Abstract
Adipose tissue (AT) inflammation is an emerging factor contributing to cardiovascular disease. STAT4 is a transcription factor expressed in adipocytes and in immune cells and contributes to AT inflammation and insulin resistance in obesity. The objective of this study was to determine the effect of STAT4 deficiency on visceral and peri-aortic AT inflammation in a model of atherosclerosis without obesity. Stat4-/-Apoe-/- mice and Apoe-/- controls were kept either on chow or western diet for 12 weeks. Visceral and peri-aortic AT were collected and analyzed for immune composition by flow cytometry and for cytokine/chemokine expression by real-time PCR. Stat4-/-Apoe-/- and Apoe-/- mice had similar body weight, plasma glucose and lipids. Western diet significantly increased macrophage, CD4+, CD8+ and NK cells in peri-aortic and visceral fat in Apoe-/- mice. In contrast, in Stat4-/-Apoe-/- mice, western diet failed to increase the percentage of immune cells infiltrating the AT. Also, IL12p40, TNFα, CCL5, CXCL10 and CX3CL1 were significantly reduced in the peri-aortic fat in Stat4-/-Apoe-/- mice. Importantly, Stat4-/-Apoe-/- mice on western diet had significantly reduced plaque burden vs. Apoe-/- controls. In conclusion, STAT4 deletion reduces inflammation in peri-vascular and visceral AT and this may contribute via direct or indirect effects to reduced atheroma formation.
Keywords: peri-aortic fat, T lymphocytes, chemokines, macrophages
Introduction
Excessive accumulation of visceral adipose tissue is independently associated with cardiovascular disease (See, et al. 2007). In addition, the fat around the conduit vessels such as aorta as well as the pericardial fat were shown to play a role in progression of atherosclerosis (Ohman, et al. 2011) and were associated with coronary atherosclerotic plaque formation in humans (Konishi, et al. 2010a; Konishi, et al. 2010b). Therefore, both visceral and perivascular adipose tissue accumulation and inflammation emerge as key contributors to atherosclerosis. The Apoe-/- mouse model of atherosclerosis displays accelerated plaque formation on high cholesterol diet. However, this mouse model neither gains weight nor develops insulin resistance as a result of high fat diet feeding (Gao, et al. 2007; Kawashima, et al. 2009). This phenotype was attributed, at least in part, to the inability of visceral adipose tissue to accumulate excess lipids resulting in a more sensitive adipocyte phenotype and reduced inflammation (Hofmann, et al. 2008; Huang, et al. 2006; Huang, et al. 2013). This raises the question whether various adipose tissue depots may contribute to the development of atherosclerosis in this model. While the contribution of visceral fat in atherosclerosis development in this model was not reported, the peri-vascular fat was proven causal for the development of atherosclerosis in Apoe-/- mice fed a western diet (Ohman et al. 2011).
The mechanisms contributing to atherosclerosis by visceral and perivascular fat are incompletely understood. In atherosclerotic Apoe-/- mice, peri-adventitial adipose tissue produces elevated levels of IL-6, IL-1α and MIP-1α,(Lohmann, et al. 2009a) and in a model of obesity with angiotensin II infusion peri-aortic AT induces inflammation and enhances aneurism formation (Police, et al. 2009). The TLR/JAK-STAT pathway is activated in human peri-vascular adipocytes from patients with atherosclerosis (Police et al. 2009). Signal transducer and activator of transcription 4 (STAT4) is downstream of the Jak/Tyk kinases and upon phosphorylation in response to IL-12 or other cytokines, induces expression of genes involved in proliferation and differentiation of various hematopoietic and non-hematopoietic cells (Darnell 1997; Horvath and Darnell 1997; Imada and Leonard 2000; Leonard and Lin 2000). STAT4 is expressed in T and NK cells and has a prominent role for IL-12 induced Th1 cell differentiation and for NK cell activation (Good, et al. 2009; Kaplan 2005; Watford, et al. 2004). IL-12 is also highly expressed in rodent and human atherosclerotic lesions and several studies have shown that approaches to reduce IL-12 levels prevent atherosclerosis (Davenport and Tipping 2003; Eid, et al. 2009; Hauer, et al. 2005; Zhang, et al. 2006; Zhao, et al. 2002). Importantly, recent findings indicate that STAT4 has a determinant role for optimal human Th1 lineage development (Chang, et al. 2009). Our group showed that STAT4 is markedly activated in the balloon injured carotid artery of the obese Zucker rat, and that an IL-12 signaling inhibitor can reduce STAT4 activation and vascular injury responses (Pei, et al. 2006). In addition, STAT4 deficient mice are protected from developing insulin resistance on a high fat diet, in part due to reduced immune cell trafficking in visceral adipose tissue and reduced pro-inflammatory cytokine production by adipocytes (Dobrian, et al. 2013). Collectively, these results suggest that activation of STAT4 may participate in vascular inflammatory responses in part via modulation of adipose tissue inflammation. To directly address this hypothesis we examined the effect of STAT4 deficiency on visceral and peri-aortic adipose tissue inflammation in Stat4-/-Apoe-/- mice, a model of atherosclerosis lacking the confounding effects of insulin resistance and obesity. A key finding is the significant effect of STAT4 deficiency on immune composition as well as pro-inflammatory cytokine and chemokine production mainly in the peri-aortic fat. The anti-inflammatory effect of STAT4 deficiency was significant in the mice fed a high cholesterol diet and was associated with the reduced atherosclerotic plaque burden suggesting that activation of this pathway in adipose tissue may be a contributor to accelerated diet-related atherosclerosis.
Materials and Methods
Animals and diets
All procedures involving animals were approved by the IACUC of Eastern Virginia Medical School and University of Virginia at Charlottesville. Female Stat4-/-Apoe-/- or Apoe-/- mice, were bred in our colonies and at 8-10 weeks of age were either fed a western diet (0.15% cholesterol, Harlan, Madison, WI) or were maintained on regular rodent chow for 12 weeks (n=6-10 mice/group). All of the mice were between 20-22 weeks of age at euthanasia. Mice were housed in a pathogen-free facility, and food and water were provided ad libitum throughout the experiment. Body weights were measured weekly.
Lipids, glucose and insulin were measured in all of the groups in non-fasted terminal plasma. Triglycerides, total cholesterol, LDL and HDL cholesterol were measured using a colorimetric kit from Wako (Richmond, VA). Plasma glucose was measured according to manufacturer’s instructions using a colorimetric method (BioVision Inc., Milpitas, CA). Insulin was measured by ELISA using a commercially available kit (Mercodia, Winston Salem, NC).
Stromal vascular fraction preparation
Samples of peri-gonadal (visceral) and peri-aortic adipose tissue (0.1-0.3g) were digested with collagenase as described before with minor modifications (Cole, et al. ; Weisberg, et al. 2003). The floating adipocytes were collected and washed and the infranatant was removed and centrifuged at 500x g, for 5 minutes to pellet the stromal vascular fraction (SVF). SVF was used for flow cytometry to determine immune cell content.
