Abstract
Quercetin (QT) and taxifolin (TF) are structurally similar plant-derived flavonoids that have antioxidant properties and act as free radical scavengers. The objective of this study was to investigate effects of QT and TF on nuclear maturation of porcine oocytes. Effects of TF at 0, 1, 10, and 50 μg/mL on oocyte nuclear maturation (polar body extrusion) were investigated. After incubation for 44 h, there were no significant differences between the treatment and control groups except in the 50 μg/mL group which was significantly lower (59.2%, p<0.05) than the other groups (control: >80%). After parthenogenetic activation, further in vitro development of QT- or TF-treated vs control oocytes was investigated. A significantly higher proportion of QT-treated (1 μg/mL) oocytes developed into blastocysts compared to controls (24.3% vs 16.8%, respectively); however, cleavage rate and blastocyst cell number were not affected. The TF-treated group was not significantly different from controls. Levels of reactive oxygen species (ROS) and intracellular glutathione (GSH) in oocytes and embryos in a culture medium supplemented with QT or TF were measured. Both treatment groups had significantly lower (p<0.05) levels of ROS than controls, however GSH levels were different only in QT-treated oocytes. We conclude that exogenous flavonoids such as QT and TF reduce ROS levels in oocytes. Although at high concentration (50 μg/mL) both QT and TF appear to be toxic to oocytes.
Keywords: In vitro Maturation, Porcine Oocyte, Antioxidants, Quercetin, Reactive Oxygen Species [ROS]
INTRODUCTION
Increasing the efficiency of systems for in vitro production of porcine embryos is very important because pigs have high biomedical value for areas such as xenotransplantation and as models for stem cell research (Telugu et al., 2011). However, despite intensive efforts, the yield and quality of in vitro matured (IVM) oocytes and embryos derived from them are still low compared with in vivo produced embryos. Improvements can be made by altering the culture conditions for oocyte maturation and embryo development, including the external oxygen concentration (Kang et al., 2012). Oxidative stress originating from high external oxygen concentration can produce reactive oxygen species (ROS), which may be responsible for damaging embryos and inducing early embryonic developmental blocks (Guerin et al., 2001). Therefore, supplementing maturation and culture media with antioxidants such as β-mercaptoethanol, cysteine and cysteamine can help to protect against defective embryo development (Abeydeera et al., 1998; de Matos and Furnus, 2000).
Flavonoids are a class of plant secondary metabolites and are most commonly known for their antioxidant activity in vitro (Bagchi et al., 1999). Quercetin (QT) (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one) and taxifolin (TF) (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one) are plant-derived flavonoids mainly found in fruits and vegetables. It has been reported that QT and TF have anti-oxidative, anti-mutagenic and anti-inflammatory activities (Naderi et al., 2003; Tang et al., 2006; Yuan et al., 2006; Kwon et al., 2011) due to free radical scavenging. Also, TF is not mutagenic and has low toxicity compared to the related compound QT (Makena et al., 2009). Our previous study on porcine oocytes demonstrated that exogenous QT is beneficial for nuclear maturation during IVM and subsequent embryo development by reducing ROS levels (Kang et al., 2013).
However, little information is available on the effect of flavonoids on oocyte maturation and embryonic development in pigs. The objective of this study was to examine the effect of QT and TF treatment during IVM and in vitro culture (IVC) on oocyte maturation and the development of parthenogenetically activated (PA) embryos. To this end, we observed nuclear maturation of oocytes, embryo cleavage and blatocyst formation of PA embryos, as well as intracellular levels of glutathione (GSH) and ROS in pig oocytes and embryos.
