Abstract
Background
The aim of this study was to examine the relationship between family history of alcohol use disorder and striatal dopamine using positron emission tomography (PET) imaging.
Methods
Participants were 84 healthy, 18–30 year old, social drinkers recruited via fliers and newspaper advertisements. At assessment, participants completed measures of lifetime personal and family substance use and psychiatric symptoms. Participants underwent two consecutive PET scans using the D2/D3 dopamine (DA) receptor radioligand [11C]raclopride. Scans were preceded by intravenous saline and amphetamine 0.3mg/kg, providing measures of baseline [11C]raclopride binding potential (BPND) and change in [11C]raclopride (ΔBPND). Subjective ratings of stimulant drug effects were collected during scans. Subjects were classified as family history positive (FHP) if they reported any first degree relative with alcohol use disorder (AUD) and family history negative (FHN) if no first degree relatives had history of AUD.
Results
Participants were predominantly White (69.0%) and male (62.1%). Baseline [11C]raclopride BPND was generally higher in FHP compared with FHN subjects across striatal subdivisions. There were no differences in ΔBPND across regions. Negative subjective drug effects were more pronounced in FHP than FHN subjects. While FHN subjects evidenced the expected positive relationship between ΔBPND and positive subjective drug effects, this relationship was disrupted in FHP subjects.
Conclusion
There are key differences in DA status and subjective stimulant drug experiences as a function of family AUD history. These findings have important implications for understanding risk for AUD development in FHP offspring.
Keywords: family history, mesolimbic dopamine, positron emission tomography (PET), alcohol use disorder, amphetamine, dopamine release
Introduction
Alcohol use disorders (AUD) are common and contribute substantially to global disease burden (Hasin et al., 2007, Rehm et al., 2009). A family history of alcoholism is associated with increased likelihood of AUD development in offspring, with genetic factors conferring 50–60% of risk (McGue, 1999, Schuckit, 2009). Yet, exact mechanisms underlying this increased risk are complex, multifactorial and largely undetermined.
Preclinical and human studies have demonstrated that psychoactive substances, including alcohol, stimulants and opioids, increase dopamine concentrations in the striatum (Chiara and Imperato, 1988, Leshner and Koob, 1999, Boileau et al., 2003, Martinez et al., 2003, Volkow et al., 2004, Pierce and Kumaresan, 2006, Constantinescu et al., 2008, Spreckelmeyer et al., 2011), a key factor in rewarding effects of abused substances. Moreover, the dorsal striatum provides circuitry that consolidates habit-based learning, a form of cognition often more pronounced in persons with substance use disorders (Smith and Graybiel, 2014). In healthy young adults, baseline dopamine (DA) D2 receptor availability in the nucleus accumbens was positively correlated with subjective scores of intoxication following alcohol administration (Yoder et al., 2005). In alcohol dependent persons compared to healthy controls, multiple studies have demonstrated lower levels of dopamine receptor availability and dopamine release (Hietala et al., 1994, Volkow et al., 1996, Heinz et al., 2004, Martinez et al., 2005), changes that persist following detoxification (Volkow et al., 2002b, Volkow et al., 2007).
These differences in baseline dopamine receptor availability and release may predate development of alcohol misuse, and contribute to risk for AUD development. Genetic studies have identified associations between dopamine receptor D2 gene polymorphisms and alcohol dependence. The Taq1A polymorphism is among the most widely studied. While there is inconsistency in individual studies, meta-analyses have confirmed a modest increased risk of alcohol dependence (OR 1.20 −1.38) associated with the A1 allele (Munafo et al., 2007, Smith et al., 2008, Le Foll et al., 2009).
Yet, findings on the relationship between family history of alcoholism and DA D2 receptor levels are inconsistent. Using positron emission tomography (PET) imaging with [11C]raclopride, Volkow and colleagues (Volkow et al., 2006) found higher levels of baseline DA D2 receptors in caudate and ventral striatum of nonalcoholic participants with a high density of alcoholism in their families (i.e., father and at least two second-degree relatives) compared to subjects with no first or second degree alcoholic relatives. In contrast, using similar PET procedures, Munro and colleagues (Munro et al., 2006a) found no association between family history and baseline DA D2 binding potential or amphetamine-induced change in dopamine receptor binding potential (ΔBPND) on PET imaging; however, family history status was more variable in this study. Recently, using PET imaging with [11C]raclopride, Casey and colleagues reported decreased amphetamine-induced ΔBPND in multigenerational family history positive (FHP) young adults with extensive personal histories of alcohol and drug use compared to both drug naïve and drug exposed family history negative (FHN) controls (Casey et al., 2013).
This study used [11C]raclopride to explore the relationship between family history of AUD and striatal dopamine at baseline and following amphetamine administration in FHP compared with FHN young adults, with very limited alcohol and drug exposure. Our sample includes participants from the 2006 Munro study, but the current sample more than doubles the original report. This expanded sample allows us to refine the definition of FHP, provides increased power to detect differences as well as the opportunity to explore potential associations between striatal dopamine and subjective responses to amphetamine.
Materials and Methods
Participants
Participants were 84 healthy young adults, ages 18–30, recruited via fliers and newspaper advertisements in the Baltimore area. Study exclusion criteria included: a current Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM IV) axis I disorder (American Psychiatric Association, 2000); drinking greater than 30 standard alcohol drinks per month; past 30-day illicit drug use; positive urine drug screen or alcohol breathalyzer at time of initial assessment or day of study procedures; reported maternal alcoholism; a medical condition prohibiting completion of study procedures; use of any medications in the past 30 days; or past 6-month treatment with antidepressant, appetite suppressant, dopamine, glucocorticoid, estrogen, neuroleptic, opiate or sedative hypnotic medications. Additionally, women who were pregnant, lactating, or using hormonal birth control or hormone replacement medications were excluded. The study was approved by the Johns Hopkins University Institutional Review Board; all participants provided written informed consent.