Flow cytometry
Counted SVF cells were incubated for 30 minutes, at room temperature with one of the following combinations of fluorophore-conjugated primary antibodies: Cocktail1 (for macrophage phenotyping): CD11b-Pacific Blue, CD45-PerCP, Cd11c- PE, F4/80-Alexa 647 and CD206-FITC; Cocktail 2 (for T cell phenotyping): CD3-Pacific Orange; CD4-APC; CD8-FITC; CD45-PerCP, NK40.6-Pacific Blue. All of the antibodies were from BD Pharmigen (San Jose, CA) or from BioLegend (San Diego, CA). Cells were analyzed on a BD upgraded FACS Caliber Flow Cytometer (8-colors) using FlowJo software (Tree Star Inc. Ashland, OR).
Immunohistochemistry
Adipose tissues were fixed in 10% buffered formalin overnight then embedded in paraffin and following antigen retrieval were incubated overnight with polyclonal rabbit anti-mouse antibody for CD45 (Abcam, 1:100 dilution) or rat anti-mouse F4/80 antibody (Abcam, 1:100 dilution). Secondary antibody staining was performed using the Vectastain ABC kit (Vector Laboratories) and detected with 3,3′-diaminobenzidine (DAB). Slides were counterstained using Mayer’s hematoxylin. Sections incubated with non-immune IgG (Pierce) instead of the primary antibody were used as method controls. All pictures were captured with an Olympus microscope using 200x magnification. Quantification of the immunohistochemical data was done using a MetaMorph software ver6.3 (Molecular Devices, Downingtown, PA) with an established arbitrary threshold. Data was normalized to section area and expressed as arbitrary units.
Macrophage polarization
Peritoneal macrophages were isolated by lavage with sterile PBS and cultured in RPMI-1640 media supplemented with 10% FBS, 1% Penicillin/Streptomycin, 1% Glutamax, 1% Hepes, 0.5% NEAA, 0.5% Sodium pyruvate, and 50 μM BME. After 24 hours, the adherent cells were treated with either a combination of IFNg (150U/mL) and LPS (100 ng/mL) or with IL-4 (20 U/mL) and IL-13 (15U/mL) for additional 24 hours to induce polarization towards “classicaly” (M1) or “alternatively” (M2) activated macrophage phenotypes, respectively. Bone-marrow-derived macrophages were isolated from the femoral and tibial bones and were differentiated in culture for 7 days using RPMI-1640 media supplemented with 10% FBS, 1% Penicillin/Streptomycin, 1% Glutamax and 10ng/mL M-CSF. Subsequently, cells were treated for 24 hours to induce “classical” M1 activation (as described above) or “metabolic” activation (MMe) using a combination of glucose (30mM), insulin (10nM) and palmitate (0.4mM), as described by Kratz et al (Kratz, et al. 2014).
Real-time PCR
RNA from total adipose tissue was extracted and reverse transcribed as previously described (Dobrian, et al. 2010). Real-time PCR was performed using Taqman probes from Applied Biosystems (Carlsbad, CA). β-actin was used to normalize the data. Results were expressed as fold change by the 2-ΔΔCt method using Apoe-/- mice as a control group.
En Face Staining
The aortic trees of mice were collected, fixed in situ by perfusion with PBS containing 4% paraformaldehyde then additionally fixed for 24 hours and stained with Sudan IV (Tangirala, et al. 1995). En face stained aortic tissues were analyzed for the percent surface areas occupied by lesions using Photoshop software.
In vitro chemokine production by aortic cells in response to STAT4-dependent AT secretome
Visceral AT from Stat4-/-Apoe-/- and Apoe-/- mice was excised and minced into ~1mm3 pieces and incubated for 24 hours in DMEM media supplemented with 1% Penicillin/Streptomycin. The following day, aortic suspensions were generated by collagenase digestion from Apoe-/- mice. The aortic cells were incubated with the AT conditioned media at ~106 cells/well for 24 hours and then cells were washed, collected by centrifugation and processed for RNA extraction and gene expression analysis by real time PCR.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Software (GraphPad Software Inc., La Jolla, CA). Student’s t-test unpaired analysis was used for all data comparisons between the Stat4-/-Apoe-/- mice and Apoe-/- controls. For comparisons of more than 2 groups one-way ANOVA was used for data analysis. For experiments including two independent variables (diet and genotype) data was analyzed using 2-way ANOVA. Data were expressed as mean±SEM and the null hypothesis was rejected for a p-value <0.05.
Results
STAT4 deficiency does not change body weight, systemic lipids, glucose and insulin
Stat-4-/-Apoe-/- or Apoe-/- control mice were kept on either a chow or a western diet for 12 weeks. Mice on both chow (not shown) and western diets (Table 1) had similar body weights. Also, randomly fed plasma glucose, insulin, triglycerides, total, HDL and LDL cholesterol were similar between the two groups for both the chow fed and western diet fed mice. The lipid profile in western diet fed mice were in the expected range with significantly elevated triglycerides and LDL+VLDL plasma cholesterol levels (Table 1).
Table 1.
Body weight and plasma glucose, insulin and lipids in western diet fed mice
| Stat4-/-Apoe-/- | Apoe-/- | p-value | |
|---|---|---|---|
| Body weight (g) | 36.8±2.81 | 33.1±1.12 | >0.05 |
| Glucose (mg/dL) | 141±5.9 | 127.3±9.2 | >0.05 |
| Insulin (μg/L) | 3.5±0.8 | 3.9±0.3 | >0.05 |
| Triglycerides (mg/dL) | 172.3±14.5 | 178±12.1 | >0.05 |
| Total cholesterol (mg/dL) | 1468.3±73.7 | 1346.1±84.3 | >0.05 |
| HDL cholesterol (mg/dL) | 29.3±7.6 | 28.4± 8.2 | >0.05 |
| LDL cholesterol (mg.dL) | 1346.2±114 | 1312.4±130.3 | >0.05 |
Female Stat4-/-Apoe-/- and Apoe-/- mice were kept on western diet for 12 weeks starting at 8-10 weeks of age. Body weights were measured at the time of euthanasia. Plasma glucose, insulin, triglycerides, total cholesterol, LDL cholesterol and HDL cholesterol were measured in randomly fed mice at the time of euthanasia. Results are from 8-10 mice and were expressed as mean ±SEM.