MATERIALS AND METHODS
Unless otherwise specified, all reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Oocyte collection and in vitro maturation
Pig ovaries were collected from a local abattoir and transported to the laboratory in 0.9% (w/v) NaCl solution at 25°C to 30°C. Follicular contents from antral follicles (3 to 6 mm in diameter) were aspirated using an 18-gauge needle attached to a 10 mL disposable syringe. The contents were pooled in a conical tube at 39°C and allowed to settle for a few minutes. The sediment was aspirated and diluted with Dulbecco’s phosphate buffered saline (D-PBS; Invitrogen, Carlsbad, CA, USA) containing 100 U/mL penicillin G and 100 mg/mL streptomycin sulphate (pen-strep; Invitrogen, Carlsbad, CA, USA). Cumulus-oocyte complexes (COCs) with intact compact cumulus cell layers were selected and washed 3 times in tissue culture medium (TCM)-Hepes before being transferred to a modified TCM-199 supplemented with 10 ng/mL epidermal growth factor, 0.57 mM cystine, 0.91 mM sodium pyruvate, 5 μg/mL insulin, 1% (v/v) pen-strep, 0.5 μg/mL follicle stimulating hormone, 0.5 μg/mL luteinizing hormone and 10% porcine follicular fluid. For the first 22 h only, the IVM medium also contained Gonadotropin. The COCs were cultured at 38°C with 5% CO2 at maximum humidity. After 44 h of maturation, oocytes were denuded of cumulus cells by pipetting with 0.1% hyaluronidase in D-PBS supplemented with 0.1% polyvinyl alcohol. Then denuded oocytes were treated according to the experimental design.
Assessment of meiotic maturation of mature oocytes
After culture for 44 h, denuded oocytes were stained with Hoechst 33342 in D-PBS. The stage of meiotic maturation was determined by examination of the presence or absence of the first polar body (metaphase II) under UV light.
Parthenogenetic activation of mature oocytes and in vitro culture
At 44 hours of IVM, oocytes in metaphase II were activated. Denuded oocytes were equilibrated for 1 min in 0.26 M D-mannitol-based activation solution, supplemented with 0.1 mM MgCl2, 0.1 mM CaCl2 and 0.5 mM Hepes, then transferred to a chamber between two electrodes spaced 3.2 mm apart and overlaid with activation solution. The oocytes were activated with an electric stimulus using a single direct current pulse of 1.5 kV/cm for 60 μsec using a BTX Electro-Cell Manipulator 2001 (BTX Inc., Holliston, MA, USA). A group of approximately 20 to 30 parthenogenetically-activated (PA) oocytes were cultured in 500 μL porcine zygote medium (PZM)-3 containing 0.3% (w/v) fatty acid-free bovine serum albumin for 7 days at 39°C in a humidified atmosphere of 5% CO2 and 5% O2. Cleavage and blastocyst formation rates were checked at 48 and 168 hours of IVC, respectively.
Assessment of embryo quality
The quality of blastocysts was assessed by Hoechst staining of total cell number including inner cell mass and trophectoderm cells according to standard procedures. Briefly, after rinsing in PZM-3 medium, blastocysts were stained with Hoechst 33342 for 15 min. After rinsing in PZM-3 medium, the blastocysts were mounted on a glass slide under a cover slip and examined under an inverted microscope (Nikon Corp., Tokyo, Japan) equipped with epifluorescence.
Measurement of intracellular glutathione and reactive oxygen species levels
Oocytes were sampled after 44 h of IVM and 2 d of IVC to determine intracellular GSH and ROS levels using the dichlorohydrofluorescein diacetate (DCHFDA) and CellTracker Blue CMF2HC (4-chloromethyl-6,8-difluoro-7-hydroxycoumarin) methods previously described (Yang et al., 1998; You et al., 2010) with slight modification. Briefly, 20 oocytes and embryos from each treatment group were incubated in the dark at 39°C for 30 min in Tyrode’s albumin lactate pyruvate (TALP) medium supplemented with 10 μM DCHFDA and 10 μM CellTracker. After incubation, oocytes were washed in Hepes-buffered TALP medium, placed on a glass slide and covered with a cover slip. The fluorescence emissions from the oocytes and embryos were recorded as TIFF files using a cooled charge-coupled device (CCD) camera attached to a fluorescence microscope (Axio Photo; Carl Zeiss Jena GmbH, Berlin, Germany) with excitation filters (460 nm for ROS and 370 nm for GSH). The recorded fluorescent images were analyzed using NIH image software 1.55 (National Institutes of Health, USA) by counting the number of pixels after color inversion (Kim et al., 2006).
