Abstract
The surgically-demanding mouse orthotopic liver transplant model was first described in 1991. It has proved a powerful research tool for investigation of liver biology, tissue injury, the regulation of alloimmunity and tolerance induction and the pathogenesis of specific liver diseases. Liver transplantation in mice has unique advantages over transplantation of the liver in larger species, such as the rat or pig, since the mouse genome is well-characterized and there is much greater availability of both genetically-modified animals and research reagents. Liver transplant experiments using various transgenic or gene knockout mice has provided valuable mechanistic insights into the immuno- and pathobiology of the liver and the regulation of graft rejection and tolerance over the past 25 years. The molecular pathways identified in regulation of tissue injury and promotion of liver transplant tolerance provide new potential targets for therapeutic intervention to control adverse inflammatory responses/ immune-mediated events in the hepatic environment and systemically. Conclusion: Orthotopic liver transplantation in the mouse is a valuable model for gaining improved insights into liver biology, immunopathology and allograft tolerance that may result in therapeutic innovation in liver and other diseases.
Keywords: immune cells, ischemia-reperfusion injury, transplant tolerance
The mouse liver transplant model and its significance
Since it was first described by Qian et al1 at the University of Pittsburgh in 1991, key mechanistic insights into liver immunobiology, regulation of hepatic injury and the balance between organ transplant rejection and tolerance have accumulated using this challenging surgical model. A survey of the literature performed in October 2015 has revealed at least 70 articles describing the model to study not only mechanisms underlying liver transplant ischemia/reperfusion (I/R) injury and alloimmunity/“spontaneous” tolerance, but also other aspects of liver biology, immunology and pathology. These include liver regeneration, hepatic immune cell homeostasis, antigen (Ag) presentation, and the pathogenesis of certain liver diseases (fulminant hepatitis and hemochromatosis). Here, we provide a concise review of significant insights gained using the model over the past 25 years.
Alloimmunity and Mechanisms Underlying Liver Allograft Tolerance
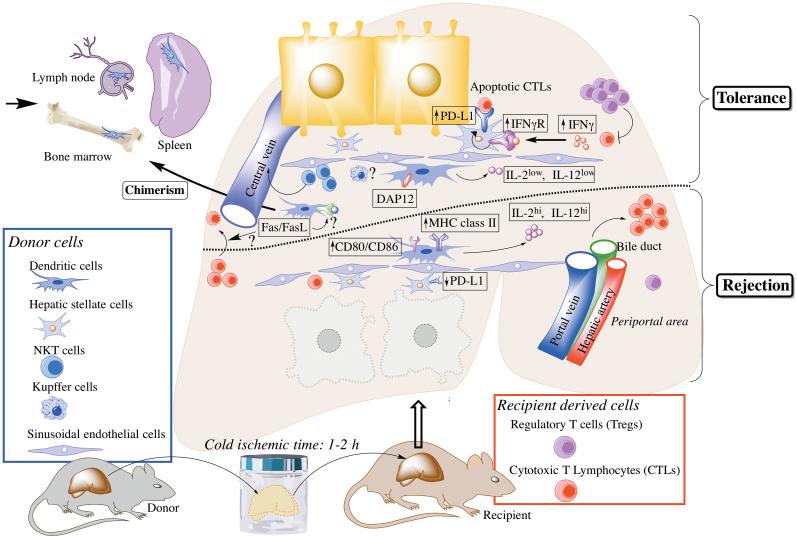
The liver is a unique anatomical and immunological site.2, 3 In mice, liver allografts are accepted indefinitely across major histocompatibility complex (MHC) barriers without immunosuppressive therapy.4 ‘Operational tolerance’ also occurs in the clinic, in approximately 20% of stable human liver allograft recipients weaned intentionally off all immunosuppressive agents.5-7 This tolerogenic effect is sufficiently robust and specific in mice that subsequent skin or heart allografts from the same donor strain are also accepted, while third-party grafts are rejected.4, 8 In addition, liver transplantation can prevent accelerated rejection after repeated skin transplantation in sensitized recipients and protect such ‘second-set’ skin grafts permanently.9 Although other types of organ allograft (i.e. kidneys or hearts) may also induce tolerance in mice, liver allografts exert a much stronger and reproducible effect.9, 10 Much effort has been made to delineate the cellular and molecular basis of allograft tolerance, with a particular focus on the roles of donor-derived or host immune cells. Thus, for example, the Fas/FasL system appears to play an important role in host immune regulation, enabling liver allograft acceptance by deletion of donor-specific cytotoxic T cells (CTL).11 The cells and molecular mechanisms that have been implicated in regulating the balance between tolerance and rejection in the mouse liver transplant model are summarized in Table 1 and depicted in Figure 1.
Table 1.
Observations implicating roles for donor-derived or recipient immune cells in mouse liver transplant tolerance
| Cells or Molecules | Findings regarding transplant tolerance | Reference |
|---|---|---|
| Donor-derived Cells | ||
|
| ||
| Dendritic cells | Systemic donor-derived hematopoietic cell chimerism in liver transplant host lymphoid tissues |
Qian S, Hepatology, 19949 |
| Propagation of donor DC from lymphoid tissue of liver transplant but not heart transplant recipients |
Lu L, J Exp Med, 199515 | |
| Proposed immunological role of donor-derived DC in bone marrow chimerism in host |
Thomson AW, Transplantation, 199516 | |
| Marked increases in DC within the graft induces rejection that can be reversed with IL- 12 blockade. |
Li W, J Immunol 200122 | |
| DAP12 expression by liver dendritic cells may be critical for the induction of tolerance |
Yoshida O, Am J Transplant, 201424 | |
|
| ||
| Kupffer cells | Kupffer cells are not critical for tolerance | Morita M, Hepatology, 201525 |
|
| ||
| NKT cells | NKT cells play a key immunomodulatory role | Morita M, Transpl Immunol, 200726 |
| Donor NK and NKT cells remain in liver and blood and are resistant to rejection |
Tay SS, Liver Transplant, 201314 | |
|
| ||
| Hepatic stellate cells | Upregulated PD-L1 expression by stellate cells is important in tolerance induction |
Morita M, Hepatology, 201525 |
|
| ||
| Host cells | ||
|
| ||
| Cytotoxic T cells | Apoptosis of cytotoxic T lymphocytes within the grafts may provide a mechanistic basis of allograft acceptance. |
Qian S, J Immunol 199729 |
| Regulatory T cells | Depletion of host regulatory T cells increases alloimmune responses |
Jiang X, Liver Transplant, 200631
Li W, Am J Transplant, 200832 |
In addition, expression of Fas (CD95) and FasL by various immune cells (and hepatocytes) may play an important role in regulation of anti-donor CTL responses.11
Figure 1.