Effect of STAT4 deficiency on lymphocyte population in visceral and peri-aortic adipose tissues
STAT4 is abundantly expressed in the T cells and NK cells of mice and humans where it plays an important role in Th1 polarization and in the cytotoxic response of NK cells following cytokine challenge. Therefore we compared the lymphocyte composition in the STAT4 deficient and sufficient Apoe-/- mice. Flow cytometry analysis of peri-aortic and visceral adipose tissues of Stat-4-/-Apoe-/- and Apoe-/- mice on western diet showed significantly increased numbers of CD45+ cells in the peri-aortic adipose tissue of both groups of mice (Figure 1A). While CD45+ cellularity was similar in the visceral fat of Stat-4-/-Apoe-/- and Apoe-/- mice, the numbers of CD45+ cells was significantly reduced in the peri-aortic fat of Stat-4-/-Apoe-/- compared to Apoe-/- controls (Figure 1A). Similar results were obtained by adipose tissue immunohistochemistry using a CD45 antibody (Figure 1B). To further determine the composition of the CD45+ lymphocytes we next analyzed the CD3+ T cell and NK cell populations in the visceral and peri-aortic adipose tissues of mice on chow or western diets. In the Stat-4-/-Apoe-/- and Apoe-/- chow fed mice, the relative percentages of CD3+, CD3+CD4+, CD3+CD8+ and NK cells were similar for both fat depots (not shown). In the western diet fed mice, the relative percentages of CD3+ cells in the CD45+ gate were also similar for both groups in both adipose tissue depots (Figure1C, D). However, significant differences of the relative percentages of CD4+, CD8+ and NK+ cells were found between the Stat-4-/-Apoe-/- and Apoe-/- mice in both the visceral and peri-aortic depots. In visceral and peri-aortic fat of Stat-4-/-Apoe-/- mice, we found a predominant population of CD3+CD4+ cells (~20%) compared to the low relative percentages of CD3+CD8+ cells (8.2% and 2.1% in peri-aortic and visceral fat, respectively) (Figure 1C, D). In stark contrast, the Apoe-/- mice showed high percentages of CD3+CD8+ cells in both depots (22.7% and 27.8%, in the peri-aortic and visceral fat, respectively) compared to only 2.3% CD3+CD4+ cells in peri-aortic fat and 1.9% in visceral fat (Figure 1C, D). In addition, STAT4 deficiency resulted in significantly lower percentages of NK cells in both the visceral and peri-aortic depots of Stat-4-/-Apoe-/- compared to Apoe-/- mice (Figure 1C, D). Collectively, this data indicates that in response to western diet feeding, in Stat-4-/-Apoe-/- mice, the increase in the CD3+ cells in adipose tissue is biased towards a CD4+ vs. a CD8+ population in a depot-independent fashion, while in the Apoe-/- mice there is a predominant CD8+ population in both depots. Also, the relative percentage of visceral and peri-aortic adipose tissue NK40.6+ cells is dramatically reduced in Stat-4-/-Apoe-/- compared to Apoe-/- mice on western diet.
Figure 1. Effect of STAT4 deficiency on lymphocyte numbers and relative composition in visceral and peri-aortic adipose tissue.
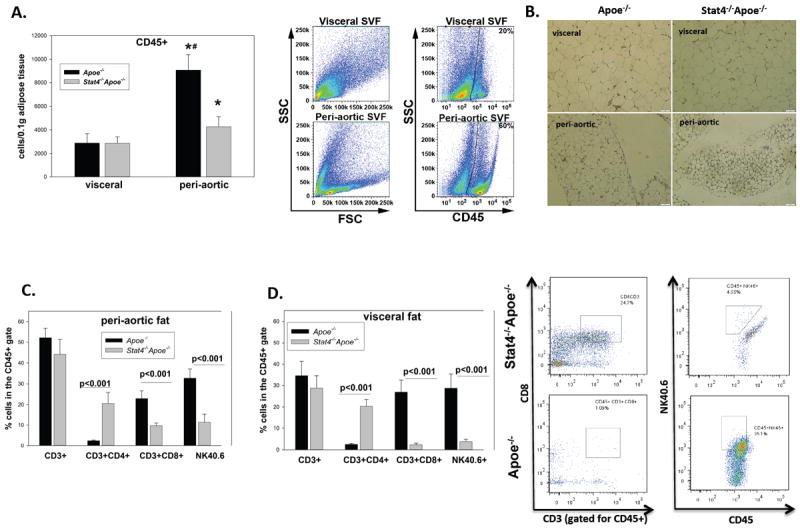
(A): CD45+ cells in peri-aortic and visceral adipose tissues were determined by flow cytometry and normalized to tissue weight; * = significant compared to visceral adipose tissue for the same mice group. Also shown is a representative gating strategy for the forward scattered and CD45+ gates in Apoe-/- mice. (B): Immunohistochemistry for CD45 in the peri-aortic and visceral fat of Stat4-/-Apoe-/- and Apoe-/- mice. Relative percentages of CD3+, CD3+CD4+, CD3+CD8+ and NK40.6+ were determined in the CD45+ gate in peri-aortic (C) and visceral (D) adipose tissues of mice on western diet. Representative FACS plots in visceral fat are also shown. Data is from n=5-7 mice/group.
Effect of STAT4 deficiency on antigen presenting cells and macrophage polarization in visceral and peri-aortic adipose tissue
The antigen presenting cells are important contributors to adipose tissue inflammation. STAT4 is expressed in activated blood monocytes and dendritic cells, and therefore STAT4 deficiency may impact on numbers and phenotype of resident adipose tissue APC directly or via indirect changes in adaptive immunity and inflammation. In contrast to C57Bl6 mice on high fat diet (Lumeng, et al. 2007a; Lumeng, et al. 2007b) visceral adipose tissue of western diet fed Apoe-/- mice has lower percentages of CD11b+F4/80+ cells (33.5%) in the CD45+ gate (Figure 2B). Virtually all of these cells were CD11c+F4/80+ canonical macrophages, characterized by a pro-inflammatory phenotype, while only ~2.5% were CD206+F4/80+ alternatively activated (M2) macrophages. In peri-aortic fat of the same mice, the percentage of CD11b+F4/80+ cells in the CD45+ gate was ~1.86%. Most of the cells had an “alternatively” activated M2 macrophage phenotype, while only ~0.6% of the cells were CD11c+F4/80+ canonical macrophages (Figure 2A,B). Compared to Apoe-/- mice on western diet, the Stat4-/-Apoe-/- mice had ~1/3 of the percentage of CD11b+F4/80+ cells and 3-3.5-fold higher percentage of CD206+F4/80+ M2 macrophages in both the visceral and peri-aortic depots (Figure 2B). Also, the relative abundance of the F4/80 positive macrophages was 2.5-fold higher in visceral fat of Apoe-/- mice compared to Stat4-/-Apoe-/- mice, as determined by immunohistochemistry (Figure 2C). Also, Stat4-/-Apoe-/- mice had virtually no CD11c+F4/80+ canonical macrophages in either of the depots (Figure 2A, B). Interestingly, Apoe-/- mice on high fat diet showed a significant increase by ~6.5-fold in the percentage of CD11c+F4/80- cells (dendritic cells) compared to Stat4-/-Apoe-/- mice in the peri-aortic but not visceral fat. Also, Apoe-/- and Stat4-/-Apoe-/- mice on chow diet have very low percentages of CD11b+F4/80+ macrophages (~3.5%) and of CD11c+F4/80- (dendritic cells) (<1%) of the total CD45+ cells in visceral fat and even lower numbers in the peri-aortic fat and no significant differences were found between the groups (not shown). Collectively, this data show that in face of dietary challenge, STAT4 deficiency leads to reduced proportion of pro-inflammatory “canonical” macrophages and increased percentage of “alternatively activated” macrophages in adipose tissue depots. These changes may result in reduced overall cytokine production and inflammation in Stat4-/-Apoe-/- mice compared to Apoe-/- controls.
