Abstract
We hypothesized that supplementing finishing diets with palm oil would promote adipogenic gene expression and stearoyl-CoA desaturase (SCD) gene expression in subcutaneous (s.c.) and intramuscular (i.m.) adipose tissues of feedlot steers. Eighteen Angus and Angus crossbred steers were assigned to three groups of 6 steers and fed a basal diet (control), with 3% palm oil, or with 3% soybean oil, for 70 d, top-dressed daily. Tailhead s.c. adipose tissue was obtained by biopsy at 14 d before the initiation of dietary treatments and at 35 d of dietary treatments. At slaughter, after 70 d of dietary treatment, tailhead s.c. adipose tissue and i.m. adipose tissue were obtained from the longissimus thoracis muscle. Palm oil increased plasma palmitic acid and soybean oil increased plasma linoleic acid and α-linolenic acid relative to the initial sampling time. Expression of AMP-activated protein kinase alpha (AMPKα) and peroxisome proliferator-activated receptor gamma (PPARγ) increased between the initial and intermediate biopsies and declined thereafter (p<0.03). SCD gene expression did not change between the initial and intermediate biopsies but declined by over 75% by the final period (p = 0.04), and G-coupled protein receptor 43 (GPR43) gene expression was unaffected by diet or time on trial. Soybean oil decreased (p = 0.01) PPARγ gene expression at the intermediate sample time. At the terminal sample time, PPARγ and SCD gene expression was less in i.m. adipose tissue than in s.c. adipose tissue (p<0.05). AMPKα gene expression was less in s.c. adipose tissue of palm oil-fed steers than in control steers (p = 0.04) and CCAAT enhancer binding protein-beta (CEBPβ) gene expression was less in s.c. and i.m. adipose tissues of palm oil-fed steers than in soybean oil-fed steers (p<0.03). Soybean oil decreased SCD gene expression in s.c. adipose tissue (p = 0.05); SCD gene expression in palm oil-fed steers was intermediate between control and soybean oil-fed steers. Contrary to our original hypothesis, palm oil did not promote adipogenic gene expression in s.c. and i.m. adipose tissue.
Keywords: Adipose Tissue, Fatty Acids, Gene Expression, Palm Oil, Stearoyl-coenzyme A Desaturase
INTRODUCTION
Foods high in oleic acid (18:1n-9), including oleic acid-enriched beef, reduce risk factors for cardiovascular disease (Adams et al., 2010; Gilmore et al., 2011;2013). Higher concentrations of oleic acid also are positively correlated with overall palatability of beef (Westerling and Hedrick, 1979), whereas increased stearic acid (18:0) is the primary determinant of fat hardness (i.e., lipid melting point; Smith et al., 1998). Therefore, increasing monounsaturated fatty acids (MUFA) and decreasing saturated fatty acid (SFA) would increase healthfulness, palatability, and fat softness of animal products. The conversion of SFA to MUFA by the fatty acid Δ9 desaturase, stearoyl-CoA desaturase, accounts for the majority of MUFA in muscle and adipose tissues, i.e., the edible portions of beef carcasses (St. John et al., 1991; Duckett et al., 2009). Stearoyl-CoA desaturase (SCD) is encoded by the SCD gene, and previous studies have indicated that SCD gene expression and/or SCD activity is elevated in adipose tissues containing a high concentration of MUFA (Archibeque et al., 2005; Chung et al., 2007; Duckett et al., 2009).
We recently demonstrated that the SFA palmitic acid (16:0) and stearic acid (18:0) strongly promoted CCAAT enhancer binding protein-beta (CEBPβ) and carnitine-palmitoyl-CoA transferase-1beta (CPT1β) gene expression in intramuscular (i.m.) preadipocytes (Choi et al., 2015). Conversely, the MUFA oleic acid (18:1cis-9) and the polyunsaturated fatty acids (PUFA) linoleic (18:2n-6) and α-linolenic acid (18:3n-3) depressed SCD gene expression. Therefore, we hypothesized that supplementing the diets of beef cattle with palm oil, rich in palmitic acid, would increase adipogenic gene expression in subcutaneous (s.c.) and i.m. adipose tissues. We previously demonstrated that, relative to soybean oil, feeding 3% palm oil to cattle increased s.c. adipocyte size and the activities of nicotinamide adenine dinucleotide phosphate (NADP)-malate dehydrogenase and glucose-6-phosphate dehydrogenase (Choi et al., 2013). Additionally, palm oil, which also is rich in oleic acid, decreased the ratio of palmitoleic acid (16:1n-7) to stearic acid (an index of SCD activity), so we further hypothesized the palm oil would decrease SCD gene expression. Soybean oil also was tested because it is rich in PUFA and also would be expected to decrease SCD gene expression.
In this study, we documented specific gene expression in s.c. and i.m. adipose tissue depots over the period of time when steers typically accumulate carcass adipose tissue, carnitine, and the genes CEBPβ, SCD, peroxisome proliferator-activated receptor gamma (PPARγ), AMP-activated protein kinase alpha (AMPKα), and G-coupled protein receptor 43 (GPR43) were selected as genetic markers of adipose tissue differentiation. The CEBPβ gene product is involved in adipocyte proliferation, metabolism, and differentiation, and binds to promoter regions of adipogenic genes (Cao et al., 1991). Similarly, the PPARγ gene product is required for proper adipose tissue development (Tontonoz et al., 1994) and is expressed abundantly in differentiating bovine adipocytes (Smith et al., 2009). AMPKα activity inactivates energy-consuming processes such as fatty acid biosynthesis, while activating energy-producing processes such as fatty acid oxidation (Hardie, 2007). In response to activation by volatile fatty acids, GPR43 activates cellular responses that subsequently depress triacylglycerol turnover (Hardie, 2007), thereby promoting adiposity. Acetate and propionate activate the GPR43 receptor (Brown et al., 2003), which leads to a reduction in lipolysis (Ge et al., 2008). This in turn increases lipid accumulation in adipocytes and promotes metabolism of fatty acids and glucose in other tissues (Kimura et al., 2013). These effects may be attenuated by AMPKα (Yin et al., 2003), as expression of AMPKα promotes fatty acid oxidation by up-regulating PPARγ coactivator-1alpha (PGC-1α) gene expression (Wan et al., 2014). Because palm oil increases carcass adiposity and lipogenesis in s.c. adipose tissue (Choi et al., 2013), we hypothesized that palm oil would increase the expression of genes associated with adipogenesis (CEBPβ, PPARγ, GPR43, and SCD), and decrease AMPKα gene expression. We specifically investigated the effects of palm and soybean oil on adipose tissue depots because s.c. adipose tissue is readily accessible by biopsy technique, and because both s.c. and i.m. adipose tissues are important components of beef and beef products.