Assessments
Participants were screened by telephone for preliminary study inclusion criteria and subsequently scheduled for an in-person assessment, including medical history, physical examination and collection of standard laboratory and diagnostic studies. Master’s-level interviewers administered The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; (Bucholz et al., 1994)) to determine the absence or presence of DSM IV axis I disorders in the proband. Participants also completed the Perceived Stress Scale (Cohen et al., 1983) as part of the assessment battery.
Family history of AUD in 1st and 2nd degree relatives was first assessed using the Family Tree Questionnaire (FTQ (Mann et al., 1985)). For all first or second degree relatives scored 3 (suspected) or 4 (definite) on the FTQ, the proband provided additional information on specific alcohol-related problems using the Family History Assessment Module (FHAM) for alcohol (Rice et al., 1995). FHAM responses were used to classify relatives for alcohol abuse and or dependence.
Participants were classified as family history positive (FHP, N=24) if they reported an AUD history (abuse and/or dependence) in at least one first degree relative (father or sibling). Of these, 21 (87.5%) had a father with an AUD, with a mean number of affected first degree relatives of 1.3 (SD 1.27). Though they did not contribute to the classification of FHP, we also examined the number of affected second degree relatives (grandparents, aunts and uncles). Amongst the 24 participants, there was a total of 45 2nd degree relatives with an AUD, with a mean of 1.9 (SD 1.45). Overall, the mean number of 1st and 2nd degree relatives with an AUD in the FHP group was 3.1 (SD 1.39).
All other participants were classified as FHN (N=60); thus, FHN participants could report an AUD in a 2 degree relative. We also conducted sensitivity analyses examining only those FHN subjects with no first or second degree affected family members (N=30).
Procedures
MRI Magnetic Resonance Image Assessment and Mask Fitting, PET Scanning Procedures, Data Acquisition and Volumes of Interest
Detailed magnetic resonance image (MRI) assessment and mask fitting, PET scanning and data acquisition procedures have been described previously (Oswald et al., 2005, Munro et al., 2006a) and are provided in Supplement 1. Briefly, MRI images were obtained using a spoiled gradient sequence (SPGR) for anatomical identification of brain structures. Participants were instructed not to ingest alcohol, drugs or over-the-counter medications for 48 hours prior to admission and were admitted the day before PET procedures. After a calorie-controlled, caffeine-free breakfast, PET images were acquired on the 3D GE Advance whole-body PET scanner (GE Medical Systems, Waukesha, WI). After a 10-minute attenuation scan employing a rotating germanium-68 source, participants underwent 2 consecutive 90-minute PET scans with [11C]raclopride, a benzamide antagonist at the DA D2 and D3 receptors, which has previously been shown to be sensitive to stimulant-induced changes in brain dopamine concentration (Volkow et al., 1994, Endres et al., 1997, Laruelle, 2000). The first scan was preceded at −5 minutes by an intravenous saline injection; the second scan was preceded at −5 minutes by 0.3 mg/kg amphetamine delivered over 3 minutes. The scanning image protocol consisted of up to 30 scan acquisitions in 3-D mode, starting from a 15-second duration and increasing to 5 minutes in length over the 90-minute period. Participants were under continuous cardiovascular monitoring during scans.
Each PET frame was reconstructed to 35 transaxial images of 128 × 128 matrices by a back-projection algorithm using the manufacturer-provided software and correcting for attenuation, scatter, and dead time. PET frames were coregistered to the frame taken at 20 minutes by means of the mutual information theory as implemented in SPM2 (Maes et al., 1997, Friston and Penny, 2003) to reduce head motions between frames.
For statistical analyses, we defined six volumes of interest (VOIs): anterior and posterior putamen (aPU and pPU), anterior and posterior caudate nucleus (aCN and pCN), right and left ventral striatum (RtvS and LtvS). Based on prior research showing left and right striatal asymmetry (Larisch et al., 1998), separate analyses were conducted for the right and left hemispheres of the ventral striatum. Binding potential (BPND) and change in binding potential (ΔBPND) were measured in each VOI.
Modeling of PET Outcome Measures
BPND (Innis et al., 2007) was estimated via the simplified reference tissue model with 2 parameters (SRTM2) (Lammertsma and Hume, 1996, Wu and Carson, 2002) and the multilinear reference tissue method (MRTM2) (Ichise et al., 2003) using cerebellum as the reference tissue (Lammertsma and Hume, 1996). Specific binding of [11C]raclopride is thought to be negligible in the cerebellum because the cerebellum is nearly devoid of DA D2/D3 receptors (Breier et al., 1997). The VOIs defined on MRI were transferred to PET images to obtain time-activity curves of regions. ΔBPND was estimated as the percent change in BP from the placebo scan to the amphetamine scan ([(BPplacebo−BPamphetamine)/BPplacebo] × 100), with lower BP values during the amphetamine scan indicating greater levels of endogenous dopamine. Although release of endogenous dopamine is thought to be the biggest factor contributing to amphetamine-induced changes in [11C]raclopride BP, “dopamine release,” the term typically used in the PET literature, probably results from several different mechanisms, which also include dopamine reuptake blockade, reverse transport of dopamine through the dopamine transporter (Schmitz et al., 2001), as well as possible actions on endogenous opioid systems (Schad et al., 2002). Therefore, we use the term ΔBPND.