Figure 2. Effect of STAT4 deficiency on macrophage abundance, polarization and inflammatory profile.
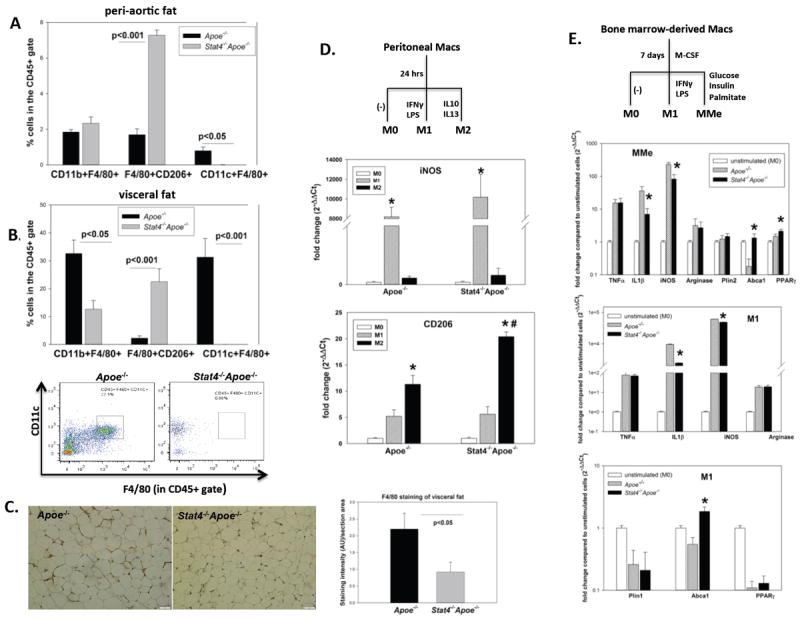
Relative composition of macrophages in peri-aortic fat (A) and visceral fat (B) of mice on western diet (n=5-7); representative FACS plots for CD11c+F4/80+ are also shown. (C): representative micrographs and quantitation of F4/80 staining in visceral adipose tissue of Stat4-/-Apoe-/- and Apoe-/- mice on western diet (n=4/group); AU=arbitrary units. (D): M1 and M2 macrophage marker gene expression in peritoneal macrophages were measured by real-time PCR and expressed as fold change compared to unstimulated macrophages (M0); n=3 independent experiments performed in duplicate; * = significantly different compared to M1/M2 and M0; # = significantly different compared to Apoe-/- group. (E): M1 and MMe (metabolically activated) markers were measured in bone marrow derived macrophages by real-time PCR; results represent mean ±SEM from n=4 independent experiments performed in duplicate; * = significant compared to Apoe-/- group. Statistical analysis for (D) and (E) was performed using One-way ANOVA.
To determine if the differences found in the phenotype of adipose tissue macrophages upon STAT4 deficiency are due to intrinsic differences in response to cytokines or metabolic cues, we isolated peritoneal and bone marrow-derived macrophages and induced in vitro polarization with various combinations of cytokines or metabolic activators. Peritoneal macrophages isolated from Apoe-/- and Stat-4-/-Apoe-/- mice were treated in vitro with either LPS+IFNγ or with IL4+IL13 to drive M1 or M2 differentiation (Figure 2D). The un-stimulated macrophages showed similar expression of iNOS (M1 marker) and CD206 (M2 marker). Also, macrophages from both groups responded robustly to cytokine challenge and showed significantly increased M1 and M2 markers upon respective stimulations (Figure 2D). Interestingly, CD206 expression was significantly increased by ~1.8 fold in macrophages of Stat4-/-Apoe-/- mice compared to Apoe-/- controls following in vitro treatments (Figure 2D) paralleling the phenotype of resident adipose tissue macrophages found in vivo. Bone marrow-derived macrophages (BMDM) from both mice groups were also generated by in vitro culture of bone marrow cells in presence of M-CSF. Recent evidence showed that in vitro metabolic challenge induces a distinct macrophage phenotype that resembles the M1 classically activated macrophages but also expresses anti-inflammatory markers such as PPARγ. Similar to peritoneal macrophages, unstimulated BMDM from both mice groups had similar expression of all of the tested genes (not shown). Also, BMDM from both mice groups showed robust activation of several M1 markers such as iNOS and arginase along with pro-inflammatory cytokines such as TNFα and IL1β (Figure 2E). In addition, M1 macrophages from Stat4-/-Apoe-/- mice showed decreased iNOS and IL1b expression compared to Apoe-/- mice (Figure 2E). Interestingly, expression of Abca1 gene was also significantly increased in M1 macrophages from Stat4-/-Apoe-/- mice compared to controls, suggesting a potential ability of these macrophages to limit lipid loading by upregulating cellular cholesterol efflux (Figure 2E). BMDM were also treated with a combination of insulin/glucose/palmitic acid to achieve “metabolic activation” (MMe) (Kratz et al. 2014). MMes from Stat4-/-Apoe-/- mice showed reduced activation of the pro-inflammatory markers iNOS and TNFa compared to Apoe-/- controls along with increased expression of the anti-inflammatory genes PPARg and the Abca1 transporter (Figure 2E). No differences were found in expression of TNFa, arginase and Plin2 (Figure 2E). These findings indicate that macrophages from Stat4-/-Apoe-/- mice assume a lower pro-inflammatory phenotype compared to Apoe-/- mice in response to both M1 and MMe polarization.
Role of STAT4 deficiency on adipose tissue cytokines and chemokines expression
We further investigated whether the reduced pro-inflammatory phenotype of macrophages from Stat4-/-Apoe-/- mice found both in vivo and in vitro is mirrored by a reduction in pro-inflammatory cytokine and chemokine gene expression in adipose tissues. We found that in Apoe-/- mice on western diet, gene expression of IL12p40 was 2-fold increased in visceral fat and ~6-fold increased in the peri-aortic fat compared to Stat4-/-Apoe-/- mice (Figure 3A, B). In the peri-aortic fat there was also ~4-fold increased TNFα gene expression in Apoe-/- mice compared to. Stat4-/-Apoe-/- mice. Expression of PPARγ was reduced ~2-fold in Apoe-/- mice in both fat depots compared to Stat4-/-Apoe-/- mice (Figure 3A, B). In addition, expression of CCL5, CXCL10 and CX3CL1 were all significantly increased in the peri-aortic fat of the Apoe-/- mice compared to Stat4-/-Apoe-/- mice while expression of CCL2 and CXCL16 was similar between groups (Figure 3C).