MATERIALS AND METHODS
Study design
The experimental procedures were approved by the Texas A&M University Animal Care and Use Committee, Office of Research Compliance. We previously reported carcass quality, fatty acid composition, and de novo fatty acid synthesis in s.c. adipose tissue taken at slaughter in this same group of cattle (Choi et al., 2013). Briefly, 15 Angus and 13 Angus×Brahman crossbred steers from the Texas A&M University Research Center at McGregor, TX were grazed on native pasture until 12 mo of age. Steers were assigned to three groups of 9 or 10 steers at an average body weight (BW) of 364.5±7.6 kg. Steers were blocked by breed type, stratified by BW, and assigned randomly to dietary treatments within block and strata, such that resultant treatment groups consisted of 5 Angus steers and 4 to 5 Angus×Brahman crossbred steers, balanced for BW. Steers were adapted to a corn/milo finishing diet (Table 1) and Calan gates over a 14-d period and then fed the finishing diet free choice until they achieved a BW of 473.6±7.9 kg (approximately 15 mo of age). Steers then were fed the basal diet without additional fat (control), the basal diet with 3% palm oil, or the basal diet with 3% soybean oil, added as top dressings. The palm oil contained 43.6% palmitic acid, 4.6% stearic acid, 37.1% oleic acid, 10.4% linoleic acid, and 0.2% α-linolenic acid. The soybean oil contained 10.7% palmitic acid, 4.6% stearic acid, 22.8% oleic acid, 51.8% linoleic acid, and 6.9% α-linolenic acid. When mixed with the basal diet, the palm oil diet contained 20.4 g/kg palmitic acid and 21.1 g/kg oleic acid and the soybean oil diet contained 16.1 g/kg oleic acid, 28.9 g/kg linoleic acid, and 2.6 g/kg α-linolenic acid (Table 2). Thus, palm oil was considered a source of supplementary palmitic and oleic acid, whereas soybean oil was a source of oleic, linoleic, and α-linolenic acid.
Table 1.
Ingredients and chemical composition of the corn-based, finishing diet of Angus steers
| Variable | Basal (control) diet |
|---|---|
| Ingredients | |
| Ground milo | 20.00 |
| Ground corn | 48.05 |
| Cottonseed meal | 6.00 |
| Cottonseed hulls | 15.00 |
| Molasses | 7.50 |
| Limestone | 0.96 |
| Trace mineralized salt1 | 0.56 |
| Dicalcium phosphate | 0.23 |
| Potassium chloride | 0.16 |
| Zinc oxide | 0.01 |
| Ammonium sulfate | 0.25 |
| Vitamin premix2 | 0.08 |
| R-15003 | 1.20 |
| Total percentage | 100.00 |
| Nutritional composition (% as fed4) | |
| Dry matter | 89.13 |
| Crude protein | 11.16 |
| Calcium | 0.52 |
| Phosphorous | 0.36 |
| Energy content | |
| NEm (Mcal/kg) | 1.78 |
| NEg (Mcal/kg) | 1.17 |
NEm, net energy of maintenance; NEg, net energy of gain.
Trace mineralized salt: NaCl, 98%; Zn, 0.35%; Mn 0.28%; Fe, 0.175%; Cu, 0.035%; I, 0.007%; Co, 0.0007%.
Vitamin premix: vitamin A, 2,200,000 IU/kg; vitamin D, 1,100,000 IU/kg; vitamin E, 2,200 IU/kg.
R-1500: 1.65g monensin sodium (Rumensin) per kg.
Calculated values based on NRC (2000).
Table 2.
Fatty acid content (g/kg feed) of the basal (control) diet and diets containing 3% added palm oil or 3% soybean oil
| Fatty acid | Diet (g/kg feed) | ||
|---|---|---|---|
|
| |||
| Control1 | Palm oil2 | Soybean oil2 | |
| 14:0 | 0.14 | 0.46 | 0.15 |
| 16:0 | 6.53 | 20.45 | 9.75 |
| 16:1cis-9 | 0.12 | 0.17 | 0.14 |
| 18:0 | 1.01 | 2.47 | 2.18 |
| 18:1cis-9 | 9.23 | 21.06 | 16.08 |
| 18:1cis-11 | 0.34 | 0.54 | 0.79 |
| 18:2n-6 | 13.44 | 16.78 | 28.97 |
| 18:3n-3 | 0.60 | 0.66 | 2.69 |
Basal diet contained 31.9 g total lipid/kg.
Palm oil and soybean oils were added at 3% of the diet, added as top dressing.
Steers were fed the experimental diets for 10 wk until they attained an average body weight of 564.5±8.0 kg. Biopsy samples of s.c. adipose tissue were taken as described previously (Martin et al., 1999) 14 d before the initiation of dietary treatment and at 35 d of dietary treatment. Briefly, the area was shaved and washed with iodine solution and ethanol. Three injections of lidocaine were used to anesthetize the area and a triangular flap of skin was lifted and underlying s.c. adipose tissue was removed. The flap was stapled shut and rinsed with 70% ethanol.