Drug Assays and Subjective Drug Effects
In a subset of participants, blood was collected for amphetamine measurement at 15, 25, 55, 85 and 90 minutes following amphetamine injection. Plasma amphetamine levels were assessed by gas chromatography mass spectroscopy (Quest Diagnostics Lyndhurst, NJ).
On a 5-point visual analog scale (VAS; 0=least and 4=most), participants verbally rated the extent to which they were experiencing each of 10 stimulant drug effects. VAS ratings were collected 5 minutes before and 3, 6, 10, 15, 25, 55, and 85 minutes during the placebo and amphetamine PET scans. First, each participant’s peak value across time points was identified for each item. Then a factor analysis on peak scores was performed to reduce the dimension of the data and to uncover underlying causes or factors. Using the iterated principal factor method, the analysis based on 9 items yielded a positive (high, rush, good effect, liking, desire for drug) and negative factor (anxious, dizziness, dry mouth, distrust); the item fidgety did not clearly load on either factor and was excluded. Factor scores are a latent continuum ranging from approximately −3 to +3. In our sample, the calculated factor scores ranged from −2.1 to 2.8.
Statistical Analyses
Demographic and baseline characteristics of FHP and FHN subjects were compared using chi-square or Fisher’s exact tests for categorical variables and t-tests or a non-parametric equivalent method for continuous variables. For the six selected VOIs, separate ANCOVA models were constructed to examine the relationship between AUD family history and placebo BPND and ΔBPND. We have previously reported significant sex effects on ΔBPND (Munro et al., 2006b), and so included sex as a covariate in analyses. Past 90-day binge drinking status (binge vs. no binge; binge defined as > 3 standard drinks for female and > 4 standard drinks for male subjects) also was added to the model as a covariate as there was a trend of a higher likelihood of baseline binge drinking in FHP vs FHN subjects. Adaptive Holm procedure (Q) was used to correct p values for multiple comparisons over the six VOIs (Hochberg and Benjamini, 1990). Finally, we conducted sensitivity analyses and repeated the models using a more stringent definition of FHN in which no first or second degree relative was classified with AUD (N=30).
We also examined subjective drug effects as a function of family history status. We compared positive and negative factor scores for FHN and FHP participants using ANCOVA models. Again sex and baseline binge status were added to the models as covariates, and the adaptive Holm procedure was used for multiple comparison correction.
Finally, we examined the relationship between ΔBPND and subjective factor scores as a function of family history status, adjusting for sex and baseline binge drinking status. All analyses were performed using SAS 9.3.
Results
Baseline Characteristics
Overall, participants were in their early twenties (mean age 22.8 years, SD 3.14), predominantly White (69.0%), majority men (60.7%), and had greater than a high school education (mean years 14.8, SD 1.78) (see Table 1). There were no demographic differences between FHP and FHN participants. More than half of participants (58.3%) reported at least one episode of binge drinking in the previous 90 days. Three-quarters (75.0%) of the FHP participants reported at least one binge drinking episode during the past 90 days compared to 51.7% of FHN subjects; however, this difference just failed to achieve statistical significance (p=0.050). Seven of 84 participants (8.3%) reported smoking more than 100 cigarettes in their lifetime, of which five were FHN and two were FHP. The mean duration of smoking for these 7 participants was 22.7 months. Four of the seven (4.8% of the total sample), were current smokers; three FHN and 1 FHP. There were no family history differences in mean lifetime or current measures of smoking exposure. With respect to drug use, FHP were more likely than FHN participants to report ever using marijuana (78.3% vs. 43.1%, p = 0.004). There was no family history difference in the number of subjects who reported > 21 episodes of marijuana use in the past year; mean use was 2.16 times for FHN and 0.57 for FHP subjects. There were no other differences in lifetime or past year drug use between FHP and FHN participants.
Table 1.
Demographic and psychosocial characteristics of participants.
| Total | FHNa | FHPb | p value | |
|---|---|---|---|---|
| Sample Size | 84 | 60 | 24 | |
| Age (Mean, SD) | 22.8 (3.14) | 22.7 (3.21) | 23.1 (2.98) | .534 |
| Race (n, %) | .456 | |||
| % White | 58 (69.0) | 40 (66.7) | 18 (75.0) | |
| % Non-White | 26 (31.0) | 20 (33.3) | 6 (25.0) | |
| Sex | .437 | |||
| Men | 51 (60.7) | 38 (63.3) | 13 (54.2) | |
| Women | 33 (39.3) | 22 (36.7) | 11 (45.8) | |
| Education (Mean years, SD) | 14.8 (1.78) | 14.7 (1.87) | 15.0 (1.57) | .604 |
| PSSc score (Mean, SD) | 10.0 (5.76) | 9.8 (5.61) | 10.4 (6.24) | .714 |
| Binge in last 90 days | .050 | |||
| No Binges | 35 (41.7) | 29 (48.3) | 6 (25.0) | |
| ≥1 Binge | 49 (58.3) | 31 (51.7) | 18 (75.0) | |
| Drinks per drinking episode (Mean, SD) | 3.0 (2.02) | 2.8 (2.16) | 3.2 (1.65) | .710 |
| Drinking episodes per week (Mean, SD) | 2.4 (2.61) | 2.1 (2.25) | 3.3 (3.25) | .058 |
| Smoked > 100 cigarettes lifetime | ||||
| Lifetime duration of smoking (Mean months, SD) | 22.7 (9.78) | 25.2 (10.73) | 16.5 (2.21) | .330 |
| Marijuana use ever (n, %) | 43 (51.9) | 25 (43.1) | 18 (78.2) | .004 |
| Marijuana use > 21 times in past year (n, %) | 2 (2.4) | 1 (1.7) | 1 (4.2) | .063 |
Family history negative
Family history positive
Perceived Stress Scale
Dopamine Binding Potential (BPND)
After confirming that SRTM2 and MRTM2 yielded essentially identical BPND values (SRTM2 = 1.0·MRTM2 − 0.009; R2 >0.999; using data from all scans), BPND values given by MRTM2 were used in further analyses. In general, FHP participants had a higher mean BPND than FHN participants across VOIs (Table 2, upper section). The difference was statistically significant in the pCN (2.32 (0.077) vs. 2.14 (0.047), Q = 0.043) and the RtvS (2.35 (0.061) vs. 2.17 (0.037), Q = 0.017).