Cellular and molecular mechanisms implicated in regulating the balance between tolerance and rejection in the mouse liver transplant model. Donor-derived cells, such as dendritic cells (DC) are found in lymphoid tissues in long-surviving, fully allogeneic mouse liver transplant recipients. Donor-derived DC and NKT cells, but not Kupffer cells, have been reported to play an important role in the establishment of mouse liver transplant tolerance. Enhanced production of IL-12 by donor DC (as the result of Flt3L administration to the liver donor) or exogenous IL-2 can promote cytotoxic T lymphocytes (CTL) and leads to graft rejection. The transmembrane adaptor protein DNAX-activating protein of 12kDa (DAP12) expression by the allograft appears to be a critical molecule that regulates the induction of liver transplant tolerance since its absence results in enhanced host CTL and reduced regulatory T cells. Similarly, IFNγ signaling appears to be required for mouse liver allograft acceptance; host-derived IFNγ induces programed death ligand-1 (PD-L1) up-regulation on donor-derived stellate cells that in turn, promote apoptosis of graft-infiltrating CTLs. The Fas/FasL pathway also appears to play a critical role in mouse liver transplant tolerance, but the exact mechanisms have not yet been elucidated.
(i) The role of donor-derived hematopoietic cells in mouse liver transplant tolerance
While whole liver allografts have well-established tolerogenic properties, purified mouse hepatocytes are immunogenic and elicit rejection,12, 13 suggesting that non-parenchymal cells play a key role in liver transplant tolerance. These donor-derived hematopoietic cells migrate rapidly from the graft following transplantation and are found in the blood as early as 1.5 hours post-transplant and in the spleen and lymph nodes (LN) 1.5 to 24 hours post-transplant.14 Interestingly, distinct leukocyte populations exhibit different migration patterns. Thus, donor T and B cells migrate to LN and spleen, whereas natural killer (NK) cells and NKT cells remain in the liver and blood. Reductions in donor T cells in both LN and spleen suggest that these lymphocytes may be rejected rapidly, whereas persistence of NK and NKT cells in the blood and the liver suggests that these cells are resistant to rejection.14
An important role of donor-derived hematopoietic cells was further suggested by the observation that long-surviving, fully allogeneic mouse liver transplant recipients exhibit systemic hematopoietic cell chimerism (i.e. the presence of donor-derived leukocytes in host tissues) several months post-transplant.9 Donor-derived cells are found most readily in host lymphoid tissues (e.g. the spleen, mesenteric LN and thymus).15 Small numbers are also found in small bowel, skin, kidney, tongue, heart and lung.9 Donor cells can also be detected, although the frequency of cells expressing donor MHC class I is very low (1%) in host bone marrow (BM) two weeks after mouse liver transplantation in the absence of immunosuppressive therapy.15, 16 These donor-derived populations include not only innate immune cells (dendritic cells [DC] and macrophages), but also T and B lymphocytes that may also have immunoregulatory properties.9
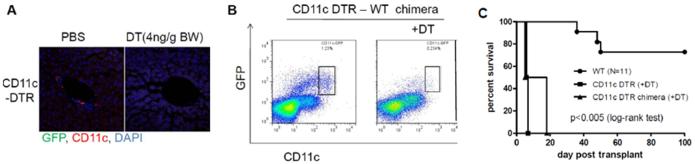
Significantly, donor-derived professional Ag-presenting cells (DC) can be propagated from lymphoid tissue of mouse liver allograft recipients but not from mice that acutely reject cardiac allografts from the same donor strain in the absence of immunosuppression.15 This suggests that liver tolerogenicity may depend on donor-derived hematopoietic cells (in particular DC17) that can inhibit host adaptive immunity.15, 18-20 Confirmation of the crucial importance of donor liver DC in promotion of mouse liver transplant tolerance comes from experiments in which conventional CD11c+ DC have been depleted selectively from liver allografts prior to their transplantation. As shown in Figure 2, livers from DC-depleted donors are rejected acutely, whereas those from wild-type (WT) donors are accepted indefinitely. On the other hand, when livers with dramatically increased numbers of interstitial DC (the result of Fms-like tyrosine kinase 3 ligand administration) are transplanted into WT recipients, they are also rejected acutely.21, 22 However, in contrast to normal livers, these allografts exhibit marked up-regulation of IL-12 production by interstitial donor DC.22 When IL-12 is neutralized, graft survival is markedly prolonged associated with inhibition of intra-graft donor-specific cytotoxic T cell (CTL) and NK cell activities and with CD8+ T cell apoptosis in the graft and lymphoid tissue. These results indicate that, in this experimental setting, marked increases in numbers of migratory liver-derived DC that exhibit enhanced IL-12 production can switch tolerance to rejection.22
Figure 2.