After 70 d of dietary treatment, the steers were transported to and harvested at the Texas A&M University Rosenthal Meat Science and Technology Center. The 8th to 11th longissimus thoracis rib section was removed immediately after the hide was removed (approximately 20 min postmortem). The entire rib muscle section was placed in oxygenated 37°C, Kreb Henseleit buffer containing 5 mM glucose and transported to the laboratory. Intramuscular adipose tissue was dissected from the muscle, snap frozen in liquid nitrogen, and stored at −80°C until used for RNA extraction. Subcutaneous adipose tissue was removed from the tailhead region, adjacent to previous biopsy sites, snap frozen, and stored at −80°C.
RNA extraction and real-time polymerase chain reaction
Gene expression in s.c. and i.m. adipose tissues was measured using the primers as described previously (Smith et al., 2012). Total RNA was extracted with Tri Reagent (Sigma Chemicals, St. Louis, MO, USA), and the concentration of RNA was quantified with a NanoDrop ND-100 Spectrophotometer (Thermo Scientific, Washington, DC, USA). Quantitative real-time polymerase chain reaction (qPCR) was used to analyze the expression of CEBPβ, SCD, PPARγ, AMPKα, and GPR43. Primers used for qPCR are listed in Table 3. The 40S ribosomal protein S9 (RPS9) was used as an endogenous gene expression control. Complementary DNA (cDNA) was produced from 1 μg RNA using TaqMan Reverse Transcriptase Reagents (Applied Biosystems, Foster City, CA, USA) by the protocol recommended by the manufacturer. Random hexamers were used as primers in cDNA synthesis. Measurement of the relative quantity of the cDNA of interest was carried out using TAMRA PCR Master Mix (Applied Biosystems, USA), appropriate forward and reverse primers, and 1 μL of the cDNA mixture. Assays were performed in the GeneAmp 5700 Sequence Detection System (Applied Biosystems, USA) using thermal cycling parameters recommended by the manufacturer (40 cycles of 15 s at 95°C and 1 min at 60°C). Titration of each set of mRNA primers against increasing amounts of cDNA yielded linear responses with slopes between −2.8 and −3.0. In order to reduce the effect of assay-to-assay variation in the qPCR assay, all values were calculated relative to a calibration standard run on every qPCR assay. Commercially available eukaryotic RPS9 DNA primers and probes were used as an endogenous control (Applied Biosystems, USA; GeneBank Accession #X03205). The ABI Prism 7000 detection system (Applied Biosystems, USA) was used to perform the assay utilizing the thermal cycling variables recommended by the manufacturer (50 cycles of 15 s at 95°C and 1 min at 60°C).
Table 3.
Forward and reserve primers and probes for real-time polymerase chain reaction for specific gene mRNA
| Maker gene | Gene no. | Sequence (5′ to 3′) | |
|---|---|---|---|
| RPS9 | DT860044 | Forward | GAGCTGGGTTTGTCGCAAAA |
| Reverse | GGTCGAGGCGGGACTTCT | ||
| Taqman probe | 6FAM-ATGTGACCCCGCGGAGACCCTTC-TAMRA | ||
| AMPKα | NM_001109802 | Forward | ACCATTCTTGGTTGCTGAAACTC |
| Reverse | CACCTTGGTGTTTGGATTTCTG | ||
| Taqman probe | 6FAM-CAGGGCGCGCCATACCCTTG-TAMRA | ||
| CEBPβ | NM_176788 | Forward | CCAGAAGAAGGTGGAGCAACTG |
| Reverse | TCGGGCAGCGTCTTGAAC | ||
| Taqman probe | 6FAM-CGCGAGGTCAGCACCCTGC-TAMRA | ||
| GPR43 | FJ562212 | Forward | GGCTTTCCCCGTGCAGTA |
| Reverse | ATCAGAGCAGCGATCACTCCAT | ||
| Taqman probe | 6FAM-AAGCTGTCCCGCCGGCCC-TAMRA | ||
| PPARγ | NM_181024 | Forward | ATCTGCTGCAAGCCTTGGA |
| Reverse | TGGAGCAGCTTGGCAAAGA | ||
| Taqman probe | 6FAM-CGCGAGGTCAGCACCCTGC-TAMRA | ||
| SCD | AB075020 | Forward | TGCCCACCACAAGTTTTCAG |
| Reverse | GCCAACCCACGTGAGAGAAG | ||
| Taqman probe | 6FAM-CCGACCCCCACAATTCCCG-TAMRA |
RPS9, ribosomal protein S9; AMPKα, AMP-activated protein kinase alpha; CEBPβ, CCAAT enhancer binding protein-beta; GPR43, G-coupled protein receptor 43; PPARγ, peroxisome proliferator-activated receptor gamma; SCD, stearoyl-CoA desaturase.
Fatty acid composition of the diet and adipose tissue
Total lipids of the diets (5 g) and i.m. adipose tissue (100 mg) were extracted and fatty acid methyl esters (FAME) were prepared as described previously (Archibeque et al., 2005). The diets were sampled at three intervals and the data pooled (Table 2). FAME were analyzed by GC-FID (model CP-3800 equipped with a CP-8200 auto-sampler; Varian Inc, Palo Alto, CA, USA). Separation of FAME was accomplished on a fused silica capillary column (100 m×0.25 mm ID) (model CP-3800, Varian Inc, USA) with the helium as carrier gas (flow rate = 1.7 mL/min). One microliter of sample was injected with the split ratio of 100:1 at 270°C. Oven temperature was set at 165°C for 65 min and then increased to 235°C (°C/min) and held for 15 min. Flame ionization detector (FID) detected the signal at 270°C. An authentic standard (GLC 68D, Nu-chek Prep, Waterville, MN, USA) was used to confirm the identity of each peak.