Effect of flavonoid on cumulus cell steroidogenesis
IVM media of both the first and second day of culture were collected, centrifuged at 1,500 rpm for 3 min and the supernatants were stored at 20°C until assayed for progesterone (P4) and estradiol −17β (E2) by validated redioimmunoassays. All samples were analyzed with assistance of the Neodin Veterinary Laboratory (Seoul, Repulic of Korea; http://www.vetlab.co.kr).
Experimental design
In order to determine effective concentrations of TF for improving oocyte maturation (Experiment 1), it was included in the IVM medium (TCM-199) at four concentrations (0, 1, 10, or 50 μg/mL) during the entire maturation culture period of 44 h. In Experiment 2, we evaluated the effects of including QT or TF in the IVM medium compared with a non-treated control group on the parthenogenetic development of embryos. In Experiment 3, we assessed the effects of these QT and TF concentrations in the IVM medium on the ROS and GSH levels in oocytes and embryos.
Statistical analysis
All statistical analyses were performed using Prism4 software (GraphPad, La Jolla, CA, USA). One-way analysis of variance was used to determine significant differences in the data followed by a Tukey test to determine statistical differences among groups. Significant differences among the treatments were determined when p<0.05. Data are expressed as means±standard error of the mean.
RESULTS
Effect of TF on porcine oocyte nuclear maturation
A total of 746 oocytes were used in five replicates to evaluate the effects of TF on nuclear maturation during IVM. The polar body extrusion rate was not significantly different among the control and the treatment groups at 1 or 10 μg/mL, but it was significantly lower (p<0.05) in the group containing 50 μg/mL TF (59.2%±7.9%) compared with the control (80.5%±3.5%) and the other treatment groups (Table 1).
Table 1.
Effects of taxifolin during oocyte IVM on the frequency of first polar body extrusion (nuclear maturation)
| Concentration (μg/mL) | Oocytes (n) | Oocytes with polar body extrusion (n) | Polar body extrusion rate (%±SEM) |
|---|---|---|---|
| 0 (Control) | 181 | 146 | 80.54±3.5a |
| 1 | 192 | 162 | 84.43±2.6a |
| 10 | 194 | 152 | 78.50±3.2a |
| 50 | 179 | 107 | 59.21±7.9b |
IVM, in vitro matured; SEM, standard error of the mean.
Polar bodies were counted by Hoechst staining after 44 h maturation in vitro.
Within a column, values with different superscripts are significantly different (p<0.05).
Effects of QT and TF on PA embryo development
Based on the results from Experiment 1 and our previous study (Kang et al., 2013), 1 μg/mL of QT or TF were used in Experiment 2. A total of 800 oocytes that underwent IVM in media supplemented with QT or TF were PA in nine replicates. QT or TF had no effect on the first cleavage frequency or the cell number per blastocyst (Table 2). However, a significantly greater (p<0.05) proportion of blastocysts developed from oocytes when the IVM medium was supplemented with 1 μg/mL QT (Table 2). Further treatment with QT or TF during IVC did not have any stimulatory effect on embryonic development (data not shown).
Table 2.
Effects of QT or TF treatment of porcine oocytes on subsequent development in vitro
| Treatment (1 μg/mL) | Oocytes examined, n | Cleavage n (%)*≥ 2 cell | Blastocysts n (%±SEM) | No. of cells per blastocyst (means±SEM) |
|---|---|---|---|---|
| Control | 265 | 182 (68.80) | 44 (16.84±2.0)a | 49.75±3.7 |
| QT | 266 | 188 (70.87) | 64 (24.30±2.3)b | 48.00±2.6 |
| TF | 269 | 198 (73.58) | 47 (17.74±1.9)a | 51.86±4.5 |
QT, quercetin; TF, taxifolin; SEM, standard error of the mean.
Percentages are based on the number of oocytes examined.
Within a column, values with different superscripts are significantly different (p<0.05).