Profound depletion of interstitial (periportal) CD11c+ conventional myeloid (m)DC following diptheria toxoid (DT) administration in (A) the liver of a B6 CD11c-DTR mouse and (B) the liver of a chimeric B6 mouse in which only the hematopoietic cells were CD11c-DTR. (C) Survival of liver allografts from mDC-depleted B6 CD11c-DTR donor mice or from mDC-depleted B6 donor mice (CD11c-DTR chimeras) in which only the hematopoietic cells were CD11c+-DTR. Recipients were wild-type C3H mice. Experiments were conducted under an Institutional Animal Care and Use Committee-approved protocol (#130590681).
Several molecular mechanisms, whereby donor hematopoietic and other liver cells may promote transplant tolerance, have been identified. Recently, the transmembrane adaptor protein DNAX-activating protein of 12kDa (DAP12) has been shown to regulate the maturation of liver DC, their ability to migrate to host lymphoid tissue and their T cell allostimulatory capacity.23, 24 Thus, freshly-isolated DAP12−/− liver mDC exhibit a more mature phenotype, enhanced secretion of proinflammatory cytokines and greater naïve T cell allostimulatory activity than WT liver DC. Following liver transplantation from DAP12−/− (B6; H2b) donors to WT C3H (H2k) recipients, donor liver mDC migrate in greater numbers to host spleens, associated with increased pro-inflammatory cytokine (IL-6, IL-12p40, perforin, granzyme B, tumor necrosis factor [TNF]α, and IFNγ) expression in the grafts. Moreover, DAP12−/− liver grafts fail to induce tolerance and are rejected acutely.24 These results suggest that DAP12 expression in donor livers is important in regulating donor DC migration to host lymphoid tissue, modulation of alloreactive T cell responses and the induction of tolerance.
The role of other liver-resident APC in the induction of transplant tolerance has also been investigated. Thus, the function of hepatic macrophages (Kupffer cells [KC]) has been examined by selectively depleting these cells in donor mouse livers by clodronate liposome administration. However, these KC-depleted liver allografts are still accepted spontaneously,25 indicating that donor KC are not critical for induction of liver transplant tolerance in mice.
Donor-derived NKT cells may play an important role in the establishment of mouse liver transplant tolerance. Thus, when liver grafts from NKT (Jα281) knockout (NKT KO) mice on the C57BL/6 (B6; H2kb) background are transplanted into WT BALB/c (H2kd) mice (NKT KO→WT), only 50% of the recipients survive, whereas 91% of WT B6 donor grafts survive in WT BALB/c (WT→WT) recipients. WT recipients of NKT KO livers also exhibit extensive lymphocytic infiltration of portal triads and bile duct epithelium, which is largely absent in the WT→WT combination.26 These results indicate that donor-derived NKT cells, like DC, may play a key immunomodulatory role in development of mouse liver allograft tolerance.
A recent report 25 shows that IFNγ signaling, reported earlier to be an absolute requirement for mouse liver allograft acceptance,27 up-regulates co-inhibitory B7-H1 (= programed death-ligand 1; PD-L1) expression on donor-derived CD45− hepatic stellate cells and that apoptosis of graft-infiltrating CD8+ T cells is important in tolerance induction. The findings indicate that T effector cell-derived IFNγ may be critical in initiating a negative feedback loop via PD-L1, that, in turn, promotes apoptotic elimination of T effectors and tolerance induction.25 These results are in accord with previous studies from the same group in which PD-L1−/− liver transplantation resulted in rejection.28
(ii) The role of host immune cells in mouse liver transplant tolerance
Most donor passenger leukocytes exit mouse liver grafts and are rapidly replaced by recipient leukocytes within 1.5 hours of transplantation.14 Along with an initial, non-specific increase in myeloid cells (predominantly neutrophils and Gr1+ monocytes), liver allografts exhibit increased numbers of T and B cells.14 By day 4 post-transplant, 95% of T cells (mainly CD4+) are recipient-derived.29 At later time points (days 7 and 14), CD8+T cells predominate. Alloreactive CTL observed on day 4 post-transplant decline gradually over time29 associated with increases in apoptotic graft-infiltrating T cells that appear to contribute to tolerance induction. Thus, systemic administration of mouse IL-2 that reduces apoptotic cells within the graft-infiltrating cell population, increases CTL activity and induces acute graft rejection.29, 30
The role of host regulatory T cells (Treg) in mouse liver allograft acceptance has also been studied.31, 32 Thus, CD4+CD25+ Foxp3+ T cells are increased in the grafts and host spleens compared to naïve mice between days 5 and 100 post-transplant.32 They appear to be important in the induction of tolerance since their selective depletion (but not depletion of donor Treg) abrogates tolerance.31, 32 Moreover, depletion of host CD4+CD25+ T cells increases CD4+ and CD8+T cell responses with reduced apoptosis of graft-infiltrating T cells.31, 32
Liver transplant ischemia/reperfusion (I/R) injury
Graft I/R injury remains as an important problem during liver transplantation.33, 34 Que et al35 developed a mouse model to study liver cold I/R injury by performing orthotopic liver transplantation with various cold preservation times. Thirty-day graft survival was 100%, 100%, 88% and 0% for 1, 4, 8, 16 hr preservation, respectively. Prolonged cold preservation induced more IL-6 expression and BrdU uptake in the grafts and resulted in severe liver injury. Shen et al36 found that hepatic artery reconstruction reduced cold I/R injury and resulted in less CD4+ T cell infiltration, with reduced cytokine expression and hepatocellular damage.
Xie et al37 have reported that partial liver grafts display more severe liver cold I/R injury compared with whole grafts. While expression of chemokines, including T cell activation gene-3 (TCA-3, CCL1), is higher in partial grafts, neutralization of TCA-3 decreases neutrophil and T cell infiltration and attenuates cold I/R injury.
