG antibodies and ABC, are summarized in Table 1.
Table 1.
Relationships between nanoparticle properties and ABC. Properties of the first-dose PEGylated liposomes or micelles responsible for the induction of ABC, examples, and original research articles are summarized. ABC – Accelerated blood clearance, PEG – polyethylene glycol.
| PROPERTY | EXAMPLES | REFERENCES |
|---|---|---|
| Size | Direct correlation found between antigen size and induction of anti-PEG antibody. 1. PEGylated liposomes induce anti-PEG antibody more effectively than PEGylated micelles. 2. Large PEGylated micelles (50.2 nm) induce ABC, while small PEGylated micelles (9.7-31.5 nm) do not. |
(Kaminskas et al., 2011) (Koide et al., 2008) |
| Charge | No correlation found between nanoparticle charge and ABC induction. | (Ishida et al., 2004) |
| PEG density | Controversial results: 1. A 5 mol% PEG-lipid concentration induced the strongest ABC. Increasing the PEG-lipid concentration to 10 or 15 mol% attenuated ABC. 2. Using 9 mol% PEG liposomes induced stronger ABC than 3 %mol PEGylated liposomes. |
(Ishida et al., 2005) (Li et al., 2012) |
| PEG length | No direct correlation found between PEG length and ABC enhancement. Increasing mPEG molecular weight from 2000 to 5000 did not enhance ABC. |
(Ishida et al., 2005) |
| PEG terminal groups | Direct correlation exists between increased hydrophobicity and antibody induction. Terminal groups are listed in the order of increasing hydrophobicity and antigenicity: SH<OH< OCH3<O(CH3)3 | (Arima et al., 2008; Sherman et al., 2012; Saifer et al., 2014) |
| Cargo | 1. Cytotoxic cargo – ABC not induced 2. Rapid release cytotoxic cargo – ABC induced 3. Immunostimulatory cargo – ABC enhanced |
(Charrois and Allen, 2003; Ishida et al., 2006a; Cui et al., 2008; Koide et al., 2010) (Li et al., 2012) (Semple et al., 2005; Judge et al., 2006; Tagami et al., 2010; Hashimoto et al., 2014a) |
| Dosing schedule | Controversial results: 1. An interval of ≤ 7 days between the first and second doses is optimal for ABC induction. 2. ABC was observed in 10 days from the first injection |
(Dams et al., 2000; Kaminskas et al., 2011; Saadati et al., 2013) (Ishida et al., 2004) |
Several reports have suggested that ABC can also be mediated by antibodies that recognize the liposomes themselves. For example, injecting an initial dose of conventional (non-PEGylated) liposomes was shown to induce ABC of a second dose composed of PEGylated liposomes (Ishida et al., 2005; Wang et al., 2007).
Other studies have also reported the presence of naturally occurring PEG antibodies in healthy donor volunteers (Richter and Akerblom, 1984; Leger et al., 2001; Armstrong et al., 2007). The percentage of healthy donors who tested positive for anti-PEG immunoglobulins varies drastically between studies: 0.2% according to one (Richter and Akerblom, 1984) and 22–25% according to others (Leger et al., 2001; Armstrong et al., 2007). One possible explanation for this difference is an increase in the population's exposure to PEGylated products through food and cosmetics (Garay et al., 2012). Another reason stems from the methodology used to detect the antibody (Dobrovolskaia, 2013; Schellekens et al., 2013). Furthermore, it has been suggested that the specificity of PEG antibodies detected in vitro does not necessarily reflect their in vivo activity and, therefore, results should be interpreted with caution (Hashimoto et al., 2014b).
Two types of experimentally obtained PEG antibodies have been reported: antibodies specific for ethoxy (OCH2CH2) repeats (i.e. the “PEG backbone”) and antibodies that recognize PEG terminal groups (Cheng et al., 1999; Tsai et al., 2001; Hashimoto et al., 2014b). Other work has suggested that PEG backbone-specific antibodies preferably interact with the less dense “mushroom” conformation of PEG, while terminal group-specific antibodies tend to react with PEG in its denser “brush” configuration, due to the reduced accessibility of the ethoxy repeats (Hashimoto et al., 2014b). The hydrophobicity of PEG terminal groups is thought to be an important antigenicity factor because hydroxy-terminated PEG has been shown to be less antigenic than methoxy-PEG, which, in turn, was shown to be less antigenic than t-butoxy-PEG (Sherman et al., 2012; Saifer et al., 2014). Studies comparing the immunogenicity of methoxy-terminated and thiol-terminated groups showed that thiol-PEG was significantly less immunogenic (Arima et al., 2008).