Intracellular levels of ROS and GSH in porcine oocytes and embryos
In Experiment 3, 430 oocytes were used in four replicates to evaluate the effect of QT or TF during IVM and IVC on the levels of ROS and GSH. QT increased intracellular GSH levels and decreased ROS generation in mature oocytes and in cultured embryos (p<0.05). TF also reduced the ROS levels in mature oocytes (p<0.05) but not in cleavage stage embryos, while GSH levels were not significantly different in oocytes or embryos compared with the control group (Figures 1 and 2).
Figure 1.
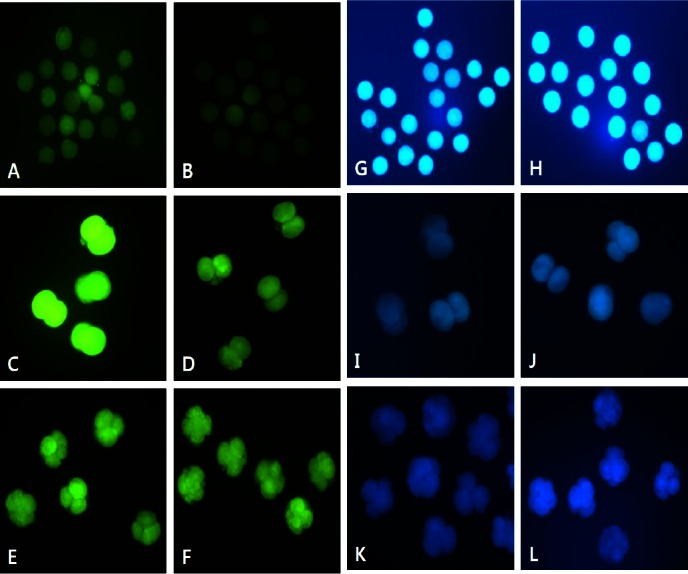
Epifluorescent photomicrographic images of in vitro matured porcine oocytes (A, B, G, and H) and embryos at the 2-cell (C, D, I, and J) and 4-cell stages (E, F, K, and L) developed after 2 d of in vitro culture. Oocytes and embryos were stained with DCHFDA (A–F) and CellTracker Blue (G–L) to detect reactive oxygen species and intracellular levels of glutathione, respectively (Original magnification ×200). Oocytes and embryos derived from the culture in medium supplemented without (A, C, E, G, I, and K) or with (B, D, F, H, J, and L) 1 μg/mL quercetin.
Figure 2.
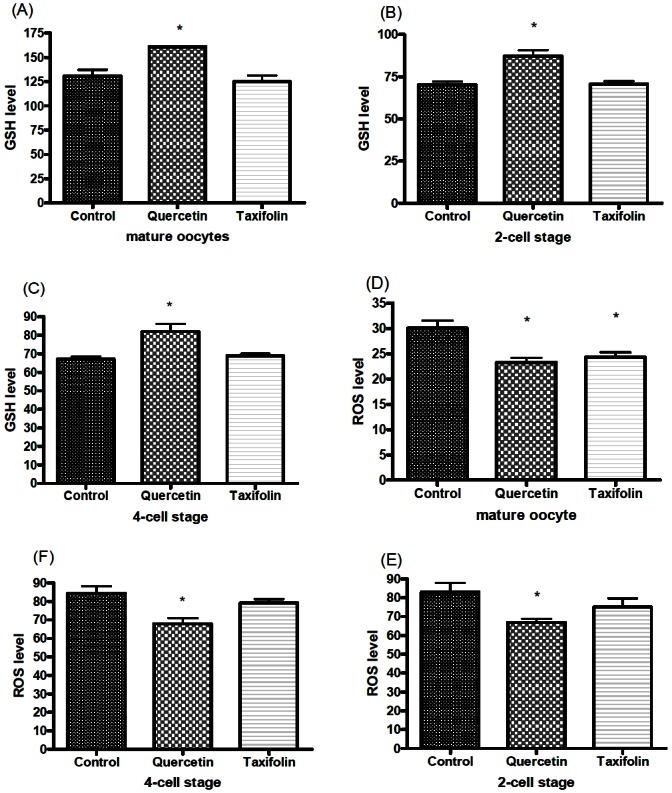
Measurement of intracellular glutathione (GSH) and reactive oxygen species (ROS) levels in in vitro matured (IVM) oocytes and in parthenogenetically activated embryos. The symbol (*) indicates a significant difference (p<0.05). Values shown on the Y-axis are number of pixels.