Statistical analyses
Data were analyzed using the general linear mixed models (GLMM) procedures of IBM SPSS version 20.0 (IBM Co., Armonk, NY, USA) as appropriate for completely randomized designs. The model tested the main effects of diet, time on feed, and the diet×time on feed interaction. At slaughter, the adipose tissue depot (s.c. vs i.m.), diet, and adipose tissue×diet effects were tested. Means were separated by the Fisher’s protected least significant difference and considered different at p<0.05, although trends (p≤0.10) also are discussed.
RESULTS
Palm oil had no effect on average daily gain (ADG), average daily intake (ADI), or the gain:feed ratio (G:F), whereas soybean oil depressed ADG (p = 0.02), ADI (p = 0.04) and G:F (p = 0.05), relative to control and palm oil-fed steers (Table 4). However, final BW (p = 0.19) and carcass weight (p = 0.45) were unaffected by supplemental dietary oils. Marbling scores tended (p<0.09) to be different between palm oil-fed steers (Modest09) and soybean oil-fed steers (Small55). Neither palm oil nor soybean oil significantly affected 12th thoracic rib fat thickness or kidney, pelvic, and heart fat (p≥0.15).
Table 4.
Growth and carcass characteristics of feedlot steers fed a basal finishing diet (n = 10) or diets supplemented with 3% palm oil (n = 9) or 3% soybean oil (n = 9)
| Item | Treatment1 | SEM | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Control | Palm oil | Soybean oil | |||
| Initial live weight (kg) | 469.5 | 470.9 | 478.2 | 7.9 | 0.29 |
| Final live weight (kg) | 567.7 | 571.4 | 553.6 | 8.0 | 0.19 |
| ADG (kg/d) | 1.06a | 1.10a | 0.76b | 0.06 | 0.02 |
| ADI (kg/d) | 10.2a | 10.0a | 9.0b | 0.3 | 0.04 |
| G:F | 0.10a | 0.11a | 0.08b | 0.01 | 0.05 |
| Marbling score1 | 479.0 | 508.9 | 455.6 | 14.1 | 0.09 |
| 12th rib fat thickness (cm) | 1.87 | 1.95 | 1.72 | 0.09 | 0.16 |
| Ribeye area (cm2) | 81.8 | 81.1 | 80.8 | 1.2 | 0.37 |
| KPH (%) | 2.30 | 2.61 | 2.50 | 0.12 | 0.15 |
SEM, standard error of the mean; ADG, average daily gain; ADI, average daily intake; G:F, gain:feed ratio; KPH, kidney, pelvic, and heart fat.
Marbling score: 400, small; 500, modest.
Means in rows not bearing a common superscript differ, p<0.05.
The proportion of palmitic acid in plasma increased between the initial and intermediate sampling periods in the palm oil-fed steers (diet×time p = 0.002) (Figure 1). There also was a main diet effect for plasma palmitic acid (p = 0.002); palmitic acid was highest in palm oil-fed steers and lowest in soybean oil-fed steers. Plasma oleic acid was lowest in the soybean oil-fed steers (diet p = 0.04), whereas plasma linoleic acid and α-linolenic acid were highest in soybean oil-fed steers at the intermediate and final sampling periods (diet×time p = 0.05 and 0.01, respectively) (Figure 1). Dietary treatment effects were not significant for stearic acid, palmitoleic acid, or cis-vaccenic acid (18:1n-7; p≥0.17) (data not shown in tabular form).
Figure 1.
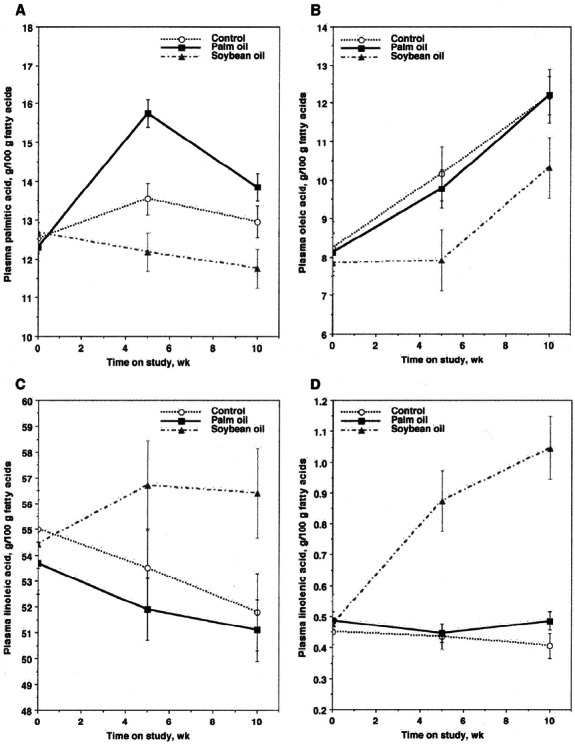
Plasma palmitic acid (A), oleic acid (B), linoleic acid (C), and α-linolenic acid (D) in steers fed a corn-based, control diet or the corn-based diet containing 3% palm oil or 3% soybean oil. p-values: (A) diet p = 0.002, time p = 0.001, diet×time p = 0.002; (B) diet p = 0.04, time p = 0.0001, diet×time p = 0.38; (C) diet p = 0.004, time p = 0.24, diet×time p = 0.05; (D) diet p = 0.001, time p = 0.06, diet×time p = 0.01.