Figure 3. Expression of pro-inflammatory cytokines and chemokines in Stat4-/-Apoe-/- and Apoe-/- mice on western diet.
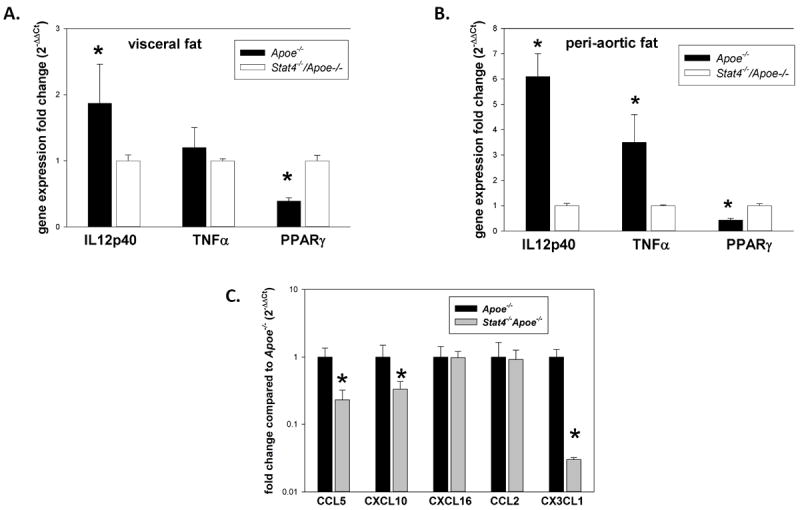
Cytokine gene expression was measured in visceral (A) and peri-aortic (B) adipose tissue using real-time PCR; results are expressed as fold change in gene expression in Apoe-/- mice compared to Stat4-/-Apoe-/- group and represent mean±SEM of n=8 mice/group; Chemokine expression in peri-aortic adipose tissue (C) was also measured by real-time PCR in n=6 mice /group; * = significantly different (p<0.05) compared to Stat4-/-Apoe-/- group.
Pro-inflammatory response of aortic cells to STAT4-deficient visceral AT secretome
To determine whether STAT4 may effect aortic wall pro-inflammatory gene expression via the STAT4 dependent adipose tissue secretome we examined in vitro the response of aortic cells from Apoe-/- mice to conditioned media (CM) generated from visceral AT of Stat4-/-Apoe-/- or Apoe-/- controls. Briefly, we excised and incubated AT from Stat4-/-Apoe-/- and Apoe-/- mice in Dulbecco’s modified Eagle media, and collected the 24-hour CM. The CM was further incubated for 24 hours with fresh preparations of aortic cell suspensions from Apoe-/- mice (Figure 4A). Following incubation, aortic cells were washed and collected by centrifugation and gene expression of various macrophage and T cell chemokines and activation markers were performed by real time PCR (Figure 4B). Gene expression for CX3CL1, CCL5, TNFα, IL12p40 was not detectable in Apoe-/- aortic cells incubated with either of the CM. However, in cells incubated with Apoe-/- CM, mRNA expression of CCL2 was significantly higher compared to cells incubated with Stat4-/-Apoe-/- CM (Figure 4B). This result shows that visceral fat secretome may induce chemokine expression in aortic cells, at least in part, via a STAT4-dependent mechanism. Interestingly, we found no significant difference in the expression of the macrophage activation marker I-Ab (MHC-II) between aortic cells incubated with AT CM from Stat4-/-Apoe-/- or Apoe-/- mice (Figure 4B). This result suggests that visceral adipose tissue secretome can directly induce increased expression of some of the pro-inflammatory genes in cells of the aortic wall and that STAT4 deficiency may limit inflammation.
Figure 4. Stat4-/-Apoe-/- visceral adipose tissue secretome decreases gene expression of CCL2 in Apoe-/- aortic cells.
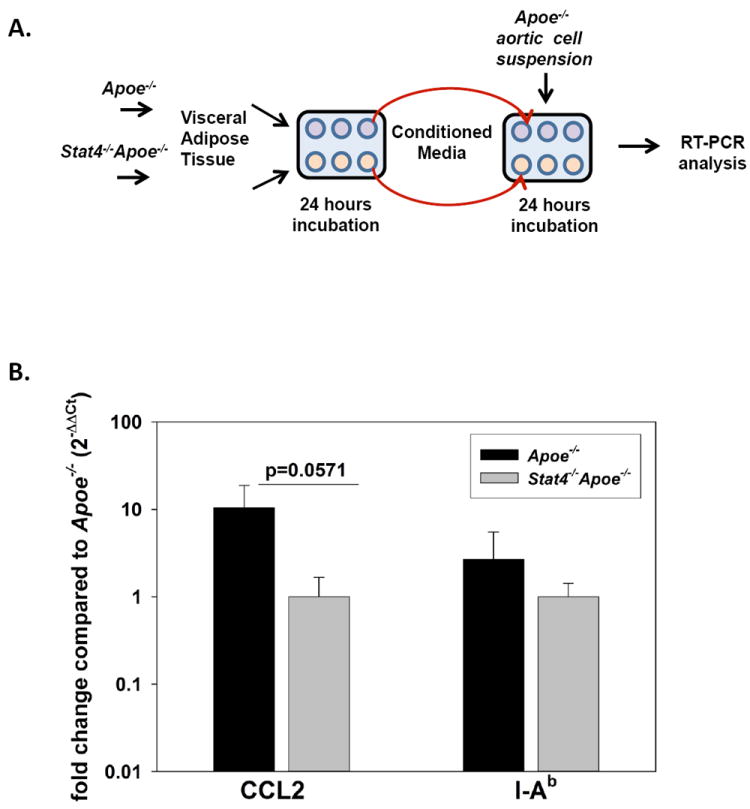
(A) Experimental schematic demonstrating the approach. See Materials and Methods for more detailed description. (B) Real time PCR analysis of CCL2 and I-Ab in aortic cell suspensions following incubation with visceral AT-CM from either Stat-4-/-Apoe-/- or Apoe-/- mice. Data is expressed as 2-ΔΔCt normalized to 18s as a housekeeping gene. Results represent mean±SEM from n=4 independent experiments performed in duplicate.