Table 2.
Mean (SEM) baseline binding potential and change in dopamine binding potential as a function of family history of alcohol use disorder (AUD).
| FHPa vs all FHNb | FHPa vs FHNc with no 1st or 2nd degree | |||||
|---|---|---|---|---|---|---|
| FHP | FHN | Q valued | FHP | FHN | Q valued | |
| Sample Size | 24 | 60 | 24 | 30 | ||
| Baseline Binding Potentiale | ||||||
| Anterior Putamen | 3.38 (0.062) | 3.27 (0.038) | 0.113 | 3.39 (0.064) | 3.21 (0.054) | 0.039 |
| Posterior Putamen | 3.57 (0.078) | 3.43 (0.047) | 0.142 | 3.58 (0.078) | 3.41 (0.066) | 0.121 |
| Anterior Caudate Nucleus | 2.98 (0.056) | 2.89 (0.034) | 0.163 | 2.99 (0.06) | 2.85 (0.051) | 0.106 |
| Posterior Caudate Nucleus | 2.32 (0.077) | 2.14 (0.047) | 0.043 | 2.31 (0.083) | 2.13 (0.07) | 0.097 |
| Left Ventral Striatum | 2.36 (0.063) | 2.25 (0.039) | 0.162 | 2.36 (0.069) | 2.21 (0.059) | 0.105 |
| Right Ventral Striatum | 2.35 (0.061) | 2.17 (0.037) | 0.017 | 2.36 (0.067) | 2.12 (0.057) | 0.012 |
| Change in Dopamine Binding Potentiald | ||||||
| Anterior Putamen | 13.07 (1.228) | 11.37 (0.749) | 0.953 | 13.42 (1.363) | 11.23 (1.156) | 0.469 |
| Posterior Putamen | 20.63 (1.455) | 20.12 (0.888) | 1 | 20.81 (1.599) | 19.74 (1.357) | 0.822 |
| Anterior Caudate Nucleus | 6.6 (1.175) | 5.81 (0.717) | 1 | 7.01 (1.299) | 5.58 (1.102) | 0.822 |
| Posterior Caudate Nucleus | 9.79 (2.075) | 6.8 (1.266) | 0.88 | 10.5 (2.254) | 5.57 (1.913) | 0.217 |
| Left Ventral Striatum | 13.59 (1.638) | 11.64 (0.999) | 1 | 13.73 (1.86) | 11.52 (1.578) | 0.758 |
| Right Ventral Striatum | 13.48 (1.754) | 11.75 (1.07) | 1 | 13.71 (1.858) | 11.44 (1.577) | 0.731 |
Subjects classified as family history positive (FHP) had at least one 1st degree family member with AUD.
Subjects classified as family history negative (FHN) had no 1st degree family members with AUD. May have had 2nd degree relatives with AUD.
Subjects classified as FHN had no 1st or 2nd degree family members with AUD.
Adaptive Holm procedure (Q) was used to correct the p values for multiple comparisons.
Analyses adjusted for sex and binge drinking.
Using the more stringent definition of FHN in which no first or second degree family member was classified with AUD, results were similar. FHP participants had higher BPND across brain regions compared with FHN subjects. Differences were significant in the aPU (3.39 (0.064) vs. 3.21 (0.054), Q=0.039), and the RtvS (2.36 (0.067) vs. 2.12 (0.057), Q=0.012), and there was a trend in the pCN (2.31 (0.083) vs. 2.13 (0.070), Q=0.097). Stratified analyses by gender (not shown) for both the main and sensitivity analyses suggest that the significant findings were attributable to baseline differences in men rather than women.
Change in Dopamine Binding Potential (ΔBPND)
Area under the amphetamine plasma curves were analyzed in a subset of participants and no difference was observed as a function of family history (FHP AUC M=2424, SD = 292; FHN AUC M=2327, SD=729; p=0.639). There were no significant family history differences for ΔBPND in any of the six striatal brain regions regardless of which definition of FHN was used for analyses (Table 2, lower section).
Subjective Effects of Amphetamine
Table 3 displays the results of participants’ ratings of positive and negative drug effects. FHP subjects had higher peak negative drug effect ratings than FHN subjects (0.25 (0.108) vs −0.02 (0.064), Q = 0.038). Similar effects were obtained using the more stringent definition of FHN. No differences in positive drug effects were observed as a function of AUD family history. Similar to what we found with BPND, stratified analyses by gender (not shown) suggest that these differences in subjective effects are primarily explained by differences in men as opposed to women.