Molecular regulation of I/R injury
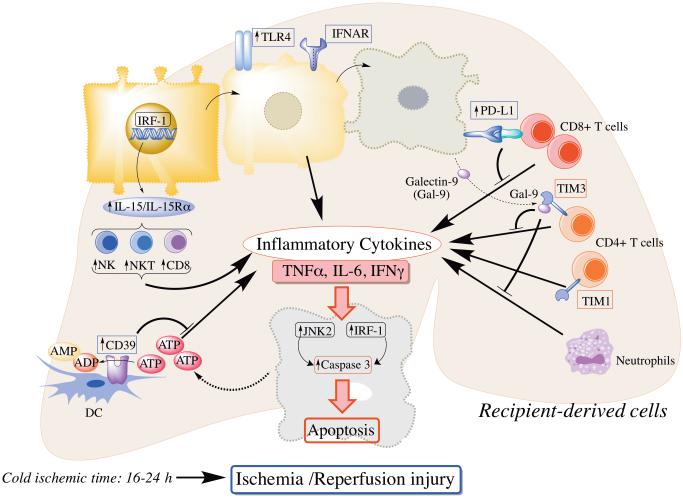
Molecular mechanisms that have been implicated in liver I/R injury based on studies in the mouse liver transplantation model are summarized Table 2 and depicted in Figure 3. Interferon regulatory factor-1 (IRF-1), a ubiquitous nuclear transcription factor induced by several agents (cytokines, hormones, viruses and retinoic acid) regulates cell proliferation, apoptosis and immune responses. Cold I/R induces IRF-1 expression in hepatocytes that upregulates death ligands (TNFR apoptosis-inducing ligand [TRAIL]; FasL)/death receptors (DR5; Fas) in the liver. IRF-1-deficient livers express neither death ligands nor receptors and display reduced cold I/R.38 A recent report39 has revealed a critical role of IRF-1. IL-15 is essential for development of T cells, NK cells and NKT cells and is produced by hepatocytes upon IRF-1 induction through cold I/R. IRF-1KO liver grafts are protected from cold I/R due to less induction of lymphocytes via IL-15. Interestingly, administration of IL-15/IL-15Rα complexes partially restores cold I/R injury by increasing T cells, NK cells and NKT cells.39
Table 2.
Specific molecular pathways implicated in liver I/R injury based on studies in mouse liver transplantation
| Molecule | Significance regarding I/R injury | Reference |
|---|---|---|
| Interferon regulatory factor-1 (IRF-1) |
IRF-1-deficient livers express neither death ligands nor death receptors and exhibit less cold I/R compared with WT livers |
Ueki S, Hepatology, 201038 |
| IRF-1 promotes liver transplant I/R injury via hepatocyte IL- 15/IL-15Ra production |
Yokota S, J Immunol 201539 | |
|
| ||
| T-cell activation gene-3 (TCA-3, CCL1) |
Neutralization by anti-TCA3 monoclonal antibody decreases neutrophil and T cell infiltration and attenuates cold I/R injury |
Xie JF Transplantation, 200637 |
|
| ||
| Toll-like receptor 4 (TLR4) | TLR4 KO liver grafts are protected from cold I/R injury | Shen XD, Liver Transpl, 200741 |
|
| ||
| JNK2 | JNK pathway induces mitochondrial depolarization and promotes hepatocyte death during cold I/R |
Theruvath TP, Am J Transpl, 200840 |
|
| ||
| B7 homolog1 (B7-H1) | Hepatocytes increase B7-H1 expression after cold I/R and induce T cell apoptosis in the graft |
Ueki S, Hepatology, 201145 |
|
| ||
| NTPDase-1 (CD39) | CD39 overexpression in liver grafts attenuates cold I/R injury | Pommery S, Hepatology, 201342 |
| CD39 deficiency exacerbates cold I/R. | Yoshida O, Hepatology, 201343 | |
|
| ||
| T cell immunoglobulin mucin- 1,3 (TIM-1,3) |
Anti-TIM-1 antibody treatment abolishes neutrophil/macrophage activation and reduces cold I/R injury |
Zhang Y, Am J Transplant, 201348 |
| TIM-3/Gal-9 activation protects livers from cold I/R injury | Liu Y, Am J Transplant, 201549 | |
|
| ||
| Type I interferon (IFN) | Type I IFN receptor (IFNAR) KO grafts that lack type I IFN signaling show less cold I/R injury compared with WT grafts. |
Shen XD, Am J Transplant, 201246 |
|
| ||
| Tumor necrosis factor-alpha (TNFα) |
Donor liver TNFR1 protects against cold I/R injury, whereas recipient TNFR1 promotes cold I/R injury |
Conzelmann LO, Transplantation, 200647 |
Figure 3.
Molecular mechanisms implicated in liver I/R injury based on studies in the mouse liver transplant model. Toll-like receptor (TLR4) and IFNAR are critical molecules involved in mouse liver transplant ischemia-reperfusion (I/R) injury. Interferon regulatory factor-1 (IRF-1) up-regulates IL-15/IL-15Ra production by hepatocytes, that in turn, activates donor-derived NK, NKT and CD8+ T cells to produce inflammatory cytokines. I/R-stressed hepatocytes release molecules such as adenosine triphosphate (ATP) and galectin-9. CD39 on donor liver-derived DC hydrolyzes extracellular ATP and ameliorate liver injury. Galectin-9 binds to T cell immunoglobulin mucin (TIM)3 on CD4+T cells and negatively regulates CD4+T cell activation and neutrophil infiltration, while TIM1 on activated CD4+T cells promotes liver injury. Following I/R injury, hepatocytes up-regulated programed death ligand-1 (PD-L1) and induce apoptosis of infiltrating CD8+T cells, thus preventing exacerbation of I/R injury. IRF-1, along with Jun N-terminal kinase (JNK)2, is involved in inducing apoptosis of hepatocytes.