Contribution of accidental nanoparticle contaminants to immunogenicity of recombinant protein therapeutics
Therapeutic protein immunogenicity, its contributing factors and mechanisms, and the regulatory requirements involved have already been given substantial attention in the current literature (Schellekens, 2002; Chamberlain and Mire-Sluis, 2003; Rosenberg, 2003; Hermeling et al., 2004; Swanson, 2006; De Groot and Scott, 2007; Carpenter et al., 2010; Sauerborn et al., 2010; Buttel et al., 2011; Parenky et al., 2014; Tatarewicz et al., 2014). Therefore, we will review herein the contribution of accidentally introduced particulate materials to the immunogenicity of protein therapeutics. These materials are heterogeneous in composition and size, and include both micro- and nano-sized particles. The source of particulate contaminants in therapeutic proteins may be internal (formed by protein aggregation) or external (introduced during manufacturing). Protein aggregation can be promoted by agitation during transportation or through binding to submicron particles (Schellekens, 2002; Jiskoot et al., 2012); (Liu et al., 2012); (Tyagi et al., 2009); (Bee et al., 2009); (Thirumangalathu et al., 2009; Krayukhina et al., 2015). Glass and cellulose fibers, tungsten and stainless steel fragments, and silicon oil are common particulate contaminants, which can be introduced into therapeutic protein formulations from gowns worn in GMP production facilities, shed from filters used in the manufacturing process, or via leaching from vial closures, valves, connectors, filters, and tubing lines (Carpenter et al., 2009; Tyagi et al., 2009; Fradkin et al., 2011); (Bee et al., 2009). Several studies have been conducted to mimic the triggering of protein aggregation by contaminating particles and to understand the source of contamination. For example, incubating recombinant human interferon beta with 14-μm stainless steel microparticles significantly increased the immunogenicity of this therapeutic protein (Van Beers et al., 2012). It has been demonstrated that sterilizing glass vials triggers a delamination process, which results in the shedding of glass particles (Ennis et al., 2001). Lyophilizing and storing recombinant human platelet-activating factor acetylhydrolase (rhPAF-AH) in such vials resulted in the formation of aggregates after the protein was reconstituted with sterile water for injection. Filtering the protein solution after reconstitution and rinsing the vial with nanopure water after sterilization to remove glass particles prevented the formation of protein aggregates. In contrast, adding 12-nm silica nanoparticles to the reconstituted protein exacerbated protein aggregation (Chi et al., 2005). The presence of residual tungsten particles from the pins used to manufacture the barrels of glass syringes was also shown to induce the aggregation of therapeutic proteins (Liu et al., 2010; Seidl et al., 2012). Tungsten particles rapidly bound erythropoietin alfa, resulting in its partial denaturation and aggregation (Seidl et al., 2012).
Immunogenicity of therapeutic proteins delivered using nanoparticles
The contribution of contaminating nano- and microparticles to the immunogenicity of therapeutic proteins has prompted some experts in this field to express logical concerns about the suitability of nanoparticle carriers for the delivery of therapeutic proteins (Carpenter et al., 2010). However, existing data demonstrate that, in contrast to the particulate contaminants, the nanomaterials used to carry therapeutic proteins are engineered to avoid or reduce immunogenicity. For example, the application of PEGylated gold nanoparticles for the delivery of recombinant tumor necrosis factor alpha reduced the systemic toxicity (fever and hypotension) of this cytokine and did not lead to the formation of protein-specific antibodies (Libutti et al., 2010). Encouraging results have been obtained from using nanoparticles to solve the immunogenicity problem of recombinant FVIII, which is used to treat hemophilia A. The major drawback to using this product in the clinic is that antibodies are generated against it, neutralizing its activity (Klinge et al., 2002). Its large size, its ability to bind phospholipids, and the presence of multiple domains and several universal CD4+ epitopes, along with a conformation prone to form aggregates, make this protein extremely immunogenic (Ramani et al., 2008a). Antibodies to therapeutic FVIII are observed in up to 25% of treated patients (Klinge et al., 2002). Incorporating FVIII into PS-containing liposomes reduced its immunogenicity (Ramani et al., 2008a; Ramani et al., 2008b). Furthermore, encapsulating FVIII in the liposomes significantly reduced temperature-induced aggregation of this protein (Ramani et al., 2008a).
Another example of reducing protein immunogenicity by using a nanotechnology-based carrier is the use of the streptococcal protein streptokinase for thrombosis intervention. The therapeutic efficacy of streptokinase is limited by neutralizing antibodies generated in response to thrombosis treatment or that naturally occur in patients as consequence of previous streptococcal infections (Kunamneni et al., 2007). Using liposomal or polymeric nanoparticles to deliver streptokinase shielded this protein from immune recognition without affecting its efficacy (Nguyen et al., 1989; Nguyen et al., 1990; Perkins et al., 1997; Leach et al., 2003). Since certain nanoparticle physicochemical properties determining their effects on the immune system may increase protein immunogenicity, such nanoparticles are inappropriate carriers for the delivery of therapeutic proteins. Both careful selection of the nanoparticle carrier and optimization of its physicochemical properties are necessary to achieve the desirable reduction of immunogenicity of protein therapeutics.