Table 3.
Mean (SEM) positive and negative subjective factor scores as a function of family history of alcohol use disorder (AUD).
| FHPa vs all FHNb | FHPa vs FHNc with no 1st or 2nd degree | |||||
|---|---|---|---|---|---|---|
| FHP | FHN | Q valued | FHP | FHN | Q valued | |
| Positive drug effects factore | −0.05 (0.110) | 0.07 (0.065) | 0.313 | −0.06 (0.118) | 0.19 (0.097) | 0.115 |
| Negative drug effects factore | 0.25 (0.108) | −0.02 (0.064) | 0.038 | 0.26 (0.104) | −0.1 (0.086) | 0.010 |
Subjects classified as family history positive (FHP) had at least one 1st degree family member with AUD.
Subjects classified as family history negative (FHN) had no 1st degree family members with AUD. May have had 2nd degree relatives with AUD.
Subjects classified as FHN had no 1st or 2nd degree family members with AUD.
Adaptive Holm procedure (Q) was used to correct the p values for multiple comparisons.
Analyses adjusted for sex and binge drinking.
Correlation of Subjective Effects and BPND and ΔBPND
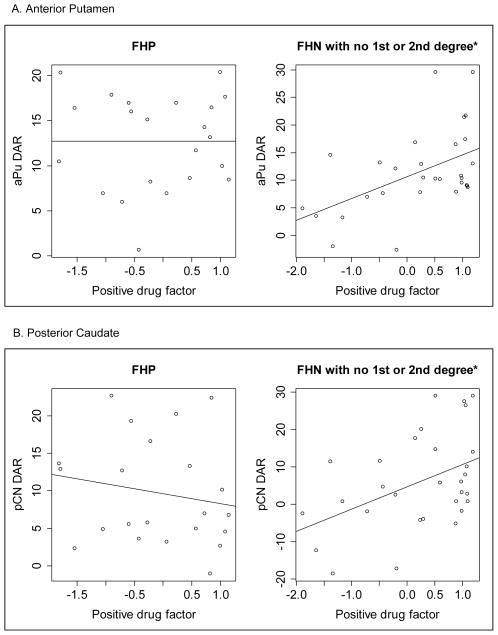
Figure 1 shows the relationship between positive drug effects factor scores and ΔBPND in FHP (left panels) and FHN subjects (right panels; defined as no first or second degree affected relatives). There was a significant positive relationship between positive subjective factor ratings and magnitude of ΔBPND in the aPU (Beta = 3.53, p=0.015) and the pCN (Beta = 5.15, p=0.039) in FHN subjects. In contrast, FHP subjects did not evidence this relationship in either region (aPU Beta = −0.32, p=0.793; pCN Beta = −1.51, p=0.382). Table 4 shows the correlation results adjusted for sex and binge drinking. For the FHN subjects, positive drug ratings and ΔBPND were significantly correlated in both regions in the adjusted analyses. There continued to be no evidence of relationship in FHP subjects after adjustment. Neither positive nor negative subjective factor ratings were related to baseline BPND in FHP and FHN subjects. Analyses stratified by gender (not shown) revealed that men were responsible for this relationship between positive drug effects and ΔBPND. There were no significant relationships between positive drug effects and ΔBPND in women.
Figure 1.
Correlation of change in dopamine binding potential (ΔBPND) and subjective amphetamine-induced positive drug effects by family history of alcohol use disorder in A) the anterior putamen (aPU), and B) posterior caudate (pCN). Family history positive (FHP) subjects had at least one 1st degree family member with AUD and family history negative (FHN) subjects had no 1st or 2nd degree family members with AUD. * p ≤ 0.05.
Table 4.
Correlation of change in dopamine binding potential (ΔBPND) and subjective amphetamine-induced positive and negative drug effects by family history of alcohol use disorder, adjusted by sex and binge drinking
| FHPa | FHNb with no 1st or 2nd degree | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive drug effects factorc | Negative drug effects facorc | Positive drug effects factorc | Negative drug effects factorc | |||||
| Slope | P value | Slope | P value | Slope | P value | Slope | P value | |
| Anterior Putamen | 0.001 | 0.999 | −1.509 | 0.171 | 3.529 | 0.017 | 0.592 | 0.809 |
| Posterior Caudate Nucleus | −0.847 | 0.619 | 2.479 | 0.088 | 5.192 | 0.041 | 2.182 | 0.598 |
Subjects classified as family history positive (FHP) had at least one 1st degree family member with AUD.
Subjects classified as family history negative (FHN) had no 1st or 2nd degree family members with AUD.
Analyses adjusted for sex and binge drinking.
Discussion
Prior brain imaging research on the relationship between AUD family history and striatal dopamine binding potential has been equivocal, probably resulting from differences across studies in definitions of family history status, prior alcohol and drug exposure in the probands, inclusion of sex as a covariate in analyses, and typically small sample sizes (Wiesbeck et al., 1995). The present study represents the largest sample studied to date using PET imaging to examine baseline dopamine binding potential (BPND), amphetamine-induced change in dopamine binding potential (ΔBPND), and positive and negative subjective drug effects simultaneously, as a function of family history of alcoholism.