The Jun N-terminal kinase (JNK) signaling pathway is activated under conditions of stress and functions to enhance immune responses and apoptosis induction. During cold I/R, it induces mitochondrial depolarization and promotes hepatocyte death. JNK-deficient mouse liver grafts display reduced cold I/R injury, with less necrosis/apoptosis compared with WT grafts.40
Many other gene products, including Toll-like receptors (TLR), other molecules expressed by immune cells, secreted cytokines and chemokines, appear to regulate I/R injury in the mouse liver transplant model. TLR4 deficiency in donor grafts reduces neutrophil/lymphocyte infiltration after cold liver I/R through weak induction of chemokines and pro-inflammatory cytokines. On the other hand, TLR4 KO grafts show increased expression of hemoxygenase-1 (HO-1) that attenuates immune responses. Thus, TLR4 KO liver grafts are protected from cold I/R injury.41 The cell surface ectonucleotidase CD39, an ecto-nucleoside triphosphate diphosphohydrolase family member, that hydrolyzes extracellular adenosine triphosphate released from damaged or dead cells can regulate inflammation. Its overexpression in liver grafts attenuates cold I/R injury42 an effect also achieved by portal infusion of CD39-expressing liver DC.43 Moreover, CD39 deficiency in donor livers promotes inflammatory injury and allograft rejection.44 B7-H1 is also expressed on liver APC and parenchymal cells, including hepatocytes. Hepatocytes increase B7-H1 expression after cold I/R and thus induce T cell apoptosis in the graft. While WT livers that express B7-H1 during cold I/R are protected against excessive T cell attack, B7-H1 KO liver grafts fail to induce T cell apoptosis and exhibit more severe cold I/R injury.45
Type-I IFNs (IFNα and IFNβ) produced by innate immune cells, including liver plasmacytoid DC, are multifunctional cytokines that activate or regulate immune responses. IFN receptor (IFNAR) KO grafts that lack type-I IFN signaling cannot recruit neutrophils or macrophages and show reduced cold I/R injury. Interestingly, silencing HO-1 cancels the protective effect of IFNAR deficiency against cold I/R injury.46 TNFα, also plays an important role during tissue damage and inflammation. When TNF receptor-1 (TNFR1) KO livers are transplanted with cold preservation into WT recipients, they exhibit severe necrosis, apoptosis and neutrophil infiltration. Interestingly, donor liver TNFR1 appears to protect against cold I/R injury, whereas host TNFR1 promotes cold I/R injury.47
T cell immunoglobulin (Ig) mucin-1 (TIM-1) is a member of the T cell Ig and mucin (TIM) domain family that is expressed on T cells and involved in T cell differentiation/activation. Cold I/R increases TIM-1 expression on activated CD4+ T cells within the liver. Blockade of TIM-1 signaling using mAb abolishes neutrophil/macrophage activation, alters cytokine profiles toward T helper (Th) 2 cells, promotes Treg and reduces cold liver I/R injury.48 TIM-3/Galactin-9 (Gal-9) signaling regulates CD4+ immune responses and while TIM-3 blockade enhances Th1 cell polarization and exacerbates hepatic cold I/R injury, TIM-3/Gal-9 activation protect mouse livers from cold I/R injury.49
Applications of mouse liver transplantation to other aspects of liver immunobiology/pathology
(i) The origin of liver macrophages (Kupffer cells) and the immunologic role of TLR4 expression and Ag presentation in the liver
Using the mouse liver transplant model, Crispe and colleagues50-53 identified two distinct KC subsets in mouse liver,- a radio-resistant, residual, non-BM-derived (sessile) population and a BM-derived KC population, by performing BM transplantation after sublethal irradiation. Only the BM-derived population was recruited into inflammatory foci in response to CD8+ T cell activation. To confirm this finding, liver transplantation was performed from congenic CD45.2 mice to CD45.1 recipients. As in the BM transplant experiment, a nearly equivalent distribution of BM-derived and sessile KCs was found 4 weeks after transplantation.52
The same group investigated the role of TLR4 expression in the liver by studying recipients of TLR4-deficient livers. These recipients received TCR transgenic (tg) OT-1 (CD8+) T cells and were primed with SIINFIKL (ovalbumin-derived peptide)-pulsed WT DCs. Six weeks later, SIINFEKL peptide was injected and clonal expansion of the OT-1 T cells determined. In recipients of TLR4-deficient livers, OT1 T cells were increased in the spleen, LN and liver compared with mice given WT grafts. Thus TLR4 expression in the liver controls the systemic immune response by trapping activated CD8+ T cells, regulating circulating CD8+ T cell numbers and down-modulating the extent of CD8+ T cell memory responses.53
To investigate the influence of restricted intrahepatic Ag presentation by hepatic cell types on immune reactivity, the same group transplanted livers from congenic B6.SJL mice (CD45.1 background, expressing MHC class I molecule Kb) into bm8 animals (CD45.2 background, expressing the Kbm8 mutation in the Kb molecule). To minimize the possibility of extrahepatic Ag presentation by donor-derived “passenger” leukocytes, BM chimeras were created as liver donors. Thus, B6.SJL mice were reconstituted with BM of the prospective liver transplant recipient strain, bm8. Using these chimeric liver grafts, only the graft parenchymal cells expressed the WT MHC class I molecule Kb and were competent to present the SIINFEKL peptide Ag. TCR tg CD8+ OT-I cells were adoptively transferred to the transplant recipients and SIINFEKL injected for 3 consecutive days. This restricted hepatic Ag presentation model was compared to control transplant recipients that expressed systemic Kb Ag. OT-I expansion was similar to or exceeded the expansion seen in control mice. Thus, Ag presentation in the liver was sufficient to promote activation and differentiation of CD8+ cells.50 Further investigation suggested that Ag-specific CD8+ T cells remain functionally competent after localization to the liver and retain their function after transfer or transplant. Investigation of naïve liver graft recipients indicated that those grafts that had previously experienced infection released trapped Ag-specific CD8+ T cells that functioned in a recall response.51
(ii) Small-for-size syndrome (SFSS) and liver regeneration
Use of partial liver grafts from either deceased or living donors expands the limited donor pool.54 However, insufficient liver mass may lead to small-for-size syndrome (SFSS) i.e. liver failure characterized by coagulopathy, hyperbilirubinemia, and encephalopathy.54 To elucidate mechanisms underlying SFSS, a few groups have developed reduced-size liver transplantation in the mouse.55-58 Liver injury is worse with smaller graft size; 30% partial liver recipients have the most severe liver injury compared to 50% partial or whole liver transplantation two days post-transplant.55, 56 Consistent with the extent of liver injury, 30% partial liver transplantation is associated with high mortality (100% within 4 days). By contrast, all mice that received 50% partial liver grafts survived for 100 days.56 The critical liver mass necessary for recipient survival and robust liver regeneration is estimated to be in the range 30 to 50%, similar to observations in humans.55, 56
Investigation of mechanisms underlying SFSS has revealed that KC inactivation with either gadolinium chloride or pentoxifylline reduces TNFα signaling and liver injury, improving recipient survival and hepatic regeneration.57 Similar effects have been observed using TFNR1−/− mice, suggesting that KC-dependent TNFα signaling plays a critical role in development of SFSS and liver regeneration after reduced-size liver transplantation.57
Amphiregulin (AR) is a ligand of the epidermal growth factor receptor (EGFR) and appears to promote liver regeneration. AR mRNA and protein expression are reduced significantly in 30% compared to 50% reduced size liver grafts in association with increased TNFα, IL-6 mRNA expression, impaired liver regeneration, worse liver function and decreased recipient survival.58 Exogenous AR partially reverses these effects, suggesting a therapeutic strategy to enhance regeneration in reduced size grafts.