Conclusion and future directions
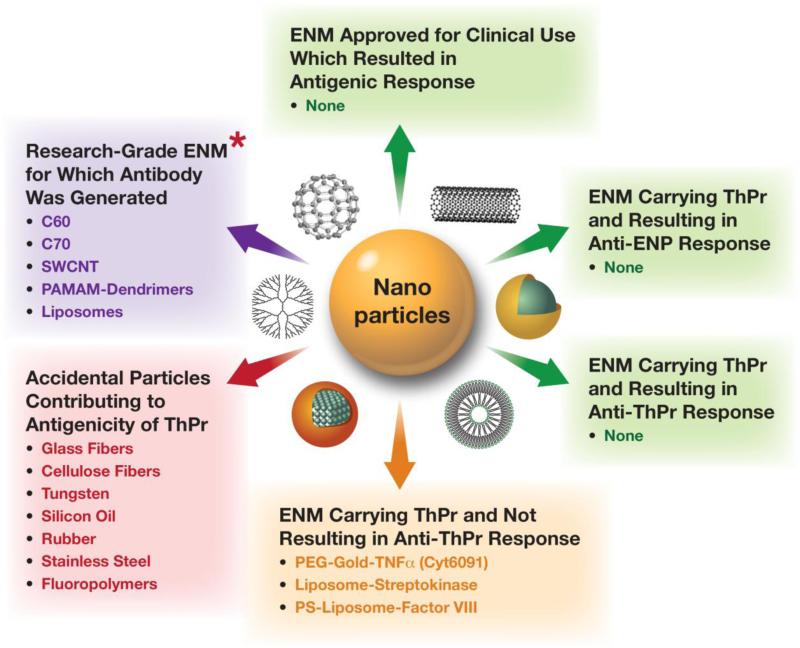
During the past decade, our understanding of nanoparticle immunogenicity has evolved from anecdotal reports describing the generation of the particle-specific antibodies to uncovering the differences between particle types, determining the roles of their physicochemical properties, and developing approaches to enhance desirable and avoid undesirable immunogenicity (Fig. 2). It is now well recognized that most engineered nanomaterials (e.g. PAMAM dendrimers, and some fullerene derivatives) are not immunogenic per se even when they are injected in the presence of strong adjuvants (Roberts et al., 1996; Masalova et al., 1999; Andreev et al., 2000b; Dykman et al., 2004; Agashe et al., 2006). The conjugation of polymeric, carbon-based, and colloidal metal nanoparticles to a protein carrier, and immunization in the presence of strong adjuvant, are important conditions required for the generation of antibodies specific to these nanomaterials (Chen et al., 1998; Braden et al., 2000; Erlanger et al., 2001; Lee et al., 2001b; Lee et al., 2004). The generation of antibodies against lipid-based nanoparticles (liposomes and micelles) depends on the presence of TLR ligands or repetitive structures, and occurs via a mechanism different than that involved in antibody generation against protein-conjugated nanoparticles. These mechanisms (TI and TD, respectively) are not unique to nanoparticles. Antibodies can be generated against the nanoparticle core, terminal groups, and surface coatings. Antibody response to PEG, one of the most popular nanoparticle surface coatings, contributes to accelerated particle clearance from circulation (via the ABC phenomenon) and alteration of the particle's pharmacokinetics profile (Ishida et al., 2004; Ishida et al., 2005; Ishida et al., 2006a; Ishida et al., 2006b; Ishida et al., 2006c; Ishida et al., 2007; Ishida et al., 2008; Ishida and Kiwada, 2008; Ishihara et al., 2010). PEGylated liposomes can be used as example of the immunogenic nanoparticles, while colloidal gold serves as example of the antigenic nanoparticles (Alving, 1984; Watanabe et al., 2008). Thus far, there are no studies demonstrating engineered nanoparticles carrying therapeutic proteins causing the formation of protein- or nanoparticle-specific antibodies. Furthermore, other work has shown that the application of nanotechnology-based carriers can overcome the problematic immunogenicity of certain therapeutic proteins (Perkins et al., 1997; Ramani et al., 2008a; Ramani et al., 2008b; Libutti et al., 2010). In contrast to the nanomedicine field, in which the physicochemical properties of nanoparticles can be tuned to either stimulate the immune system or avoid its recognition, the biotechnology field has experienced a negative impact from accidentally introduced nanomaterials (e.g. cellulose and glass fibers, tungsten and stainless steel fragments, and silicon oil), since contamination of therapeutic protein formulations with these nano-sized particulates has been shown to contribute to protein immunogenicity (Jiang et al., 2009; Carpenter et al., 2010; Liu et al., 2010; Fradkin et al., 2011; Mire-Sluis et al., 2011; Jiskoot et al., 2012). A graphic summary of these data is presented in Fig. 3.
Fig. 2.
Timeline of understanding of nanoparticle antigenicity. Our understanding of nanoparticle immunogenicity has evolved from anecdotal reports describing the generation of the particle-specific antibodies to uncovering the differences between particle types, determining the roles of their physicochemical properties, and developing approaches to enhance desirable and avoid undesirable immunogenicity. Future research will focus on methodologies and mechanisms, and leveraging this knowledge for the development of safe nanomedicines. PCP – Physicochemical properties.
Fig. 3.
Nanoparticle antigenicity. Current data about nanoparticles and antibody response are summarized. * – Immunization required a strong adjuvant and either conjugation to a protein carrier or the presence of a TLR agonist. ENM – Engineered nanomaterial, ThPr – Therapeutic protein; SWCNT – Single-wall carbon nanotubes, PAMAM – Polyamidoamine, PEG – Polyethylene glycol, PS – Phosphatidyl serine, TNF – Tumor necrosis factor.
Future research in this area should focus on developing methods for isolating and characterizing undesirable nanoparticulate contaminants, uncovering the mechanisms of undesirable immunogenicity and antigenicity, improving the mechanistic understanding of desirable immunogenicity, and applying this knowledge to design safe nanomedicines and biotechnology-derived pharmaceutics.