The diet×time interaction was not significant for any gene measured in this study, so only the main effects of diet, time, and adipose tissue depot are reported. AMPKα and PPARγ gene expression in s.c. adipose tissue increased between the initial and intermediate biopsies and declined thereafter (p = 0.01; data pooled across diets) (Table 5). SCD gene expression did not change between the initial and intermediate s.c. adipose tissue biopsies but declined by over 75% between the intermediate and final periods (p = 0.04). There were no differences across time on study for CEBPβ or GPR43 gene expression.
Table 5.
Gene expression in biopsy samples of tailhead subcutaneous adipose tissue (initial, d–14; intermediate, d 35) and tailhead subcutaneous adipose tissue taken at slaughter (d 70) (data pooled across diets)
| Gene | Day1 | SEM | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| d–14 | d 35 | d 70 | |||
| AMPKα | 1.58b | 4.44a | 2.09b | 0.43 | 0.01 |
| CEBPβ | 1.35 | 1.69 | 1.78 | 0.22 | 0.16 |
| GPR43 | 3.81 | 1.66 | 3.12 | 0.54 | 0.12 |
| PPARγ | 2.86b | 9.44a | 1.10b | 1.10 | 0.01 |
| SCD | 2.45a | 2.89a | 0.86b | 0.36 | 0.04 |
AMPKα, AMP-activated protein kinase alpha; CEBPβ, CCAAT enhancer binding protein-beta; GPR43, G-coupled protein receptor 43; PPARγ, peroxisome proliferator-activated receptor gamma; SCD, stearoyl-CoA desaturase.
Initial, 14 d prior to initiating feeding trial; Intermediate, 35 d on feeding trial; Slaughter, 70 d on feeding trial.
Means in rows not bearing a common superscript differ, p<0.05.
At the intermediate sampling period (d 35), soybean oil decreased PPARγ gene expression (Table 6). Palm oil tended (p = 0.08) to increase AMPKα gene expression. Neither palm oil nor soybean oil affected CEBPβ, GPR43, or SCD gene expression at the intermediate sampling period (p≥0.15). However, CEBPβ gene expression was numerically lowest in s.c. adipose tissue of the soybean oil-fed steers, which was consistent with the low level of PPARγ gene expression observed adipose tissue of the soybean oil-fed steers.
Table 6.
Diet main effects for gene expression in subcutaneous adipose tissue of feedlot steers fed a basal finishing diet (n = 6) or the basal diet supplemented with 3% palm oil (n = 6) or 3% soybean oil (n = 6) for 35 d (intermediate sample)
| Gene | Treatment | SEM | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Control | Palm oil | Soybean oil | |||
| AMPKα | 4.30 | 6.65 | 2.82 | 0.90 | 0.08 |
| CEBPβ | 1.17 | 2.46 | 1.37 | 0.35 | 0.17 |
| GPR43 | 3.47 | 1.05 | 0.91 | 0.65 | 0.15 |
| PPARγ | 13.21a | 13.84a | 2.91b | 2.11 | 0.01 |
| SCD | 3.25 | 2.39 | 3.01 | 0.68 | 0.31 |
AMPKα, AMP-activated protein kinase alpha; CEBPβ, CCAAT enhancer binding protein-beta; GPR43, G-coupled protein receptor 43; PPARγ, peroxisome proliferator-activated receptor gamma; SCD, stearoyl-CoA desaturase.
Means in rows not bearing a common superscript differ, p<0.05.
There were greater proportions of SFA in i.m. adipose tissue and correspondingly lesser proportions of MUFA in i.m. adipose tissue, relative to s.c. adipose tissue (Table 7). Palm oil decreased palmitoleic acid in s.c. adipose tissue and a similar tendency was observed in i.m. adipose tissue (p = 0.10). Similarly, the lowest proportion of cis-vaccenic acid was observed in s.c. and i.m. adipose tissues of palm oil-fed steers (p≤0.04). Intramuscular adipose tissue of soybean oil-fed steers tended (p = 0.10) to have a greater proportion of linoleic acid than control and palm oil-fed steers.
Table 7.
Tissue and diet effects for fatty acid composition and gene expression in subcutaneous and intramuscular adipose tissues taken at slaughter from feedlot steers fed a basal finishing diet (n = 6) or the basal diet supplemented with 3% palm oil (n = 6) or 3% soybean oil (n = 6)
| Fatty acid (g/100 g total fatty acids) | Subcutaneous adipose tissue1 | Intramuscular adipose tissue | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Control | Palm oil | Soybean oil | SEM | p-value | Control | Palm oil | Soybean oil | SEM | p-value | |
| 16:02 | 27.91 | 26.95 | 26.70 | 0.37 | 0.09 | 31.88 | 32.22 | 31.43 | 0.19 | 0.14 |
| 16:1n-73 | 5.00a | 3.73b | 4.30ab | 0.24 | 0.05 | 3.66 | 3.32 | 3.62 | 0.15 | 0.10 |
| 18:02 | 10.43b | 12.55a | 12.58a | 0.54 | 0.02 | 14.72 | 15.00 | 14.82 | 0.47 | 0.39 |
| 18:1n-93 | 42.94 | 42.70 | 42.85 | 0.60 | 0.22 | 34.92 | 35.91 | 35.03 | 0.54 | 0.26 |
| 18:1n-73 | 1.83a | 1.36b | 1.46b | 0.07 | 0.01 | 0.97a | 0.50b | 0.66ab | 0.09 | 0.04 |
| 18:2n-6 | 1.84 | 1.90 | 2.04 | 0.08 | 0.18 | 1.67 | 1.63 | 1.88 | 0.07 | 0.10 |
| 18:3n-33 | 0.08 | 0.07 | 0.10 | 0.01 | 0.22 | 0.02 | 0.01 | 0.01 | 0.01 | 0.17 |
| 18:2cis-9, trans-113 | 0.67 | 0.60 | 0.65 | 0.04 | 0.25 | 0.04 | 0.04 | 0.01 | 0.02 | 0.17 |
| AMPKα | 2.78a | 2.19b | 2.46ab | 0.25 | 0.04 | 2.16 | 1.93 | 1.84 | 0.21 | 0.31 |
| CEBPβ | 1.63ab | 1.14b | 2.72a | 0.35 | 0.03 | 1.51ab | 0.74b | 1.98a | 0.41 | 0.01 |
| GPR43 | 2.99 | 3.83 | 3.08 | 0.92 | 0.24 | 3.50 | 4.89 | 1.65 | 1.06 | 0.17 |
| PPARγ3 | 1.00 | 1.26 | 1.02 | 0.21 | 0.31 | 0.58 | 0.43 | 0.41 | 0.09 | 0.25 |
| SCD3 | 1.85a | 1.04ab | 0.66b | 0.14 | 0.05 | 0.08 | 0.13 | 0.04 | 0.03 | 0.16 |
SEM, standard error of the mean; AMPKα, AMP-activated protein kinase alpha; CEBPβ, CCAAT enhancer binding protein-β; GPR43, G-coupled protein receptor 43; PPARγ, peroxisome proliferator-activated receptor gamma; SCD, stearoyl-CoA desaturase.