Similar to Volkow and colleagues (Volkow et al., 2006), we found that social drinkers with a positive family history of alcoholism but no personal history of excessive drinking or alcohol or drug-related problems had significantly higher baseline [11C]raclopride BPND, consistent with higher DA D2/D3 receptor availability, compared with FHN social drinkers. Specifically, we noted significant differences in the right ventral striatum, a region associated with reward, drive and motivation, and in the posterior caudate and anterior putamen, striatal regions associated with cognition. As suggested by Volkow, higher DA D2/D3receptors in our sample with increased genetic and/or epigenetic risk for development of AUD but without evidence of current alcohol problems may be protective against AUD. This explanation is supported by preclinical studies demonstrating lower striatal DA D2 levels in selectively bred alcohol-preferring rats compared to nonalcohol-preferring rats (Stefanini et al., 1992, McBride et al., 1993), increased administration of alcohol in alcohol-preferring rats in the presence of a DA D2 receptor antagonist (Levy et al., 1991), and substantial reductions in alcohol intake of alcohol preferring rats after artificially increasing DA D2 receptor levels using an adenoviral vector (Thanos et al., 2001). These findings highlight the potentially critical role of baseline DA D2 receptor availability as a contributor to alcoholism risk and are consistent with this “protection” hypothesis.
We found significant differences in DA D2/D3 receptor availability in the right ventral striatum but not the left. This asymmetry is consistent with previous research demonstrating a preponderance of DA D2 receptors in the right compared to the left striatum (Larisch et al., 1998). Prior research suggests that right-sided neural pathways play a critical role in decision making (Bechara, 2005, Mohr et al., 2010). Right but not left ventral striatum ΔBPND has been associated with unpredictable monetary rewards in healthy controls (Martin-Soelch et al., 2011), gambling in both healthy controls and pathological gamblers (Joutsa et al., 2012) and gambling severity in pathological gamblers (Joutsa et al., 2012). Recent brain imaging research has shown a relationship between high impulsivity, a personality factor with a well-established association with substance use disorders, and blunted right ventral striatum activity (Oswald et al., 2007, Beck et al., 2009). Of particular relevance to the current study, Casey and colleagues (2014) reported blunted amphetamine-induced ΔBPND in very high risk young adults with multigenerational FH of substance use disorders and personal current regular alcohol and drug use compared to FHN controls; no group differences were observed in baseline BPND. They also observed a relationship between age of first alcohol use, another well-known risk factor for AUD development, and magnitude of amphetamine-induced ΔBPND, such that earlier onset was associated with smaller ΔBPND response. Taken together it can be postulated that protection is conferred by baseline differences in DA D2/D3 receptors, particularly in the right ventral striatum, via neural mechanisms regulating reward and decision making. In contrast, risk, as measured by FH, impulsivity, age of drinking onset and current use, is associated with blunted dopamine activity. It is also important to consider the agent used to provoke ΔBPND. While the Casey et. al. paper demonstrated blunted ΔBPND in FHP subjects in response to amphetamine administration, Setiawan and colleagues (2014) observed increased ΔBPND in FHP participants when alcohol was used to stimulate the dopaminergic system.
In the present study, FHP and FHN subjects did not differ in magnitude of amphetamine-induced ΔBPND, yet we observed significant differences between FHP and FHN participants in patterns of subjective drug effect ratings following amphetamine administration. Specifically, FHP subjects rated negative drug effects significantly higher than FHN subjects, and there was a tendency for FHP subjects to rate positive drug effects lower than FHN subjects although this observation did not reach statistical significance. It is possible that observed differences in baseline DA D2/D3 receptor availability account for these findings. Previous studies have shown that DA D2/D3 receptor availability is inversely related to subjective liking of methylphenidate (Volkow et al., 2002a). Importantly, only FHN participants demonstrated the expected positive relationship between positive subjective drug effects and magnitude of amphetamine-induced ΔBPND (Drevets et al., 2001, Oswald et al., 2005). Indeed, this relationship was completely disrupted in FHP probands. Taken together, it appears that higher DA D2 receptor levels are associated with greater negative drug effects and a disruption of the positive relationship between ΔBPND magnitude and subjective drug reward.
An alternative explanation for the elevated baseline [11C]raclopride BPND is that it results from low tonic levels of endogenous synaptic dopamine in FHP participants. This dopamine deficiency model (Blum et al., 2000, Bowirrat and Oscar-Berman, 2005) hypothesizes that increased AUD risk results from the reduced ability of FHP persons to generate dopamine which results in reduced drug responsiveness. This would theoretically lead to increased consumption of alcohol/drugs to achieve comparable pleasurable effects as FHN persons. Unfortunately, findings derived from high-specific activity raclopride scans cannot differentiate whether high BPND is a reflection of a low dopamine state versus high D2/D3 receptor expression level. Future studies in FHP and FHN subjects could employ high- and low-specific activity raclopride scans, which would provide a comparison of Bmax between the two groups.
Our study employed amphetamine to interrogate the dopamine system rather than alcohol, highlighting the breadth of drug use risk conferred by a positive family history of alcoholism. An earlier study by our group observed differences in self-reported rates of alcohol, marijuana, sedative and cocaine use in high density FHP compared with FHN subjects surveyed on local college campuses (McCaul et al., 1990). Interestingly, FHP respondents also reported a younger age at first marijuana use, experience with less commonly used drugs, and more personal drug-related problems. In our current study, FHP subjects were more likely to report experimenting with marijuana despite careful screening for drug use. There is strong evidence that genetic risk for substance use disorders is largely nonspecific and impacts across a wide range of drug classes (Kendler et al., 2003, Ystrom et al., 2014). Thus, our observation of low DA D2 in high-risk individuals may underpin risk across many different drug classes.