(iii) The role of the liver in pathogenesis of liver disease (hemochromatosis, fulminant hepatitis)
There is controversy regarding the primary organ responsible for excess tissue iron deposition in hereditary hemochromatosis (HH). Hepcidin is the master regulator of systemic iron homeostasis produced by the liver and normally down-regulates the expression of ferroportin, the main iron exporter expressed in hepatocytes, duodenal enterocytes and macrophages.59 In HH, a mutation in the hemochromatosis HFE gene causes abnormally low hepcidin levels, which up-regulates ferroportin and leads to high blood iron levels. When HFE WT livers are transplanted in HFE KO mice (HH phenotype), liver hepcidin mRNA expression, serum transferrin saturation and liver iron content are normalized and iron content in the spleen and intestine improve.60 Conversely, transplantation of HFE KO livers into WT recipients, results in the HH phenotype. These findings indicate that hepatic HFE and downstream hepcidin expression are crucial in the pathogenesis of HH.
Mouse liver transplantation has been used to determine the relative importance of hepatic versus systemic Fas (CD95) expression in the development of fulminant hepatitis (FH).61 Fas-mutant (MRL-lpr/lpr) liver transplantation into WT mice (MRL-lpr/lpr→WT) results in a Fas defect only in the liver, whereas WT liver transplantation into MRL-lpr/lpr mice (WT →MRL-lpr/lpr) results in a systemic Fas defect with Fas expression only in the liver. FH can be induced by administration of either an adenoviral vector containing FasL plasmid to promote FasL overexpression in the liver or by agonistic anti-Fas Ab. Overexpression of FasL in the liver results in severe hepatic injury, massive CD11b+ granulocyte infiltration and increased apoptotic cells in all groups except non-transplanted MRL-lpr/lpr mice. Thus, FasL-induced FH occurrs when Fas is expressed either in the liver or the host. However, agonistic anti-Fas-induced FH requires Fas expression in the liver. All graft recipients that received Fas-mutated liver grafts (both MRL-lpr/lpr→WT group and MRL-lpr/lpr→MRL-lpr/lpr) survived and exhibited no apoptosis or CD11b+ granulocyte infiltration. By contrast, all those that received WT liver grafts died within 48 hr and exhibited massive apoptosis and CD11b+ granulocyte infiltration. These results indicate that experimental FH involves not only direct Fas/FasL interaction, but also recruitment of inflammatory cells.
Acknowledgments
The authors thank Ms. Miriam Freeman for excellent administrative support and Dr. Angelica Perez-Gutierrez for assistance with the preparation of the figures and tables.
Footnotes
- BM
- bone marrow
- DC
- dendritic cell
- KO
- knock-out
- NK(T) cell
- natural killer (T) cell
- WT
- wild-type
Grants and financial support: The authors’ work was supported by National Institutes of Health (NIH) grants P01 AI81678 and R56 AI126377. OY was supported by NIH institutional training grant T32 AI74490.
The authors declare no conflicts of interest.