STAT4 deficiency reduces aortic plaque burden in mice on western diet
In this study we analyzed a small sample of mice (n=4 on chow and n=8 on western diet) to confirm previous data showing reduced atherosclerosis in Stat-4-/-Apoe-/- mice compared to Apoe-/- controls (manuscript under revision). Our data shows that compared to Apoe-/- controls, the Stat-4-/-Apoe-/- mice on western diet have a prominent and significant ~41% reduction in aortic plaque area by en face staining (Figure 5). In age matched Stat-4-/-Apoe-/- mice on chow diet the plaque burden is also slightly but not significantly reduced (Figure 5). There is significant interaction between the diet and the genotype (p<0.05) indicating that STAT4 deficiency is particularly protective in the context of high dietary cholesterol challenge.
Figure 5. STAT4 deficiency reduces atherosclerotic lesion burden in Apoe-/- mice on western diet.
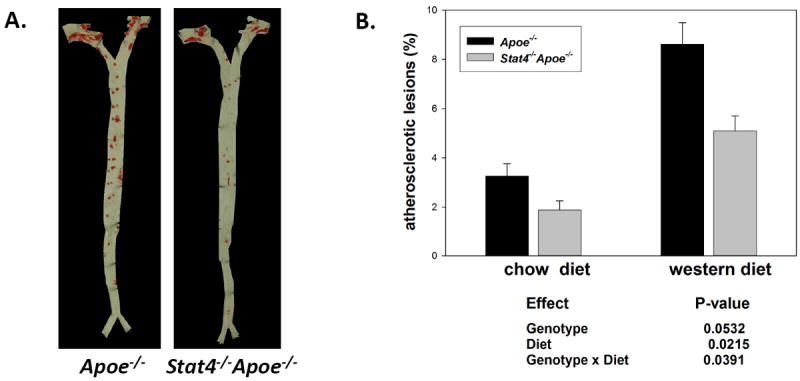
Female Stat4-/-Apoe-/- and Apoe-/- mice were kept on western diet or fed regular chow diet for 12 weeks starting at 8-10 weeks of age. (A) representative en face analysis using Sudan IV staining of whole aortas from western diet fed mice. (B) Lesion analysis from n=4 mice/group for the chow diet and from n=8 mice/group for the western diet groups. Data represent % stained area of the total aortic tissue. Statistical analysis was performed using 2-way ANOVA.
Discussion
The contribution of adipose tissue to systemic inflammation and cardiovascular disease is well documented. In this study we report for the first time the effect of STAT4 deficiency on peri-vascular and visceral adipose tissue inflammation in atherosclerotic Apoe-/- mice. STAT4 deficiency also reduces development of atherosclerosis suggesting that reduction of peri-vascular and visceral inflammation may have a contributing athero-protective role. We show that STAT4 deficiency reduces pro-inflammatory cytokines and chemokines as well as lymphocyte number and composition and macrophage polarization in peri-vascular fat of mice on western diet. In Apoe-/- mice, the increased atherosclerotic burden on western diet compared to chow is largely due to a dramatic increase in circulating VLDL+LDL cholesterol (Plump, et al. 1992). We show that protection by STAT4 deficiency is independent of circulating lipids as the STAT4 deficient mice have similarly increased levels of LDL+VLDL cholesterol to Apoe-/- controls (Table 1).
We previously showed that STAT4 deficiency improves glucose tolerance and increases insulin sensitivity in a mouse model of obesity and insulin resistance (Dobrian et al. 2013). This effect was accompanied by reduced adipose tissue inflammation due to more insulin sensitive and less hypertrophic adipocytes and reduced lymphocyte infiltration in the visceral adipose tissue. However, Apoe-/- mice are lean and insulin sensitive, even when fed a western diet (Gao et al. 2007; Kawashima et al. 2009). Therefore the anti-inflammatory effects of STAT4 in this model cannot be attributed to an improved metabolic profile or to reduced visceral adiposity. Indeed we did not find any differences in glucose, insulin or body weight between the Stat4-/-Apoe-/- mice and the Apoe-/- controls (Table 1). In this study we focused our investigation on two of the more relevant fat depots, visceral and peri-aortic, known to exert vasocrine effects in both mice and humans (Berg and Scherer 2005; Lakka, et al. 2002; Lohmann, et al. 2009b; Ouwens, et al. 2010; Yudkin, et al. 2005). While visceral adipose tissue contributes to accelerated atherosclerosis in Apoe-/- mice, the peri-vascular adipose tissue in particular plays a causative role in atherosclerotic plaque formation in this mouse model (Ohman et al. 2011; Ohman, et al. 2008). Our data shows significantly higher numbers of CD45+ lymphocytes in the peri-aortic, but not in the visceral fat of Apoe-/- compared to Stat4-/-Apoe-/- mice on western diet (Figure 1). In particular, the number of CD3+CD8+ T lymphocytes was dramatically increased in the peri-aortic fat in Apoe-/- mice on western diet compared to Stat4-/-Apoe-/- mice. Previous studies indicated that expansion of CD3+ subsets in response to IL12 stimulation is dependent on STAT4 (Yoo, et al. 2002). We found that in visceral fat of Stat-4-/-Apoe-/- mice expression of IL12p40 subunit is reduced, compared to Apoe-/- controls, suggesting lower levels of functional IL-12. In this study we also found that expression of a number of potent chemokines for T lymphocytes or monocytes, such as CCL5, CXCL10 and CX3CL1 are reduced in the peri-aortic fat of Stat4-/-Apoe-/- mice compared to controls (Figure 3). STAT4 is involved in regulation of several chemokine receptors that are preferentially expressed in Th1 cells, such as CXCR3 and CCR5 (Kim, et al. 2006). We have previously shown (Dobrian et al. 2013) that expression of these and other chemokine receptors are selectively decreased in CD8+ but not CD4+ cells of STAT4-deficient mice, compared to wild type controls. It is therefore possible that reduced chemokine/cytokine production in peri-aortic and visceral fat combined with reduced migratory capacity of CD8+ cells due to a reduction in the chemokine receptors results in a relative reduction of CD8+ cells recruited in the adipose tissues of Stat4-/-Apoe-/- mice. Cytotoxic CD8(+) T lymphocytes represent up to 50% of the total numbers of leukocytes found in advanced human plaques and dominate early immune responses in mouse lesions (Gewaltig, et al. 2008; Kolbus, et al. 2010). A recent publication showed that depletion of this lymphocyte subset in Apoe-/- mice fed a high-fat diet ameliorates atherosclerosis (Kyaw, et al. 2013). Therefore, peri-vascular and possibly visceral fat may constitute a reservoir of CD8+ cells for the aortic wall and reduction of this T cell subset may be part of the mechanism responsible for athero-protection reported in this paper and by our group in preliminary observations in Stat-4-/-Apoe-/- mice (manuscript under revision). An in-depth analysis of the atherosclerotic phenotype and related vascular mechanisms in Stat-4-/-Apoe-/- mice are under publication elsewhere.