In addition to our large sample size, the current study has several important strengths. Our definition of FHP is consistent with Diagnostic Statistical Manual-5 (American Psychiatric Association, 2013), in that relatives were considered AUD positive if they met either alcohol abuse or dependence criteria. FHP subjects averaged over three AUD family members and therefore would be considered high density and highest risk. Also notably, our large sample size enabled examination of more and less stringent definitions of FHN. In the FHN sample (N=60) used in our primary analyses, half of our FHN subjects reported at least one 2nd degree AUD affected relative. It is striking that there were very few changes in overall findings when we conducted sensitivity analyses using the more stringent definition of FHN that ruled out both 1st and 2nd degree relatives. Future investigations should consider adoption of the more inclusive diagnostic system and family history classifications.
This is the first PET study of family history effects that has had a sufficient number of female subjects to allow stratified analyses by sex. Importantly, we observed FH differences in baseline BPND as well as amphetamine-induced subjective effects exclusively in male and not female subjects. These findings are in line with our own and others reports of sex differences in striatal dopamine function (Munro et al., 2006b, Riccardi et al., 2011) as well as sex differences in response to stimulant administration in FHP and FHN participants (Gabbay, 2005). There also is evidence of menstrual cycle effects on stimulant subjective drug effects (Evans et al., 2002, White et al., 2002), highlighting the importance of investigating menstrual cycle phase in future family history studies.
Despite study strengths, our findings should be interpreted in the context of several limitations. First, data related to family member’s history of alcohol use was collected from the probands and was not corroborated via interviews with additional family members. However, this likely resulted in an underestimation of FHP participants, decreasing the likelihood of observing group differences. Additionally, while there were no differences in mean drinking frequency or intensity, FHP subjects tended to be more likely to report past 90-day binge drinking compared to FHN participants, despite our rigorous efforts to recruit FHP and FHN subjects with comparable demographic, alcohol and drug use, and psychological profiles. It is important to note that, although binge drinking rates were different as a function of FH status, rates were low and in line with those reported for young adults in this age range (Naimi et al., 2003).
The current findings provide important new insights into neural mechanisms of protective versus risk factors for substance use development. Our participants reported high-density AUD family histories but had no evidence of alcohol-related problems themselves. Although we cannot rule out development of problems in the future, our recruitment strategy may have resulted in highly resilient FHP participants, in whom high baseline DA D2/D3 receptor levels are associated with more negative drug effects and a disruption of the expected relationship between dopamine release and positive subjective drug effects. In contrast, much of the research on AUD risk factors, including FH, personal alcohol/drug use, impulsivity and age of onset, has found similar baseline DA D2/D3 receptor levels but blunted ΔBPND in at-risk subjects. Our results highlight the importance of studying persons across a range of current drinking patterns and problems to ensure a more complete understanding of the different mechanisms that may be involved in conferring risk and resilience.
Supplementary Material
Acknowledgments
We would like to thank James Brasic, MD, MHS, MPH for providing medical support for PET imaging and amphetamine injections and radiochemists, Robert F. Dannals, PhD, Hayden Ravert PhD and Danny Holt, BS. This research was supported by NIAAA grants 5K23AA020316 (Alvanzo), AA10158 (Wand) and AA12837 (McCaul) and NIDA grant K24 DA000412 (Wong). Dr. Wand reports consulting fees from Questcor Pharma in 2014. Dr. Wong reports research support from Addex, Avid, Dartneuroscience, GE, Intracellular, Johnson & Johnson, Lundbeck, Pfizer, Roche, and Takeda.
Footnotes
Author Contributions:
MM and GW were responsible for the study concept and design. AA completed preliminary analyses and wrote the initial manuscript draft. XX completed data analyses. MM, GW, HK and DW provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Drs. Alvanzo, Kuwabara, McCaul, and Xu reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Anika A. H. Alvanzo, Division of General Internal Medicine, Johns Hopkins University School of Medicine, 1830 East Monument Street, Room 8069, Baltimore, MD 21287 USA, Phone: 410-502-2048, Fax: 410-502-6952.
Gary S Wand, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21287 USA.
Hiroto Kuwabara, Department of Radiology and Radiological Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21287 USA.
Dean F. Wong, Departments of Radiology and Radiological Sciences, Psychiatry and Behavioral Sciences, and Solomon H. Snyder Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD 21287 USA.
Xiaoqiang Xu, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21287 USA.