Most engineered nanomaterials are not immunogenic per se
Generation of nanoparticle-specific antibody can be T-cell dependent or independent
Antibodies can be generated to particle core, terminal groups or surface coatings
Engineered and accidental nanomaterials have distinct contribution to immunogenicity
Tunable physicochemical properties make each nanoparticle unique
Acknowledgments
This work has been funded with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
The authors have no financial information to disclose related to this study.
References
- Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172:38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977a;252:3582–3586. [PubMed] [Google Scholar]
- Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977b;252:3578–3581. [PubMed] [Google Scholar]
- Agashe HB, Dutta T, Garg M, Jain NK. Investigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimer. J Pharm Pharmacol. 2006;58:1491–1498. doi: 10.1211/jpp.58.11.0010. [DOI] [PubMed] [Google Scholar]
- Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving CR. Natural antibodies against phospholipids and liposomes in humans. Biochem Soc Trans. 1984;12:342–344. doi: 10.1042/bst0120342. [DOI] [PubMed] [Google Scholar]
- Alving CR. Antibodies to liposomes, phospholipids and phosphate esters. Chem Phys Lipids. 1986;40:303–314. doi: 10.1016/0009-3084(86)90075-7. [DOI] [PubMed] [Google Scholar]
- Alving CR, Swartz GM, Jr., Wassef NM, Ribas JL, Herderick EE, Virmani R, Kolodgie FD, Matyas GR, Cornhill JF. Immunization with cholesterol-rich liposomes induces anti-cholesterol antibodies and reduces diet-induced hypercholesterolemia and plaque formation. J Lab Clin Med. 1996;127:40–49. doi: 10.1016/s0022-2143(96)90164-x. [DOI] [PubMed] [Google Scholar]
- Andreev SM, Babakhin AA, Petrukhina AO, Romanova VS, Parnes ZN, Petrov RV. Immunogenic and allergenic properties of fulleren conjugates with aminoacids and proteins. Doklady Biochemistry. 2000a;370:261–264. [PubMed] [Google Scholar]
- Andreev SM, Babakhin AA, Petrukhina AO, Romanova VS, Parnes ZN, Petrov RV. Immunogenic and allergenic properties of fulleren conjugates with aminoacids and proteins. Dokl Biochem. 2000b;370:4–7. [PubMed] [Google Scholar]
- Arima Y, Toda M, Iwata H. Complement activation on surfaces modified with ethylene glycol units. Biomaterials. 2008;29:551–560. doi: 10.1016/j.biomaterials.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, Garratty G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- Artzy Schnirman A, Zahavi E, Yeger H, Rosenfeld R, Benhar I, Reiter Y, Sivan U. Antibody molecules discriminate between crystalline facets of a gallium arsenide semiconductor. Nano Lett. 2006;6:1870–1874. doi: 10.1021/nl0607636. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- Banerji B, Kenny JJ, Scher I, Alving CR. Antibodies against liposomes in normal and immune-defective mice. J Immunol. 1982;128:1603–1607. [PubMed] [Google Scholar]
- Barbas CF, 3rd, Rosenblum JS, Lerner RA. Direct selection of antibodies that coordinate metals from semisynthetic combinatorial libraries. Proc Natl Acad Sci U S A. 1993;90:6385–6389. doi: 10.1073/pnas.90.14.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee JS, Chiu D, Sawicki S, Stevenson JL, Chatterjee K, Freund E, Carpenter JF, Randolph TW. Monoclonal antibody interactions with micro- and nanoparticles: adsorption, aggregation, and accelerated stress studies. J Pharm Sci. 2009;98:3218–3238. doi: 10.1002/jps.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BC, Goldbaum FA, Chen BX, Kirschner AN, Wilson SR, Erlanger BF. X-ray crystal structure of an anti-Buckminsterfullerene antibody fab fragment: biomolecular recognition of C(60). Proc Natl Acad Sci U S A. 2000;97:12193–12197. doi: 10.1073/pnas.210396197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttel IC, Chamberlain P, Chowers Y, Ehmann F, Greinacher A, Jefferis R, Kramer D, Kropshofer H, Lloyd P, Lubiniecki A, Krause R, Mire-Sluis A, Platts-Mills T, Ragheb JA, Reipert BM, Schellekens H, Seitz R, Stas P, Subramanyam M, Thorpe R, Trouvin JH, Weise M, Windisch J, Schneider CK. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals. 2011;39:100–109. doi: 10.1016/j.biologicals.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Carpenter J, Cherney B, Lubinecki A, Ma S, Marszal E, Mire-Sluis A, Nikolai T, Novak J, Ragheb J, Simak J. Meeting report on protein particles and immunogenicity of therapeutic proteins: filling in the gaps in risk evaluation and mitigation. Biologicals. 2010;38:602–611. doi: 10.1016/j.biologicals.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G, Fan YX, Kirshner S, Verthelyi D, Kozlowski S, Clouse KA, Swann PG, Rosenberg A, Cherney B. Overlooking subvisible particles in therapeutic protein products: gaps that may compromise product quality. J Pharm Sci. 2009;98:1201–1205. doi: 10.1002/jps.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain P, Mire-Sluis AR. An overview of scientific and regulatory issues for the immunogenicity of biological products. Dev Biol (Basel) 2003;112:3–11. [PubMed] [Google Scholar]