Effect of flavonoid on cumulus cell steroidogenesis
Basal steroid production by cumulus cells after 22 and 44 h of culture is shown in Table 3.
Table 3.
Effects of flavonoid (QT and TF) on cumulus cell steroidogensesis
| Treatment | Estradiol (pg/mL) | Progesterone (ng/mL) | ||
|---|---|---|---|---|
|
|
|
|||
| 22 h | 44 h | 22 h | 44 h | |
| Control | 1,326.5 | 1,161.7 | 85.0 | 323.7 |
| QT | 1,206.3 | 1,144.3 | 80.7 | 295.3 |
| TF | 1,223.8 | 1,168.0 | 75.8 | 395.2 |
QT, quercetin; TF, taxifolin; E2, estradiol-17β; P4, progesterone.
E2 and P4 production by cumulus cells after 22 and 44 h of in vitro maturation of oocytes in absence (control) or in presence of 1 μg/mL QT or 1 μg/mL TF. Data represent mean of at least four replicates.
Progesterone and estradiol levels in culture media of oocytes incubated with and without flavonoid (QT or TF) were investigated. Both concentration of flavonoid (QT and TF) didn’t significantly have no differences.
DISCUSSION
In this study, effects of the antioxidants QT and TF were examined during oocyte maturation and embryonic development following PA, and on intracellular levels of ROS and GSH. Although porcine PA embryos cannot develop beyond 29 days of gestation in vivo (Kure-bayashi et al., 2000), they may be good models to evaluate effects of exogenous factors during in vitro embryonic development. Our results showed that the rate of PA blastocyst formation from QT-treated oocytes was significantly higher than in the control group and in the TF-treated group. However, QT did improve the cleavage rate or total cell number of blastocysts compared with the other groups. Supplementing the IVM medium with TF did not improve nuclear maturation, but was effective in reducing ROS levels in mature oocytes. The inclusion of QT but not TF in the IVM medium increased PA blastocyst formation, presumably because QT reduced ROS and increased intracellular GSH more effectively than the TF treatment. However, we have found no beneficial effect of QT or TF treatment on blastocyst formation when applied only during IVC (data not shown). It is not clear whether improved embryonic development observed after treatment of oocytes with QT or TF was due to a direct action of QT or TF on embryos or to the reduction of ROS toxicity by increasing GSH.
ROS may originate from embryo metabolism and/or the embryo environment, and are detrimental to embryonic development (Johnson and Nasr-Esfahani, 1994). Many antioxidants can alleviate oxidative stress during reproductive processes, and can enhance embryonic development in vitro. In pigs, as in other mammals, several antioxidants have been used as supplements in culture media to enhance embryonic development (Kang et al., 2009), but little research has been done with flavonoids, which are well-known and powerful natural antioxidants. To the best of our knowledge, this is the first study investigating QT or TF effects on early embryonic development in vitro in pigs. Our data indicate that the optimal amount of flavonoid is concentration-specific, and while lower concentrations elicit no observable responses, excessive levels could be toxic to the oocyte.
Due to their structural similarity with estrogen, several flavonoids, including genistein and daidzein, also interact with the estrogen receptor to mediate their activity and thereby act as weak or moderate phytoestrogens (Galeati et al., 2010). Female mice treated neonatally with genistein showed multi-oocyte follicles, lacked regular estrous cyclicity and showed implantation failure, although ovulated oocytes were developmentally competent (Jefferson et al., 2009). Therefore, to confirm effects of flavonoids as phytoestrogens, we assayed for P4 and E2 by radioimmunoassay during the oocyte maturation period (44 h). For P4 and E2, steroid concentrations are expressed steadily without differences among groups (control, 1 μg/mL QT- and TF- treated group) (Table 3). Maybe it thought that QT and TF in this concentration is not effective in changing basal estradiol-17β secretion as phytoestrogens, and this finding is in agreement with the results of other flavonoids obtained by Galeati et al. (2010) in porcine granulosa cells, showing that these flavonoids in this concentration may affect very weakly on oocytes or granulosa cells as phytoestrogens.