There was also, a prominent reduction in the number of NK40.6+ cells, in Stat-4-/-Apoe-/- mice on western diet compared Apoe-/- controls, in both peri-aortic and visceral fat. Like CD8+ cells, NK cells appear to play an important role in the development of atherosclerosis (Kolbus et al. 2010; Major, et al. 2004). STAT4 is abundantly expressed in NK cells and is key for IFNγ production and increased cytotoxicity. Therefore reduction in the relative composition of NK cells in visceral and peri-aortic fat may also contribute to reduced vascular wall inflammation in STAT4-deficient mice.
Macrophages represent a major component of visceral adipose tissue in rodents (Lumeng et al. 2007a; Lumeng et al. 2007b) but are less abundant in the peri-vascular fat. In Apoe-/- mice, the number of macrophages is reportedly very low in various adipose tissue depots. We also found that only a minor proportion, of up to ~2.2% of the total CD45+ cells in peri-aortic fat of Apoe-/- mice is represented by CD11b+F4/80+ macrophages and is similar for Stat-4-/-Apoe-/- mice (Figure 2). Even in visceral fat, the proportion of CD11b+F4/80+ cells is ~30% in Apoe-/- mice and only ~10% in Stat-4-/-Apoe-/- mice. Therefore, in these mice the tropism of macrophages may be more prominent for other tissues, including the aorta. Interestingly, we found that STAT4 deficiency induces a bias towards CD206+ macrophages in both depots in vivo and that peritoneal macrophages of Stat-4-/-Apoe-/- mice have a more robust response to “alternative” activation in vitro (Figure 2). It is known that the CD206+ (alternatively activated) macrophages are a source of the anti-inflammatory IL-10 and IL-4 cytokines as opposed to the M1 macrophages that are an abundant source of IL-12, IL-6, IL-1β and other pro-inflammatory cytokines (Lee, et al. 2012). We also found that in the peri-aortic fat, gene expression of IL12p40 and TNFα was decreased in Stat4-/-Apoe-/- mice. The data suggests that STAT4 deficiency drives M2 polarization of adipose tissue macrophages without affecting the number of resident cells. This conclusion is also supported by reduced in vitro propensity of the STAT4 deficient bone marrow derived macrophages to differentiate into a M1 phenotype (Figure 2) as well as increased PPARγ expression in adipose tissue (Figure 3) known to promote M2 polarization (Bouhlel, et al. 2007; Odegaard, et al. 2007). Also, virtually the entire population of F4/80+ macrophages was CD11c+ in both visceral and peri-aortic fat of Apoe-/- mice, while in Stat-4-/-Apoe-/- mice the CD11c+ macrophages were not detectable. Canonical CD11c+ macrophages are known to have a marked pro-inflammatory phenotype in both mice and humans (Geissmann, et al. 2010) and can therefore be a source of circulating cytokines and chemokines impacting on immune cell recruitment and inflammation in the vascular wall. Importantly, several recent studies have described a combined M1/M2 phenotype of macrophages in obesity in mice and humans suggesting they can adapt a more complex phenotype (Riboldi, et al. 2013; Zeyda, et al. 2007). Although, mice on the Apoe-/- background are lean and normoglycemic, we tested the paradigm that metabolic activation of bone marrow-derived macrophages from Stat-4-/-Apoe-/- mice may lead to phenotypically different macrophages compared to Apoe-/- cells. Indeed, in vitro stimulation of bone marrow-derived macrophages with a combination of insulin/glucose/palmitate resulted in significantly reduced expression of IL1β and iNOS in cells from Stat-4-/-Apoe-/- mice compared to Apoe-/- controls. Also, metabolic activation has been shown to up-regulate anti-inflammatory genes such as PPARγ and lead to more efficient metabolism due to an increase in Abca1 and Plin2 genes (Kratz et al. 2014). In Stat-4-/-Apoe-/- macrophages PPARγ gene expression was significantly elevated compared to Apoe-/- suggesting a more efficient anti-inflammatory response in the former in response to metabolic activation. This may be relevant especially upon western diet feeding when circulating lipids are dramatically increased and may lead to a predominant pro-inflammatory macrophage phenotype. Finally, we found that Abca1 gene expression was significantly increased in MMe macrophages of Stat-4-/-Apoe-/- mice compared to Apoe-/- controls. This finding suggests that STAT4 deficiency promotes a more efficient cholesterol efflux from macrophages and therefore could contribute to the reduced atheroma formation in Stat-4-/-Apoe-/- mice on western diet via a reduction of foam cells.
Many studies demonstrate a link between the inflammation within adipose tissue, resulting in insulin resistance and accelerated atherosclerosis (Mazzone, et al. 2008). Adipose tissue releases inflammatory cytokines and bioactive mediators that are potent inducers of structural and functional changes in the vasculature (Brown, et al. 2014). Collectively referred to as the adipose tissue secretome these factors may originate from adipocytes or other cells in adipose tissue, such as immune cells and vascular cells. We examined the effect of STAT4 sufficient and deficient adipose tissue secretome on aortic gene expression of pro-inflammatory cytokines and chemokines in Apoe-/- mice. Interestingly, we found that CCL2 expression in aortic cells of Apoe-/- mice following incubation with adipose tissue CM of Stat4-/-Apoe-/- mice is significantly lower compared to CM from Apoe-/- mice (Figure 4). This finding indicates that visceral AT secretome can support inflammation and atherosclerosis development at least through changes in the aortic expression of CCL2, one of the major chemokines necessary for MΦ recruitment into the aorta. Although we did not test the effect of peri-aortic fat secretome on aortic cells due to the limited tissue availability, the similarly immune cells composition and phenotype found between visceral and peri-aortic depots suggest comparable responses. Also, although the CM component or combination responsible for the response is unknown, the effect is STAT4 related and further studies are granted to pinpoint the mechanism.
Collectively our data show that STAT4 deficiency in Apoe-/- mice reduces immune cell infiltration, pro-inflammatory cytokine and chemokine expression and macrophage polarization in peri-aortic and visceral adipose tissue. These effects may contribute to the athero-protective effect of STAT4 deficiency in this model, particularly in mice on western diet. Potential mechanisms include reduction of the reservoir of pro-inflammatory cells or pro-inflammatory cytokines and chemokines to the aortic wall or blunted expression of pro-inflammatory genes in cells of the aortic wall. Ultimately STAT4 deficiency limits vascular inflammation potentially leading to reduced plaque burden in diet-induced accelerated atherosclerosis. Our study suggests, that selective inhibitors of the Jak/Tyk/STAT4 pathway may have possible cardiovascular health benefits beyond their current indications as therapeutics for autoimmune and chronic inflammatory diseases.
Acknowledgments
The authors wish to thank Dr. Mark Kaplan for providing the STAT4 null mice. The authors also acknowledge the excellent technical support of Lindsey Glenn, BS, Jack Lindsey, MS, Ashley James, BS and Mr. Ciriaco Villaflor.
Funding
This work was supported by the PO1 HL55798 to JLN and by the RO1 HL112605 to JLN and AD.