Data for subcutaneous adipose tissue derived from Choi et al. (2013).
Intramuscular adipose tissue > subcutaneous adipose tissue, p<0.05 (data pooled across dietary treatments).
Intramuscular adipose tissue < subcutaneous adipose tissue, p<0.05 (data pooled across dietary treatments).
Means in rows not bearing a common superscript differ, p<0.05.
In samples collected at slaughter, PPARγ and SCD gene expression was less in i.m. adipose tissue than in s.c. adipose tissue (p<0.05) (Table 6), and SCD mRNA was barely detectable in i.m. adipose tissue. Palm oil decreased AMPKα gene expression in s.c. adipose tissue (p = 0.04). CEBPβ gene expression was higher in s.c. and i.m. adipose tissue of soybean oil-fed steers than in palm oil-fed steers and was intermediate in adipose tissues of control steers (p≤0.03). Soybean oil decreased SCD gene expression in s.c. adipose tissue (p = 0.05); SCD gene expression in palm oil-fed steers was intermediate between control and soybean oil-fed steers.
DISCUSSION
In a study with Korean Hanwoo cattle (Song et al., 2010), 7% supplementary soybean oil (DM basis) depressed feed intake, ADG, and G:F, similar to results of the current study. However, Engle et al. (2000) earlier reported that 4% supplementary soybean oil had no effect on ADG, ADI, or G:F, but depressed marbling scores, whereas Ludden et al. (2009) later reported that 5% supplemental soybean oil had no effect on production or carcass traits of Gelbvieh×Angus cross bred steers. The previous studies and the current study differed in many aspects of experimental design, but in none of these studies did soybean oil increase ADG, G:F, or marbling scores.
Changes in plasma fatty acid composition indicated at least partial absorption of fatty acids provided by the oil supplements. The proportion of plasma palmitic acid was greatest in palm oil-fed steers and proportions of linoleic acid and α-linolenic acid were greatest in the soybean oil-fed steers at the intermediate and final sampling times. The increase in plasma PUFA in the soybean oil-fed steers indicated that at least a portion of these fatty acids escaped ruminal biohydrogenation. We expected plasma oleic acid to be elevated in response to palm oil supplementation, but this was not observed. Instead, there was no difference in the proportion of plasma oleic acid between the control and palm oil-fed steers, even though palm oil provided an additional 12 g oleic acid per kg of feed.
In plasma from the control and palm oil-fed steers, oleic acid steadily increased and linoleic acid decreased over time. It is not possible to discern from these data if these changes in plasma fatty acid composition reflected changes in hepatic fatty acid metabolism or ruminal biohydrogenation. Steers supplemented with soybean oil grew less efficiently, suggesting that the supplemental dietary PUFA in soybean oil were affecting ruminal fermentation. Conversely, the increase in plasma oleic acid could have been caused by a possible increase in hepatic SCD activity with time on feed, but we have not documented the effects of time on feed on SCD gene expression or SCD activity in bovine liver.
Bovine s.c. adipose tissue is the primary site for fatty acid elongase and SCD activity in cattle (St. John et al., 1991; Archibeques et al., 2005), and the fatty acid elongase and Δ9 desaturase enzymes work in concert to convert palmitic acid first to stearic acid and subsequently to oleic acid. The elevation in adipose tissue stearic acid caused by palm oil is consistent with a rapid conversion of supplementary palmitic acid to stearic acid. Alternatively, palmitic acid can be desaturated to palmitoleic acid by SCD, and palmitoleic acid subsequently can be elongated to cis-vaccenic acid. However, palm and soybean oil depressed the proportions of both palmitoleic acid and cis-vaccenic acid in s.c. adipose tissue. These data indicate that palm and soybean oil depressed Δ9 desaturation of palmitic acid, a conclusion that is consistent with the reduction in SCD gene expression caused by the supplemental oils. Palm and soybean oil had lesser but similar effects on palmitoleic and cis-vaccenic acid in i.m. adipose tissue, although there was not a detectable effect of supplemental oils on SCD gene expression in i.m. adipose tissue.
Joseph et al. (2010) reported that adding high levels of corn oil (0.62 kg/d, or approximately 6%) to the diets of Angus steers depressed SCD, PPARγ, CEBPα, fatty acid synthase, and acetyl-CoA carboxylase gene expression, but increased lipoprotein lipase, and sterol regulatory element-binding protein gene expression; these effects were attributed to the high concentration of linoleic acid in the corn oil. The decrease in SCD gene expression and increase in CEBPβ gene expression we observed in s.c. adipose tissue taken slaughter in soybean oil-supplemented steers are similar to the results reported by Joseph et al. (2010) and provide additional support for the effects of dietary PUFA on adipose tissue gene expression during growth.