Mary E. McCaul, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21287 USA.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJH, Comings DE. The reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. J Psychoactive Drugs. 2000;32:1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad J-M, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger C, Dinwiddie S, Hesselbrock V, Nurnberger JIRT, Jr, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alc. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M. Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol Psychiatry. 2014;76:23–30. doi: 10.1016/j.biopsych.2013.08.033. [DOI] [PubMed] [Google Scholar]
- Chiara GD, Imperato A. Drugs Abused by Humans Preferentially Increase Synaptic Dopamine Concentrations in the Mesolimbic System of Freely Moving Rats. Proc Natl Acad Sci. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Constantinescu CC, Yoder KK, Kareken DA, Bouman CA, O’Connor SJ, Normandin MD, Morris ED. Estimation from PET data of transient changes in dopamine concentration induced by alcohol: support for a non-parametric signal estimation method. Phys Med Biol. 2008;53:1353–1367. doi: 10.1088/0031-9155/53/5/012. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A, Eckelman WC, Carson RE. Kinetic Modeling of [11C]Raclopride: Combined PET-Microdialysis Studies. J Cereb Blood Flow Metab. 1997;17:932–942. doi: 10.1097/00004647-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Evans S, Haney M, Foltin R. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny W. Posterior probability maps and SPMs. Neuroimage. 2003;19:1240–1249. doi: 10.1016/s1053-8119(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Gabbay FH. Family history of alcoholism and response to amphetamine: sex differences in the effect of risk. Alcohol Clin Exp Res. 2005;29:773–780. doi: 10.1097/01.alc.0000164380.16043.4f. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, et al. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvälahti E, Någren K, Lehikoinen P, Sonninen P, Ruotsalainen U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology. 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow J-S, Lu J-Q, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang S-C, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Joutsa J, Johansson J, Niemelä S, Ollikainen A, Hirvonen MM, Piepponen P, Arponen E, Alho H, Voon V, Rinne JO, Hietala J, Kaasinen V. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. NeuroImage. 2012;60:1992–1999. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. The American Journal of Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Muller-Gartner HW. Left-right asymmetry of striatal dopamine D2 receptors. Nucl Med Commun. 1998;19:781–788. doi: 10.1097/00006231-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of Abuse and the Brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Levy AD, Murphy JM, McBride WJ, Lumeng L, Li TK. Microinjection of sulpiride into the nucleus accumbens increases ethanol drinking in alcohol-preferring (P) rats. Alcohol Alcohol Suppl. 1991;1:417–420. [PubMed] [Google Scholar]
- Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, Herscovitch P, Carson RE, Drevets WC. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33:1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang D-R, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Dah-Ren H, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of Cerebral Blood Flow and Metabolism. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Dyr W, Lumeng L, Li TK. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol. 1993;10:387–390. doi: 10.1016/0741-8329(93)90025-j. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE, Cromwell CC. Alcohol and drug use by college males as a function of family alcoholism history. Alcohol Clin Exp Res. 1990;14:467–471. doi: 10.1111/j.1530-0277.1990.tb00505.x. [DOI] [PubMed] [Google Scholar]
- McGue M. The Behavioral Genetics of Alcoholism. Curr Dir Psychol Sci. 1999;8:109–115. [Google Scholar]
- Mohr PNC, Biele G, Heekeren HR. Neural processing of risk. J Neurosci. 2010;30:6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Mol Psychiatry. 2007;12:454–461. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Oswald LM, Wong DF, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Striatal dopamine release and family history of alcoholism. Alcohol Clin Exp Res. 2006a;30:1143–1151. doi: 10.1111/j.1530-0277.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006b;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships Among Ventral Striatal Dopamine Release, Cortisol Secretion, and Subjective Responses to Amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, Kumar A, Brasic J, Alexander M, Ye W, Kuwabara H, Hilton J, Wand GS. Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. NeuroImage. 2007;36:153–166. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S, Doop M, Ansari MS, Schmidt D, Baldwin R. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [18F]fallypride. Synapse. 2011;65:99–102. doi: 10.1002/syn.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Schuckit MA, Begleiter H. Comparison of Direct Interview and Family History Diagnoses of Alcohol Dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Schad CA, Justice JB, Holtzman SG. Endogenous opioids in dopaminergic cell body regions modulate amphetamine-induced increases in extracellular dopamine levels in the terminal regions. J Pharmacol Exp Ther. 2002;300:932–938. doi: 10.1124/jpet.300.3.932. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. The J Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Dagher A, Schlagintweit H, Casey KF, Benkelfat C, Leyton M. Differential striatal dopamine responses following oral alcohol in individuals at varying risk for dependence. Alcoholism: Clinical and Experimental Research. 2014;38:126–134. doi: 10.1111/acer.12218. [DOI] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM. Investigating habits: strategies, technologies and models. Front in Behav Neurosci. 2014;8:1–17. doi: 10.3389/fnbeh.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Watson M, Gates S, Ball D, Foxcroft D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: a HuGE gene-disease association review. Am J Epidemiol. 2008;167:125–138. doi: 10.1093/aje/kwm281. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Paulzen M, Raptis M, Baltus T, Schaffrath S, Van Waesberghe J, Zalewski MM, Rosch F, Vernaleken I, Schafer WM, Grunder G. Opiate-induced dopamine release is modulated by severity of alcohol dependence: an [18f]fallypride positron emission tomography study. Biol Psychiatry. 2011;70:770–776. doi: 10.1016/j.biopsych.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Stefanini E, Frau M, Garau MG, Garau B, Fadda F, Gessa GL. Rapid communication: alcohol-preferring rats have fewer dopamine D2 receptors in the limbic system. Alcohol Alcohol. 1992;27:127–130. [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. 2001;78:1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Hitzemann R, Ding Y-S, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Re. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Thanos P, Logan J, Gatley SJ, Gifford A, Ding Y-S, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: Replication study. Synapse. 2002a;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding Y-S, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res: Neuroimaging. 2002b;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wiesbeck GA, Mauerer C, Thome J, Jakob F, Boening J. Alcohol dependence, family history, and D2 dopamine receptor function as neuroendocrinologically assessed with apomorphine. Drug Alcohol Depend. 1995;40:49–53. doi: 10.1016/0376-8716(95)01180-3. [DOI] [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise Reduction in the Simplified Reference Tissue Model for Neuroreceptor Functional Imaging. J Cereb Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O’Connor SJ, Wang C, Zheng Q-H, Mock B, Morris ED. Dopamine D2 receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Re. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Ystrom E, Reichborn-Kjennerud T, Neale M, Kendler K. Genetic and environmental risk factors for illicit substance use and use disorders: joint analysis of self and co-twin ratings. Behav Genet. 2014;44:1–13. doi: 10.1007/s10519-013-9626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.