References
- 1.Qian SG, Fung JJ, Demetris AV, Ildstad ST, Starzl TE. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52(3):562–4. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3(1):51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 3.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 4.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27(2):194–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 5.Demetris AJ, Lunz JG, 3rd, Randhawa P, Wu T, Nalesnik M, Thomson AW. Monitoring of human liver and kidney allograft tolerance: a tissue/histopathology perspective. Transpl Int. 2009;22(1):120–41. doi: 10.1111/j.1432-2277.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140(1):51–64. doi: 10.1053/j.gastro.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307(3):283–93. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 8.Qian S, Fung JJ, Sun H, Demetris AJ, Starzl TE. Transplantation unresponsiveness induced by liver allografts in mouse strains with various histocompatibility disparities. Transplant Proc. 1992;24(4):1605–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19(4):916–24. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahmen U, Qian S, Rao AS, Demetris AJ, Fu F, Sun H, et al. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. 1994;58(1):1–8. doi: 10.1097/00007890-199407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchiyama H, Kishihara K, Minagawa R, Hashimoto K, Sugimachi K, Nomoto K. Crucial Fas-Fas ligand interaction in spontaneous acceptance of hepatic allografts in mice. Immunology. 2002;105(4):450–7. doi: 10.1046/j.1365-2567.2002.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bumgardner GL, Li J, Heininger M, Ferguson RM, Orosz CG. In vivo immunogenicity of purified allogeneic hepatocytes in a murine hepatocyte transplant model. Transplantation. 1998;65(1):47–52. doi: 10.1097/00007890-199801150-00010. [DOI] [PubMed] [Google Scholar]
- 13.Bumgardner GL, Li J, Prologo JD, Heininger M, Orosz CG. Patterns of immune responses evoked by allogeneic hepatocytes: evidence for independent co-dominant roles for CD4+ and CD8+ T-cell responses in acute rejection. Transplantation. 1999;68(4):555–62. doi: 10.1097/00007890-199908270-00019. [DOI] [PubMed] [Google Scholar]
- 14.Tay SS, Lu B, Sierro F, Benseler V, McGuffog CM, Bishop GA, et al. Differential migration of passenger leukocytes and rapid deletion of naive alloreactive CD8 T cells after mouse liver transplantation. Liver Transpl. 2013;19(11):1224–35. doi: 10.1002/lt.23720. [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Rudert WA, Qian S, McCaslin D, Fu F, Rao AS, et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J Exp Med. 1995;182(2):379–87. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson AW, Lu L, Wan Y, Qian S, Larsen CP, Starzl TE. Identification of donor-derived dendritic cell progenitors in bone marrow of spontaneously tolerant liver allograft recipients. Transplantation. 1995;60(12):1555–9. doi: 10.1097/00007890-199560120-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–21. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 18.Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13(6):622–39. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson AW, Lu L. Are dendritic cells the key to liver transplant tolerance? Immunol Today. 1999;20(1):27–32. doi: 10.1016/s0167-5699(98)01378-4. [DOI] [PubMed] [Google Scholar]
- 20.Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60(11):1366–70. [PMC free article] [PubMed] [Google Scholar]
- 21.Steptoe RJ, Fu F, Li W, Drakes ML, Lu L, Demetris AJ, et al. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J Immunol. 1997;159(11):5483–91. [PubMed] [Google Scholar]
- 22.Li W, Lu L, Wang Z, Wang L, Fung JJ, Thomson AW, et al. IL-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J Immunol. 2001;166(9):5619–28. doi: 10.4049/jimmunol.166.9.5619. [DOI] [PubMed] [Google Scholar]
- 23.Sumpter TL, Packiam V, Turnquist HR, Castellaneta A, Yoshida O, Thomson AW. DAP12 promotes IRAK-M expression and IL-10 production by liver myeloid dendritic cells and restrains their T cell allostimulatory ability. J Immunol. 2011;186(4):1970–80. doi: 10.4049/jimmunol.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida O, Kimura S, Dou L, Matta BM, Yokota S, Ross MA, et al. DAP12 deficiency in liver allografts results in enhanced donor DC migration, augmented effector T cell responses and abrogation of transplant tolerance. Am J Transplant. 2014;14(8):1791–805. doi: 10.1111/ajt.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morita M, Joyce D, Miller C, Fung JJ, Lu L, Qian S. Rejection triggers liver transplant tolerance: Involvement of mesenchyme-mediated immune control mechanisms in mice. Hepatology. 2015;62(3):915–31. doi: 10.1002/hep.27909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita M, Fujino M, Li XK, Kimura H, Nakayama T, Taniguchi M, et al. Spontaneous tolerance involving natural killer T cells after hepatic grafting in mice. Transpl Immunol. 2007;18(2):142–5. doi: 10.1016/j.trim.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Mele TS, Kneteman NM, Zhu LF, Ramassar V, Urmson J, Halloran B, et al. IFN-gamma is an absolute requirement for spontaneous acceptance of liver allografts. Am J Transplant. 2003;3(8):942–51. doi: 10.1034/j.1600-6143.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 28.Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. 2010;10(1):40–6. doi: 10.1111/j.1600-6143.2009.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158(10):4654–61. [PMC free article] [PubMed] [Google Scholar]
- 30.Thai NL, Li Y, Fu F, Qian S, Demetris AJ, Duquesnoy RJ, et al. Interleukin-2 and interleukin-12 mediate distinct effector mechanisms of liver allograft rejection. Liver Transpl Surg. 1997;3(2):118–29. doi: 10.1002/lt.500030204. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Morita M, Sugioka A, Harada M, Kojo S, Wakao H, et al. The importance of CD25+ CD4+ regulatory T cells in mouse hepatic allograft tolerance. Liver Transpl. 2006;12(7):1112–8. doi: 10.1002/lt.20787. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008;8(8):1639–51. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 33.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181(2):160–6. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 34.Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125(5):1480–91. doi: 10.1016/j.gastro.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Que X, Debonera F, Xie J, Furth EE, Aldeguer X, Gelman AE, et al. Pattern of ischemia reperfusion injury in a mouse orthotopic liver transplant model. J Surg Res. 2004;116(2):262–8. doi: 10.1016/j.jss.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11(10):1273–81. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 37.Xie JF, Wang G, Debonera F, Han R, Dorf ME, Hancock W, et al. Selective neutralization of the chemokine TCA3 reduces the increased injury of partial versus whole liver transplants induced by cold preservation. Transplantation. 2006;82(11):1501–9. doi: 10.1097/01.tp.0000243167.11566.eb. [DOI] [PubMed] [Google Scholar]