Previous studies reported that addition of high concentrations of antioxidants to the IVM medium decreased the rate of blastocyst formation compared to treatment with low concentrations (Boquest et al., 1999), suggesting that the proper concentration of an antioxidant can contribute to the generation of high quality embryos. In our experiments, treatment with 50 μg/mL TF was detrimental to oocyte maturation, which is consistent with the result using QT in our previous study (Kang et al., 2013). One study reported that QT at 50 μg/mL inhibited progesterone production by granulosa cells, altered estradiol-17β production, and interfered with angiogenesis by inhibiting vascular endothelial growth factor production, so QT may have a negative influence on ovarian physiology (Santini et al., 2009). Both QT and TF in high concentration (at least 50 μg/mL) may have detrimental effects to oocytes by influencing culture environments.
We compared effects of QT and its analogue TF, both at 1 μg/mL, on embryo development after parthenogenetic activation (Experiment 2). Previous research has shown that using antioxidants during oocyte maturation increases cytoplasmic maturation and leads to higher rates of IVF and embryo development (Abeydeera et al., 1998). In our study, the antioxidant QT applied during IVM increased intracellular GSH levels and improved blastocyst development, which implies enhancement of cytoplasmic maturation. However, no beneficial effect of TF treatment during IVM was found on first polar body extrusion rate, showing that it could not affect nuclear maturation even though it effectively reduced ROS levels. Also, our observations that antioxidants, specifically QT, applied during oocyte maturation increases blastocyst formation, are in agreement with previous findings (Tao et al., 2004; Whitaker and Knight, 2004; You et al., 2010).
In conclusion, treatment of porcine oocytes with the flavonoid QT had a significant positive effect on embryonic development and reduced ROS generation by increasing intracellular GSH levels at low concentrations, but it was detrimental at high concentrations. It is not clear whether this concentration of QT is optimal in pigs. Therefore, further studies are needed to determine the optimal concentration of QT and to ascertain its beneficial effects on further development of pig embryos.
ACKNOWLEDGMENTS
This study was supported by IPET (#311011-05-4-SB010, #114059-03-2-SB010), MI (#10048948), Research Institute for Veterinary Science, TS corporation and the BK21 plus program.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- Abeydeera LR, Wang WH, Cantley TC, Prather RS, Day BN. Presence of beta-mercaptoethanol can increase the glutathione content of pig oocytes matured in vitro and the rate of blastocyst development after in vitro fertilization. Theriogenology. 1998;50:747–756. doi: 10.1016/s0093-691x(98)00180-0. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Carryl OR, Tran MX, Bagchi M, Garg A, Milnes MM, Williams CB, Balmoori J, Bagchi DJ, Mitra S, Stohs SJ. Acute and chronic stress-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. Mol Cell Biochem. 1999;196:109–116. [PubMed] [Google Scholar]
- Boquest AC, Abeydeera LR, Wang WH, Day BN. Effect of adding reduced glutathione during insemination on the development of porcine embryos in vitro. Theriogenology. 1999;51:1311–1319. doi: 10.1016/S0093-691X(99)00075-8. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Furnus CC. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development: Effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology. 2000;53:761–771. doi: 10.1016/S0093-691X(99)00278-2. [DOI] [PubMed] [Google Scholar]
- Galeati G, Vallorani C, Bucci D, Bernardini C, Tamanini C, Parmeggiani A, Spinaci M. Daidzein does affect progesterone secretion by pig cumulus cells but it does not impair oocytes IVM. Theriogenology. 2010;74:451–457. doi: 10.1016/j.theriogenology.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, Williams CJ. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol Reprod. 2009;80:425–431. doi: 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–38. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- Kang JT, Atikuzzaman M, Kwon DK, Park SJ, Kim SJ, Moon JH, Koo OJ, Jang G, Lee BC. Developmental competence of porcine oocytes after in vitro maturation and in vitro culture under different oxygen concentrations. Zygote. 2012;20:1–8. doi: 10.1017/S0967199411000426. [DOI] [PubMed] [Google Scholar]