In these same cattle, we reported that, relative to palm oil, feeding 3% soybean oil to cattle decreased s.c. adipocyte size and the activities of NADP-malate dehydrogenase and glucose-6-phosphate dehydrogenase (Choi et al., 2013). In a separate study, we reported that the SFA palmitic acid and stearic acid strongly promoted CEBPβ and CPT1β gene expression in i.m. preadipocytes (Choi et al., 2015), whereas oleic acid and linoleic acid depressed SCD gene expression. We conclude that the depression in SCD gene expression in s.c. adipose tissue of soybean oil-supplemented steers was caused by the high concentration of linoleic acid in soybean oil. Furthermore, supplemental palm oil did not significantly depress SCD gene expression in this study, possibly because SFA in palm oil may attenuated the effects of oleic acid on SCD gene expression in vivo (Choi et al., 2015).
At the intermediate sampling period, AMPKα and CEBPβ gene expression was numerically higher in s.c. adipose tissue of palm oil-fed steers than in adipose tissue from control or soybean oil-fed steers. Although not statistically significant, these data are similar to results with cultured preadipocytes, in which AMPKα and CEBPβ gene expression was higher in cultures containing supplemental SFA than in cultures containing unsaturated fatty acids (Choi et al., 2015). Underwood et al. (2007) reported lower AMPK phosphorylation in muscle that had abundant i.m. lipid than in muscle low in i. m. lipid. We cannot discern from this or our previous study of gene expression in adipose tissue of growing cattle (Smith et al., 2012) whether changes in AMPKα mRNA with age or supplemental oil treatment were associated with greater AMPK activity, as AMPK protein amount and phosphorylation were not measured. The current data indicate that the decline in PPARγ and SCD gene expression between d 35 and d 70 was accompanied with a decrease in AMPKα gene expression as s.c. adipose tissue became less metabolically active in the older cattle. Similarly, the somewhat lower AMPKα gene expression in soybean oil-fed steers at the intermediate sampling point was consistent with an overall depression of adipose tissue metabolism by soybean oil.
We have demonstrated previously that PPARγ and SCD gene expression and catalytic activity are in less in i.m. adipose tissue than in s.c. adipose tissue (Archibeque et al., 2005; Choi et al., 2013), and the same results for gene expression were observed in this study. SCD gene expression was barely detectable in i.m. adipose tissue, so it was not possible to demonstrate an effect of supplemental oils in this tissue. In a previous study, we demonstrated that CEBPβ and PPARγ gene expression declined markedly between 14 and 16 mo of age, whereas GPR43 gene expression increased nearly 10-fold and SCD and AMPKα gene expression did not change during this period (Smith et al., 2012). Our previous (Martin et al., 1999; Smith et al., 2012) and current studies suggest that adipogenic gene expression declines in s.c. adipose during the late phases of carcass fattening. Similarly, gene expression associated with lipid filling of adipocytes (e.g., GPR43 gene expression) is elevated in fatter cattle, promoting additional carcass adiposity.
In conclusion, this study demonstrated that palm oil and soybean oil significantly affected gene expression in bovine s.c. and i.m. adipose tissues, consistent with our previous report on the effects of palm oil on lipid synthesis in vitro and carcass adiposity (Choi et al., 2013). However, contrary to original hypothesis, soybean oil more effectively depressed SCD gene expression than palm oil in s.c. adipose tissue. In countries such as Korea in which palm oil is an agri-byproduct, palm oil may be a cost-effective feed additive. In the U.S., 33% of feedlot nutritionists use plant oils as feed ingredients (Vasconcelos and Gaylean, 2007) but, because palm oil is not used in industrial food preparation, it is not available as an agri-byproduct. Thus, it may not be cost-effective as a feed ingredient in the USA.
ACKNOWLEDGMENTS
Supported by funds from the Beef Checkoff, the Texas A&M University/NSF-China collaborative research program, and the Rural Development Administration, Korea, PJ01091003.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- Adams TH, Walzem RL, Smith DR, Tseng S, Smith SB. Hamburger high in total, saturated and trans-fatty acids decreases HDL cholesterol and LDL particle diameter, and increases TAG, in mildly hypercholesterolaemic men. Br J Nutr. 2010;103:91–98. doi: 10.1017/S0007114509991516. [DOI] [PubMed] [Google Scholar]
- Archibeque SL, Lunt DK, Gilbert CD, Tume RK, Smith SB. Fatty acid indices of stearoyl-CoA desaturase do not reflect actual stearoyl-CoA desaturase enzyme activities in adipose tissues of beef steers finished with corn-, flaxseed-, or sorghum-based diets. J Anim Sci. 2005;83:1153–1166. doi: 10.2527/2005.8351153x. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Choi SH, Gang GO, Sawyer JE, Johnson BJ, Kim KH, Choi CW, Smith SB. Fatty acid biosynthesis and lipogenic enzyme activities in subcutaneous adipose tissue of feedlot steers fed supplementary palm oil or soybean oil. J Anim Sci. 2013;91:2091–2098. doi: 10.2527/jas.2012-5801. [DOI] [PubMed] [Google Scholar]