- Chanda N, Kattumuri V, Shukla R, Zambre A, Katti K, Upendran A, Kulkarni RR, Kan P, Fent GM, Casteel SW, Smith CJ, Boote E, Robertson JD, Cutler C, Lever JR, Katti KV, Kannan R. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc Natl Acad Sci U S A. 2010;107:8760–8765. doi: 10.1073/pnas.1002143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrois GJ, Allen TM. Multiple injections of pegylated liposomal Doxorubicin: pharmacokinetics and therapeutic activity. J Pharmacol Exp Ther. 2003;306:1058–1067. doi: 10.1124/jpet.103.053413. [DOI] [PubMed] [Google Scholar]
- Chen BX, Wilson SR, Das M, Coughlin DJ, Erlanger BF. Antigenicity of fullerenes: antibodies specific for fullerenes and their characteristics. Proc Natl Acad Sci U S A. 1998;95:10809–10813. doi: 10.1073/pnas.95.18.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TL, Wu PY, Wu MF, Chern JW, Roffler SR. Accelerated clearance of polyethylene glycol-modified proteins by anti-polyethylene glycol IgM. Bioconjug Chem. 1999;10:520–528. doi: 10.1021/bc980143z. [DOI] [PubMed] [Google Scholar]
- Chi EY, Weickmann J, Carpenter JF, Manning MC, Randolph TW. Heterogeneous nucleation-controlled particulate formation of recombinant human platelet-activating factor acetylhydrolase in pharmaceutical formulation. J Pharm Sci. 2005;94:256–274. doi: 10.1002/jps.20237. [DOI] [PubMed] [Google Scholar]
- Cui J, Li C, Wang C, Li Y, Zhang L, Yang H. Repeated injection of pegylated liposomal antitumour drugs induces the disappearance of the rapid distribution phase. J Pharm Pharmacol. 2008;60:1651–1657. doi: 10.1211/jpp/60.12.0011. [DOI] [PubMed] [Google Scholar]
- Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292:1071–1079. [PubMed] [Google Scholar]
- De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA. Nanoparticles and antigenicity. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterilas. World Scientific Publishing Co. Pte. Ltd; Singapore: 2013. pp. 547–580. [Google Scholar]
- Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- Dykman LA, Sumaroka MV, Staroverov SA, Zaitseva IS, Bogatyrev VA. [Immunogenic properties of the colloidal gold]. Izv Akad Nauk Ser Biol. 2004:86–91. [PubMed] [Google Scholar]
- Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801–808. [PubMed] [Google Scholar]
- Ennis RD, Pritchard R, Nakamura C, Coulon M, Yang T, Visor GC, Lee WA. Glass vials for small volume parenterals: influence of drug and manufacturing processes on glass delamination. Pharm Dev Technol. 2001;6:393–405. doi: 10.1081/pdt-100002248. [DOI] [PubMed] [Google Scholar]
- Erlanger BF, Chen BX, Zhu M, Brus L. Binding of an anti-fullerene IgG monoclonal antibody to single wall carbon nanotubes. Nano Letters. 2001;1:465–467. [Google Scholar]
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nanovaccines against tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- Fradkin AH, Carpenter JF, Randolph TW. Glass particles as an adjuvant: a model for adverse immunogenicity of therapeutic proteins. J Pharm Sci. 2011;100:4953–4964. doi: 10.1002/jps.22683. [DOI] [PubMed] [Google Scholar]
- Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9:1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- Gershon SK, Luksenburg H, Cote TR, Braun MM. Pure red-cell aplasia and recombinant erythropoietin. N Engl J Med. 2002;346:1584–1586. doi: 10.1056/NEJM200205163462015. author reply 1584-1586. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Abu Lila AS, Shimizu T, Ishida T, Kiwada H. B cell-intrinsic toll-like receptor 7 is responsible for the enhanced anti-PEG IgM production following injection of siRNA-containing PEGylated lipoplex in mice. J Control Release. 2014a;184:1–8. doi: 10.1016/j.jconrel.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Shimizu T, Abu Lila AS, Ishida T, Kiwada H. Relationship between the Concentration of Anti-polyethylene Glycol (PEG) Immunoglobulin M (IgM) and the Intensity of the Accelerated Blood Clearance (ABC) Phenomenon against PEGylated Liposomes in Mice. Biol Pharm Bull. 2015;38:417–424. doi: 10.1248/bpb.b14-00653. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Shimizu T, Mima Y, Abu Lila AS, Ishida T, Kiwada H. Generation, characterization and in vivo biological activity of two distinct monoclonal anti-PEG IgMs. Toxicol Appl Pharmacol. 2014b;277:30–38. doi: 10.1016/j.taap.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Hendrickson O, Fedyunina N, Zherdev A, Solopova O, Sveshnikov P, Dzantiev B. Production of monoclonal antibodies against fullerene C60 and development of a fullerene enzyme immunoassay. Analyst. 2012;137:98–105. doi: 10.1039/c1an15745k. [DOI] [PubMed] [Google Scholar]
- Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W. Structure-immunogenicity relationships of therapeutic proteins. Pharm Res. 2004;21:897–903. doi: 10.1023/b:pham.0000029275.41323.a6. [DOI] [PubMed] [Google Scholar]
- Hermeling S, Schellekens H, Crommelin DJ, Jiskoot W. Micelle-associated protein in epoetin formulations: aA risk factor for immunogenicity? Pharm Res. 2003;20:1903–1907. doi: 10.1023/b:pham.0000008034.61317.02. [DOI] [PubMed] [Google Scholar]
- Ichihara M, Shimizu T, Imoto A, Hashiguchi Y, Uehara Y, Ishida T, Kiwada H. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics. 2011;3:1–11. doi: 10.3390/pharmaceutics3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release. 2006a;115:251–258. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release. 2005;105:305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J Control Release. 2006b;115:243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release. 2006c;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ichikawa T, Ichihara M, Sadzuka Y, Kiwada H. Effect of the physicochemical properties of initially injected liposomes on the clearance of subsequently injected PEGylated liposomes in mice. J Control Release. 2004;95:403–412. doi: 10.1016/j.jconrel.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kashima S, Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J Control Release. 2008;126:162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Pharm. 2008;354:56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Maeda T, Sakamoto H, Takasaki N, Shigyo M, Ishida T, Kiwada H, Mizushima Y, Mizushima T. Evasion of the accelerated blood clearance phenomenon by coating of nanoparticles with various hydrophilic polymers. Biomacromolecules. 2010;11:2700–2706. doi: 10.1021/bm100754e. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Nashed-Samuel Y, Li C, Liu W, Pollastrini J, Mallard D, Wen ZQ, Fujimori K, Pallitto M, Donahue L, Chu G, Torraca G, Vance A, Mire-Sluis T, Freund E, Davis J, Narhi L. Tungsten-induced protein aggregation: solution behavior. J Pharm Sci. 2009;98:4695–4710. doi: 10.1002/jps.21778. [DOI] [PubMed] [Google Scholar]
- Jiskoot W, Randolph TW, Volkin DB, Middaugh CR, Schoneich C, Winter G, Friess W, Crommelin DJ, Carpenter JF. Protein instability and immunogenicity: roadblocks to clinical application of injectable protein delivery systems for sustained release. J Pharm Sci. 2012;101:946–954. doi: 10.1002/jps.23018. [DOI] [PubMed] [Google Scholar]
- Judge A, McClintock K, Phelps JR, Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13:328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, McLeod VM, Porter CJ, Boyd BJ. Differences in colloidal structure of PEGylated nanomaterials dictate the likelihood of accelerated blood clearance. J Pharm Sci. 2011;100:5069–5077. doi: 10.1002/jps.22682. [DOI] [PubMed] [Google Scholar]
- Klinge J, Ananyeva NM, Hauser CA, Saenko EL. Hemophilia A--from basic science to clinical practice. Semin Thromb Hemost. 2002;28:309–322. doi: 10.1055/s-2002-32667. [DOI] [PubMed] [Google Scholar]
- Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm. 2010;392:218–223. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Koide H, Asai T, Hatanaka K, Urakami T, Ishii T, Kenjo E, Nishihara M, Yokoyama M, Ishida T, Kiwada H, Oku N. Particle size-dependent triggering of accelerated blood clearance phenomenon. Int J Pharm. 2008;362:197–200. doi: 10.1016/j.ijpharm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Krayukhina E, Tsumoto K, Uchiyama S, Fukui K. Effects of syringe material and silicone oil lubrication on the stability of pharmaceutical proteins. J Pharm Sci. 2015;104:527–535. doi: 10.1002/jps.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunamneni A, Abdelghani TT, Ellaiah P. Streptokinase--the drug of choice for thrombolytic therapy. J Thromb Thrombolysis. 2007;23:9–23. doi: 10.1007/s11239-006-9011-x. [DOI] [PubMed] [Google Scholar]
- Leach JK, O'Rear EA, Patterson E, Miao Y, Johnson AE. Accelerated thrombolysis in a rabbit model of carotid artery thrombosis with liposome-encapsulated and microencapsulated streptokinase. Thromb Haemost. 2003;90:64–70. [PubMed] [Google Scholar]
- Lee SC, Parthasarathy R, Botwin K, Kunneman D, Rowold E, Lange G, Klover J, Abegg A, Zobel J, Beck T, Miller T, Hood W, Monahan J, McKearn JP, Jansson R, Voliva CF. Biochemical and immunological properties of cytokines conjugated to dendritic polymers. Biomed Microdevices. 2004;6:191–202. doi: 10.1023/B:BMMD.0000042048.18186.ff. [DOI] [PubMed] [Google Scholar]
- Lee SC, Parthasarathy R, Duffin TD, Botwin K, Zobel J, Beck T, Lange G, Kunneman D, Janssen R, Rowold E, Voliva CF. Recognition properties of antibodies to PAMAM dendrimers and their use in immune detection of dendrimers. Biomedical Microdevices. 2001a;3:53–59. [Google Scholar]
- Lee SC, Parthasarathy R, Duffin TD, Botwin K, Zobel J, Beck T, Lange G, Kunneman D, Janssen R, Rowold E, Voliva CF. Recognition properties of antibodies to PAMAM dendrimers and their use in immune detection of dendrimers. Biomed Microdevices. 2001b;3:53–59. [Google Scholar]