- 38.Ueki S, Dhupar R, Cardinal J, Tsung A, Yoshida J, Ozaki KS, et al. Critical role of interferon regulatory factor-1 in murine liver transplant ischemia reperfusion injury. Hepatology. 2010;51(5):1692–701. doi: 10.1002/hep.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokota S, Yoshida O, Dou L, Spadaro AV, Isse K, Ross MA, et al. IRF-1 promotes liver transplant ischemia/reperfusion injury via hepatocyte IL-15/IL-15Ralpha production. J Immunol. 2015;194(12):6045–56. doi: 10.4049/jimmunol.1402505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theruvath TP, Czerny C, Ramshesh VK, Zhong Z, Chavin KD, Lemasters JJ. C-Jun N-terminal kinase 2 promotes graft injury via the mitochondrial permeability transition after mouse liver transplantation. Am J Transplant. 2008;8(9):1819–28. doi: 10.1111/j.1600-6143.2008.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, et al. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13(10):1435–43. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 42.Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, et al. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology. 2013;57(4):1597–606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida O, Kimura S, Jackson EK, Robson SC, Geller DA, Murase N, et al. CD39 expression by hepatic myeloid dendritic cells attenuates inflammation in liver transplant ischemia-reperfusion injury in mice. Hepatology. 2013;58(6):2163–75. doi: 10.1002/hep.26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida O, Dou L, Kimura S, Yokota S, Isse K, Robson SC, et al. CD39 deficiency in murine liver allografts promotes inflammatory injury and immune-mediated rejection. Transpl Immunol. 2015;32(2):76–83. doi: 10.1016/j.trim.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueki S, Castellaneta A, Yoshida O, Ozaki K, Zhang M, Kimura S, et al. Hepatic B7 homolog 1 expression is essential for controlling cold ischemia/reperfusion injury after mouse liver transplantation. Hepatology. 2011;54(1):216–28. doi: 10.1002/hep.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen XD, Ke B, Ji H, Gao F, Freitas MC, Chang WW, et al. Disruption of Type-I IFN pathway ameliorates preservation damage in mouse orthotopic liver transplantation via HO-1 dependent mechanism. Am J Transplant. 2012;12(7):1730–9. doi: 10.1111/j.1600-6143.2012.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conzelmann LO, Lehnert M, Kremer M, Zhong Z, Wheeler MD, Lemasters JJ. Graft tumor necrosis factor receptor-1 protects after mouse liver transplantation whereas host tumor necrosis factor receptor-1 promotes injury. Transplantation. 2006;82(9):1214–20. doi: 10.1097/01.tp.0000239190.95190.5e. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Ji H, Shen X, Cai J, Gao F, Koenig KM, et al. Targeting TIM-1 on CD4 T cells depresses macrophage activation and overcomes ischemia-reperfusion injury in mouse orthotopic liver transplantation. Am J Transplant. 2013;13(1):56–66. doi: 10.1111/j.1600-6143.2012.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Ji H, Zhang Y, Shen XD, Gao F, Nguyen TT, et al. Negative CD4 + TIM-3 signaling confers resistance against cold preservation damage in mouse liver transplantation. Am J Transplant. 2015;15(4):954–64. doi: 10.1111/ajt.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein I, Crispe IN. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J Exp Med. 2006;203(2):437–47. doi: 10.1084/jem.20051775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polakos NK, Klein I, Richter MV, Zaiss DM, Giannandrea M, Crispe IN, et al. Early Intrahepatic Accumulation of CD8+ T Cells Provides a Source of Effectors for Nonhepatic Immune Responses. The Journal of Immunology. 2007;179(1):201–10. doi: 10.4049/jimmunol.179.1.201. [DOI] [PubMed] [Google Scholar]
- 52.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110(12):4077–85. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.John B, Klein I, Crispe IN. Immune role of hepatic TLR-4 revealed by orthotopic mouse liver transplantation. Hepatology. 2007;45(1):178–86. doi: 10.1002/hep.21446. [DOI] [PubMed] [Google Scholar]
- 54.Dutkowski P, Linecker M, DeOliveira ML, Mullhaupt B, Clavien PA. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148(2):307–23. doi: 10.1053/j.gastro.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 55.Conzelmann LO, Zhong Z, Bunzendahl H, Wheeler MD, Lemasters JJ. Reduced-size liver transplantation in the mouse. Transplantation. 2003;76(3):496–501. doi: 10.1097/01.TP.0000076469.93443.E4. [DOI] [PubMed] [Google Scholar]
- 56.Tian Y, Graf R, Jochum W, Clavien PA. Arterialized partial orthotopic liver transplantation in the mouse: a new model and evaluation of the critical liver mass. Liver Transpl. 2003;9(8):789–95. doi: 10.1053/jlts.2003.50170. [DOI] [PubMed] [Google Scholar]
- 57.Tian Y, Jochum W, Georgiev P, Moritz W, Graf R, Clavien PA. Kupffer cell-dependent TNF-alpha signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. Proc Natl Acad Sci U S A. 2006;103(12):4598–603. doi: 10.1073/pnas.0600499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Q, Rehman H, Krishnasamy Y, Haque K, Schnellmann RG, Lemasters JJ, et al. Amphiregulin stimulates liver regeneration after small-for-size mouse liver transplantation. Am J Transplant. 2012;12(8):2052–61. doi: 10.1111/j.1600-6143.2012.04069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Babitt JL, Lin HY. The Molecular Pathogenesis of Hereditary Hemochromatosis. Semin Liver Dis. 2011;31(03):280–92. doi: 10.1055/s-0031-1286059. [DOI] [PubMed] [Google Scholar]
- 60.Garuti C, Tian Y, Montosi G, Sabelli M, Corradini E, Graf R, et al. Hepcidin expression does not rescue the iron-poor phenotype of Kupffer cells in Hfe-null mice after liver transplantation. Gastroenterology. 2010;139(1):315–22. doi: 10.1053/j.gastro.2010.03.043. e1. [DOI] [PubMed] [Google Scholar]
- 61.Li XK, Fujino M, Sugioka A, Morita M, Okuyama T, Guo L, et al. Fulminant hepatitis by Fas-ligand expression in MRL-lpr/lpr mice grafted with Fas-positive livers and wild-type mice with Fas-mutant livers. Transplantation. 2001;71(4):503–8. doi: 10.1097/00007890-200102270-00004. [DOI] [PubMed] [Google Scholar]