Footnotes
Declaration of interest
The authors have nothing to disclose. There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Han L, Goswami R, Nguyen ET, Pelloso D, Robertson MJ, Kaplan MH. Impaired development of human Th1 cells in patients with deficient expression of STAT4. Blood. 2009;113:5887–5890. doi: 10.1182/blood-2008-09-179820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55:715–721. doi: 10.1161/HYPERTENSIONAHA.109.148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrian AD, Galkina EV, Ma Q, Hatcher M, Aye SM, Butcher MJ, Ma K, Haynes BA, Kaplan MH, Nadler JL. STAT4 deficiency reduces obesity-induced insulin resistance and adipose tissue inflammation. Diabetes. 2013;62:4109–4121. doi: 10.2337/db12-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrian AD, Ma Q, Lindsay JW, Leone KA, Ma K, Coben J, Galkina EV, Nadler JL. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2010;300:E410–421. doi: 10.1152/ajpendo.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Katagiri H, Ishigaki Y, Yamada T, Ogihara T, Imai J, Uno K, Hasegawa Y, Kanzaki M, Yamamoto TT, et al. Involvement of apolipoprotein E in excess fat accumulation and insulin resistance. Diabetes. 2007;56:24–33. doi: 10.2337/db06-0144. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewaltig J, Kummer M, Koella C, Cathomas G, Biedermann BC. Requirements for CD8 T-cell migration into the human arterial wall. Hum Pathol. 2008;39:1756–1762. doi: 10.1016/j.humpath.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Good SR, Thieu VT, Mathur AN, Yu Q, Stritesky GL, Yeh N, O’Malley JT, Perumal NB, Kaplan MH. Temporal induction pattern of STAT4 target genes defines potential for Th1 lineage-specific programming. J Immunol. 2009;183:3839–3847. doi: 10.4049/jimmunol.0901411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, van Snick J, Kuiper J. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112:1054–1062. doi: 10.1161/CIRCULATIONAHA.104.533463. [DOI] [PubMed] [Google Scholar]
- Hofmann SM, Perez-Tilve D, Greer TM, Coburn BA, Grant E, Basford JE, Tschop MH, Hui DY. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes. 2008;57:5–12. doi: 10.2337/db07-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath CM, Darnell JE. The state of the STATs: recent developments in the study of signal transduction to the nucleus. Curr Opin Cell Biol. 1997;9:233–239. doi: 10.1016/s0955-0674(97)80067-1. [DOI] [PubMed] [Google Scholar]
- Huang ZH, Reardon CA, Mazzone T. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 2006;55:3394–3402. doi: 10.2337/db06-0354. [DOI] [PubMed] [Google Scholar]
- Huang ZH, Reardon CA, Subbaiah PV, Getz GS, Mazzone T. ApoE derived from adipose tissue does not suppress atherosclerosis or correct hyperlipidemia in apoE knockout mice. J Lipid Res. 2013;54:202–213. doi: 10.1194/jlr.M031906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- Kaplan MH. STAT4: a critical regulator of inflammation in vivo. Immunol Res. 2005;31:231–242. doi: 10.1385/IR:31:3:231. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Chen J, Sun H, Lann D, Hajjar RJ, Yakar S, Leroith D. Apolipoprotein E deficiency abrogates insulin resistance in a mouse model of type 2 diabetes mellitus. Diabetologia. 2009;52:1434–1441. doi: 10.1007/s00125-009-1378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Gunst KV, Sarvetnick N. STAT4/6-dependent differential regulation of chemokine receptors. Clin Immunol. 2006;118:250–257. doi: 10.1016/j.clim.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Bjorkbacka H, Fredrikson GN, Nilsson J. CD8+ T cell activation predominate early immune responses to hypercholesterolemia in Apoe(-)(/)(-) mice. BMC Immunol. 2010;11:58. doi: 10.1186/1471-2172-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Sugiyama S, Sato Y, Oshima S, Sugamura K, Nozaki T, Ohba K, Matsubara J, Sumida H, Nagayoshi Y, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010a;213:649–655. doi: 10.1016/j.atherosclerosis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Konishi M, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Matsubara J, Matsuzawa Y, Sumida H, Nagayoshi Y, Nakaura T, et al. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis. 2010b;209:573–578. doi: 10.1016/j.atherosclerosis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation. 2013;127:1028–1039. doi: 10.1161/CIRCULATIONAHA.112.001347. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Lakka TA, Tuomilehto J, Salonen JT. Abdominal obesity is associated with increased risk of acute coronary events in men. Eur Heart J. 2002;23:706–713. doi: 10.1053/euhj.2001.2889. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2012;13:371–376. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, Lin JX. Cytokine receptor signaling pathways. J Allergy Clin Immunol. 2000;105:877–888. doi: 10.1067/mai.2000.106899. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Schafer N, von Lukowicz T, Sokrates Stein MA, Boren J, Rutti S, Wahli W, Donath MY, Luscher TF, Matter CM. Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver, and pancreatic islets. Atherosclerosis. 2009a doi: 10.1016/j.atherosclerosis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Schafer N, von Lukowicz T, Sokrates Stein MA, Boren J, Rutti S, Wahli W, Donath MY, Luscher TF, Matter CM. Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver, and pancreatic islets. Atherosclerosis. 2009b;207:360–367. doi: 10.1016/j.atherosclerosis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007a;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007b;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Major AS, Wilson MT, McCaleb JL, Ru Su Y, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2351–2357. doi: 10.1161/01.ATV.0000147112.84168.87. [DOI] [PubMed] [Google Scholar]
- Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219:33–39. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Gu J, Thimmalapura PR, Mison A, Nadler JL. Activation of the 12-lipoxygenase and signal transducer and activator of transcription pathway during neointima formation in a model of the metabolic syndrome. Am J Physiol Endocrinol Metab. 2006;290:E92–E102. doi: 10.1152/ajpendo.00133.2005. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboldi E, Porta C, Morlacchi S, Viola A, Mantovani A, Sica A. Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int Immunol. 2013;25:67–75. doi: 10.1093/intimm/dxs110. [DOI] [PubMed] [Google Scholar]
- See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50:752–759. doi: 10.1016/j.jacc.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival signals to murine CD4+ T cells through phosphatidylinositol 3-kinase/Akt signaling pathway. J Immunol. 2002;169:3637–3643. doi: 10.4049/jimmunol.169.7.3637. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res. 2006;98:524–531. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]
- Zhao L, Cuff CA, Moss E, Wille U, Cyrus T, Klein EA, Pratico D, Rader DJ, Hunter CA, Pure E, et al. Selective interleukin-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atherosclerosis in a mouse model of familial hypercholesterolemia. J Biol Chem. 2002;277:35350–35356. doi: 10.1074/jbc.M205738200. [DOI] [PubMed] [Google Scholar]