- Leger RM, Arndt P, Garratty G, Armstrong JK, Meiselman HJ, Fisher TC. Normal donor sera can contain antibodies to polyethylene glycol (PEG). Transfusion. 2001;41:29S. [Google Scholar]
- Li C, Cao J, Wang Y, Zhao X, Deng C, Wei N, Yang J, Cui J. Accelerated blood clearance of pegylated liposomal topotecan: influence of polyethylene glycol grafting density and animal species. J Pharm Sci. 2012;101:3864–3876. doi: 10.1002/jps.23254. [DOI] [PubMed] [Google Scholar]
- Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Jr., Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Randolph TW, Carpenter JF. Particles shed from syringe filters and their effects on agitation-induced protein aggregation. J Pharm Sci. 2012;101:2952–2959. doi: 10.1002/jps.23225. [DOI] [PubMed] [Google Scholar]
- Liu W, Swift R, Torraca G, Nashed-Samuel Y, Wen ZQ, Jiang Y, Vance A, Mire-Sluis A, Freund E, Davis J, Narhi L. Root Cause Analysis of Tungsten-Induced Protein Aggregation in Pre-filled Syringes. PDA J Pharm Sci Technol. 2010;64:11–19. [PubMed] [Google Scholar]
- Macdougall IC, Provenzano R, Sharma A, Spinowitz BS, Schmidt RJ, Pergola PE, Zabaneh RI, Tong-Starksen S, Mayo MR, Tang H, Polu KR, Duliege AM, Fishbane S. Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N Engl J Med. 2013;368:320–332. doi: 10.1056/NEJMoa1203166. [DOI] [PubMed] [Google Scholar]
- Masalova OV, Shepelev AV, Atanadze SN, Parnes ZN, Romanova VS, Vol'pina OM, Semiletov Iu A, Kushch AA. [Immunostimulating effect of water-soluble fullerene derivatives--perspective adjuvants for a new generation of vaccine]. Dokl Akad Nauk. 1999;369:411–413. [PubMed] [Google Scholar]
- Mire-Sluis A, Cherney B, Madsen R, Polozova A, Rosenberg A, Smith H, Arora T, Narhi L. Analysis and immunogenic potential of aggregates and particles: a practical approach, part 2. BioProcess International. 2011;9:38–43. [Google Scholar]
- Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, Dawson KA. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133:2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- Murphy K. Janeway's immunobiology Garland Science. 2012 [Google Scholar]
- Nguyen PD, O'Rear EA, Johnson AE, Lu R, Fung BM. Thrombolysis using liposomal-encapsulated streptokinase: an in vitro study. Proc Soc Exp Biol Med. 1989;192:261–269. doi: 10.3181/00379727-192-42995. [DOI] [PubMed] [Google Scholar]
- Nguyen PD, O'Rear EA, Johnson AE, Patterson E, Whitsett TL, Bhakta R. Accelerated thrombolysis and reperfusion in a canine model of myocardial infarction by liposomal encapsulation of streptokinase. Circ Res. 1990;66:875–878. doi: 10.1161/01.res.66.3.875. [DOI] [PubMed] [Google Scholar]
- Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Parenky A, Myler H, Amaravadi L, Bechtold-Peters K, Rosenberg A, Kirshner S, Quarmby V. New FDA draft guidance on immunogenicity. AAPS J. 2014;16:499–503. doi: 10.1208/s12248-014-9587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins WR, Vaughan DE, Plavin SR, Daley WL, Rauch J, Lee L, Janoff AS. Streptokinase entrapment in interdigitation-fusion liposomes improves thrombolysis in an experimental rabbit model. Thromb Haemost. 1997;77:1174–1178. [PubMed] [Google Scholar]
- Ramani K, Miclea RD, Purohit VS, Mager DE, Straubinger RM, Balu-Iyer SV. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J Pharm Sci. 2008a;97:1386–1398. doi: 10.1002/jps.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani K, Purohit V, Miclea R, Gaitonde P, Straubinger RM, Balu-Iyer SV. Passive transfer of polyethylene glycol to liposomal-recombinant human FVIII enhances its efficacy in a murine model for hemophilia A. J Pharm Sci. 2008b;97:3753–3764. doi: 10.1002/jps.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- Richards RL, Aronson J, Schoenbechler M, Diggs CL, Alving CR. Antibodies reactive with liposomal phospholipids are produced during experimental Trypanosoma rhodesiense infections in rabbits. J Immunol. 1983;130:1390–1394. [PubMed] [Google Scholar]
- Richter AW, Akerblom E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol. 1984;74:36–39. doi: 10.1159/000233512. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J Biomed Mater Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Rosenberg AS. Immunogenicity of biological therapeutics: a hierarchy of concerns. Dev Biol (Basel) 2003;112:15–21. [PubMed] [Google Scholar]
- Saadati R, Dadashzadeh S, Abbasian Z, Soleimanjahi H. Accelerated blood clearance of PEGylated PLGA nanoparticles following repeated injections: effects of polymer dose, PEG coating, and encapsulated anticancer drug. Pharm Res. 2013;30:985–995. doi: 10.1007/s11095-012-0934-y. [DOI] [PubMed] [Google Scholar]