- Kang JT, Koo OJ, Kwon DK, Park HJ, Jang G, Kang SK, Lee BC. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J Pineal Res. 2009;46:22–28. doi: 10.1111/j.1600-079X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- Kang JT, Kwon DK, Park SJ, Kim SJ, Moon JH, Koo OJ, Jang G, Lee BC. Quercetin improves the in vitro development of porcine oocytes by decreasing reactive oxygen species levels. J Vet Sci. 2013;14:15–20. doi: 10.4142/jvs.2013.14.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee SH, Kim S, Jeong YW, Koo OJ, Hashem MD, Park SM, Lee EG, Hossein MS, Kang SK, Lee BC, Hwang WS. Embryotrophic effects of ethylenediaminetetraacetic acid and hemoglobin on in vitro porcine embryos development. Theriogenology. 2006;66:449–455. doi: 10.1016/j.theriogenology.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kure-bayashi S, Miyake M, Okada K, Kato S. Successful implantation of in vitro-matured, electro-activated oocytes in the pig. Theriogenology. 2000;53:1105–1119. doi: 10.1016/S0093-691X(00)00256-9. [DOI] [PubMed] [Google Scholar]
- Kwon JH, Kim SB, Park KH, Lee MW. Antioxidative and anti-inflammatory effects of phenolic compounds from the roots of Ulmus macrocarpa. Arch Pharm Res. 2011;34:1459–1466. doi: 10.1007/s12272-011-0907-4. [DOI] [PubMed] [Google Scholar]
- Makena PS, Pierce SC, Chung KT, Sinclair SE. Comparative mutagenic effects of structurally similar flavonoids quercetin and taxifolin on tester strains Salmonella typhimurium TA102 and Escherichia coli WP-2 uvrA. Environ Mol Mutagen. 2009;50:451–459. doi: 10.1002/em.20487. [DOI] [PubMed] [Google Scholar]
- Naderi GA, Asgary S, Sarraf-Zadegan N, Shirvany H. Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol Cell Biochem. 2003;246:193–196. [PubMed] [Google Scholar]
- Santini SE, Basini G, Bussolati S, Grasselli F. The phytoestrogen quercetin impairs steroidogenesis and angiogenesis in swine granulosa cells in vitro. J Biomed Biotechnol. 2009 doi: 10.1155/2009/419891. Article ID 419891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Zhang C, Zeng W, Mi Y, Liu H. Proliferating effects of the flavonoids daidzein and quercetin on cultured chicken primordial germ cells through antioxidant action. Cell Biol Int. 2006;30:445–451. doi: 10.1016/j.cellbi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Tao Y, Zhou B, Xia G, Wang F, Wu Z, Fu M. Exposure to L-ascorbic acid or alpha-tocopherol facilitates the development of porcine denuded oocytes from metaphase I to metaphase II and prevents cumulus cells from fragmentation. Reprod Domest Anim. 2004;39:52–57. doi: 10.1046/j.1439-0531.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- Telugu BP, Ezashi T, Sinha S, Alexenko AP, Spate L, Prather RS, Roberts RM. Leukemia inhibitory factor (LIF)-dependent, pluripotent stem cells established from inner cell mass of porcine embryos. J Biol Chem. 2011;286:28948–28953. doi: 10.1074/jbc.M111.229468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker BD, Knight JW. Exogenous gamma-glutamyl cycle compounds supplemented to in vitro maturation medium influence in vitro fertilization, culture, and viability parameters of porcine oocytes and embryos. Theriogenology. 2004;62:311–322. doi: 10.1016/j.theriogenology.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]
- You J, Kim J, Lim J, Lee E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology. 2010;74:777–785. doi: 10.1016/j.theriogenology.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, Du XB, Wang GQ, Yao WX, Zhao QM, Ye B, Wang R, Diao P, Zhang W, Wu HB, Zhao X, Wei YQ. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res. 2006;12:3193–3199. doi: 10.1158/1078-0432.CCR-05-2365. [DOI] [PubMed] [Google Scholar]