- Choi SH, Park SK, Johnson BJ, Chung KY, Choi CW, Kim KH, Kim WY, Smith B. AMPKα, C/EBPβ, CPT1β, GPR43, PPAR, and SCD gene expression in single- and co-cultured bovine satellite cells and intramuscular preadipocytes treated with palmitic, stearic, oleic, and linoleic acid. Asian Australas J Anim Sci. 2015;28:411–419. doi: 10.5713/ajas.14.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KY, Lunt DK, Kawachi H, Yano H, Smith SB. Lipogenesis and stearoyl-CoA desaturase gene expression and enzyme activity in adipose tissue of short- and long-fed Angus and Wagyu steers fed corn- or hay-based diets. J Anim Sci. 2007;85:380–387. doi: 10.2527/jas.2006-087. [DOI] [PubMed] [Google Scholar]
- Duckett SK, Pratt SL, Pavan E. Corn oil or corn grain supplementation to steers grazing endophyte-free tall fescue. II. Effects on subcutaneous fatty acid content and lipogenic gene expression. J Anim Sci. 2009;87:1120–1128. doi: 10.2527/jas.2008-1420. [DOI] [PubMed] [Google Scholar]
- Engle TE, Spears JW, Fellner V, Odle J. Effects of soybean oil and dietary copper on ruminal and tissue lipid metabolism in finishing steers. J Anim Sci. 2000;78:2713–2721. doi: 10.2527/2000.78102713x. [DOI] [PubMed] [Google Scholar]
- Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, Tian H, Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- Gilmore LA, Walzem RL, Crouse SF, Smith DR, Adams TH, Vaidyanathan V, Cao X, Smith SB. Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. J Nutr. 2011;141:1188–1194. doi: 10.3945/jn.110.136085. [DOI] [PubMed] [Google Scholar]
- Gilmore LA, Crouse SF, Carbuhn A, Klooster J, Calles JA, Meade T, Smith SB. Exercise attenuates the increase in plasma monounsaturated fatty acids and high-density lipoprotein cholesterol but not high-density lipoprotein 2b cholesterol caused by high-oleic ground beef in women. Nutr Res. 2013;33:1003–1011. doi: 10.1016/j.nutres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Biochemistry. Balancing cellular energy. Science. 2007;315:1671–1672. doi: 10.1126/science.1140737. [DOI] [PubMed] [Google Scholar]
- Joseph SJ, Pratt SL, Pavan E, Rekaya R, Duckett SK. Omega-6 fat supplementation alters lipogenic gene expression in bovine subcutaneous adipose tissue. Gene Regul Syst Bio. 2010;19:91–101. doi: 10.4137/GRSB.S5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden PA, Kucuk O, Rule DC, Hess BW. Growth and carcass fatty acid composition of beef steers fed soybean oil for increasing duration before slaughter. Meat Sci. 2009;82:185–192. doi: 10.1016/j.meatsci.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Martin GS, Lunt DK, Britain KG, Smith SB. Postnatal development of stearoyl coenzyme A desaturase gene expression and adiposity in bovine subcutaneous adipose tissue. J Anim Sci. 1999;77:630–636. doi: 10.2527/1999.773630x. [DOI] [PubMed] [Google Scholar]
- NRC. Nutrient Requirements of Beef Cattle. 7th rev ed. National Academy Press; Washington, DC, USA: 2000. [Google Scholar]
- Smith SB, Yang A, Larsen TW, Tume RK. Positional analysis of triacylglycerols from bovine adipose tissue lipids varying in degree of unsaturation. Lipids. 1998;33:197–207. doi: 10.1007/s11745-998-0196-8. [DOI] [PubMed] [Google Scholar]
- Smith SB, Go GW, Johnson BJ, Chung KY, Choi SH, Sawyer JE, Silvey DT, Gilmore LA, Ghahramany G, Kim KH. Adipogenic gene expression and fatty acid composition in subcutaneous adipose tissue depots of Angus steers between 9 and 16 months of age. J Anim Sci. 2012;90:2505–2514. doi: 10.2527/jas.2011-4602. [DOI] [PubMed] [Google Scholar]
- Smith SB, Kawachi H, Choi CB, Choi CW, Wu G, Sawyer JE. Cellular regulation of bovine intramuscular adipose tissue development and composition. J Anim Sci. 2009;87(14 Suppl):E72–82. doi: 10.2527/jas.2008-1340. [DOI] [PubMed] [Google Scholar]
- Song MK, Jin GL, Ji BJ, Chang SS, Jeong J, Smith SB, Choi SH. Conjugated linoleic acids content in M. longissimus dorsi of Hanwoo steers fed a concentrate supplemented with soybean oil, sodium bicarbonate-based monensin, fish oil. Meat Sci. 2010;85:210–214. doi: 10.1016/j.meatsci.2010.01.001. [DOI] [PubMed] [Google Scholar]
- St John LC, Lunt DK, Smith SB. Fatty acid elongation and desaturation enzyme activities of bovine liver and subcutaneous adipose tissue microsomes. J Anim Sci. 1991;69:1064–1073. doi: 10.2527/1991.6931064x. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Underwood KR, Tong J, Zhu MJ, Shen QW, Means WJ, Ford SP, Paisley SI, Hess BW, Du M. Relationship between kinase phosphorylation, muscle fiber typing, and glycogen accumulation in longissimus muscle of beef cattle with high and low intramuscular fat. J Agric Food Chem. 2007;55:9698–9703. doi: 10.1021/jf071573z. [DOI] [PubMed] [Google Scholar]
- Vasconcelos JT, Galyean ML. Nutritional recommendations of feedlot consulting nutritionists: the 2007 Texas Tech University survey. J Anim Sci. 2007;85:2772–2781. doi: 10.2527/jas.2007-0261. [DOI] [PubMed] [Google Scholar]
- Wan Z, Root-McCaig J, Castellani L, Kemp BE, Steinberg GR, Wright DC. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity (Silver Spring) 2014;22:730–738. doi: 10.1002/oby.20605. [DOI] [PubMed] [Google Scholar]
- Westerling DB, Hedrick HB. Fatty acid composition of bovine lipids as influenced by diet, sex and anatomical location and relationship to sensory characteristics. J Anim Sci. 1979;48:1343–1348. [Google Scholar]
- Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3-L1 adipocytes. J Biol Chem. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]