- Saifer MG, Williams LD, Sobczyk MA, Michaels SJ, Sherman MR. Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxyPEG-proteins. Mol Immunol. 2014;57:236–246. doi: 10.1016/j.molimm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Sauerborn M, Brinks V, Jiskoot W, Schellekens H. Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci. 2010;31:53–59. doi: 10.1016/j.tips.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24:1720–1740. doi: 10.1016/s0149-2918(02)80075-3. discussion 1719. [DOI] [PubMed] [Google Scholar]
- Schellekens H, Hennink WE, Brinks V. The immunogenicity of polyethylene glycol: facts and fiction. Pharm Res. 2013;30:1729–1734. doi: 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- Schuster BG, Neidig M, Alving BM, Alving CR. Production of antibodies against phosphocholine, phosphatidylcholine, sphingomyelin, and lipid A by injection of liposomes containing lipid A. J Immunol. 1979;122:900–905. [PubMed] [Google Scholar]
- Seidl A, Hainzl O, Richter M, Fischer R, Bohm S, Deutel B, Hartinger M, Windisch J, Casadevall N, London GM, Macdougall I. Tungsten-induced denaturation and aggregation of epoetin alfa during primary packaging as a cause of immunogenicity. Pharm Res. 2012;29:1454–1467. doi: 10.1007/s11095-011-0621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SC, Harasym TO, Clow KA, Ansell SM, Klimuk SK, Hope MJ. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic Acid. J Pharmacol Exp Ther. 2005;312:1020–1026. doi: 10.1124/jpet.104.078113. [DOI] [PubMed] [Google Scholar]
- Sherman MR, Williams LD, Sobczyk MA, Michaels SJ, Saifer MG. Role of the methoxy group in immune responses to mPEG-protein conjugates. Bioconjug Chem. 2012;23:485–499. doi: 10.1021/bc200551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Ishida T, Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 2013;218:725–732. doi: 10.1016/j.imbio.2012.08.274. [DOI] [PubMed] [Google Scholar]
- Smith DM, Simon JK, Baker JR., Jr. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, Heuser JE, Arbeit JM, Wickline SA, Schlesinger PH. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest. 2009;119:2830–2842. doi: 10.1172/JCI38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Fang C, Stephen Z, Veiseh O, Hansen S, Lee D, Ellenbogen RG, Olson J, Zhang M. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine (Lond) 2008;3:495–505. doi: 10.2217/17435889.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SJ. Immunogenicity issues in drug development. J Immunotoxicol. 2006;3:165–172. doi: 10.1080/15476910600908852. [DOI] [PubMed] [Google Scholar]
- Tagami T, Nakamura K, Shimizu T, Yamazaki N, Ishida T, Kiwada H. CpG motifs in pDNA-sequences increase anti-PEG IgM production induced by PEG-coated pDNA-lipoplexes. J Control Release. 2010;142:160–166. doi: 10.1016/j.jconrel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Tatarewicz SM, Mytych DT, Manning MS, Swanson SJ, Moxness MS, Chirmule N. Strategic characterization of anti-drug antibody responses for the assessment of clinical relevance and impact. Bioanalysis. 2014;6:1509–1523. doi: 10.4155/bio.14.114. [DOI] [PubMed] [Google Scholar]
- Thirumangalathu R, Krishnan S, Ricci MS, Brems DN, Randolph TW, Carpenter JF. Silicone oil- and agitation-induced aggregation of a monoclonal antibody in aqueous solution. J Pharm Sci. 2009;98:3167–3181. doi: 10.1002/jps.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai NM, Cheng TL, Roffler SR. Sensitive measurement of polyethylene glycol-modified proteins. Biotechniques. 2001;30:396–402. doi: 10.2144/01302rr03. [DOI] [PubMed] [Google Scholar]
- Tyagi AK, Randolph TW, Dong A, Maloney KM, Hitscherich C, Jr., Carpenter JF. IgG particle formation during filling pump operation: a case study of heterogeneous nucleation on stainless steel nanoparticles. J Pharm Sci. 2009;98:94–104. doi: 10.1002/jps.21419. [DOI] [PubMed] [Google Scholar]
- Van Beers MM, Gilli F, Schellekens H, Randolph TW, Jiskoot W. Immunogenicity of recombinant human interferon beta interacting with particles of glass, metal, and polystyrene. J Pharm Sci. 2012;101:187–199. doi: 10.1002/jps.22744. [DOI] [PubMed] [Google Scholar]
- Verhoef JJ, Carpenter JF, Anchordoquy TJ, Schellekens H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov Today. 2014;19:1945–1952. doi: 10.1016/j.drudis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119:236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Nakanishi T, Umetsu M, Kumagai I. Human anti-gold antibodies: biofunctionalization of gold nanoparticles and surfaces with anti-gold antibodies. J Biol Chem. 2008;283:36031–36038. doi: 10.1074/jbc.M805547200. [DOI] [PubMed] [Google Scholar]
- Xiang SD, Selomulya C, Ho J, Apostolopoulos V, Plebanski M. Delivery of DNA vaccines: an overview on the use of biodegradable polymeric and magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:205–218. doi: 10.1002/wnan.88. [DOI] [PubMed] [Google Scholar]
- Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151:458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]