Abstract
To report the clinical presentation and outcomes of a series of patients who presented with abdominal/pelvic mass or pelvic pain and were diagnosed with a gastrointestinal stromal tumor (GIST). Retrospective data were collected of all patients who presented with an abdominal/pelvic mass or pelvic pain between January 2010 and July 2015 and who were ultimately diagnosed with a GIST. The patients' medical records were reviewed. A literature review was also conducted. The event free survival and overall survival was calculated for all patients using Kaplan Meier curve (SPSS19-SPSS Inc. USA). A total ten patients were identified with GIST during the study period. Eight of ten patients had a tumor in the small intestine, one in sigmoid colon and one in base of small bowel mesentry. The mean tumor size was 13.9 cm (range, 3.9 to 24 cm). A complete resection was achieved in all 10 patients. No patient had distance metastasis. There were no intraoperative complications. One patient developed postoperative intestinal fistula and was managed conservatively. All patients were treated with imatinib after surgery. The mean follow-up time was 18 months (range, 2 to 47 months). The seven of the 10 patients (70 %) with no evidence of disease, two (20 %) lost follow up and one patient developed recurrence during follow up period and was started on sunitinib and patient died during follow up period because of disease. Gastrointestinal stromal tumors should be considered in the differential diagnosis of patients presenting with an abdominal/pelvic mass or pelvic pain in Gynaecologic oncology department. In such unusual circumstances the complete resection and appropriate adjuvant treatment results in complete durable remission.
Keywords: Adnexal mass, Ovarian tumor, Gastrointestinal stromal tumor, Incidental finding
Introduction
Gastrointestinal stromal tumors (GISTs) are the mesenchymal neoplasms of gastrointestinal tract, whose tumor cell’s normal counterpart is interstitial cell of Cajal. This serves as pacemaker of gastrointestinal motility, providing an interface between autonomic nerve stimulation and muscle layer of gastrointestinal wall. [1] The crude incidence is suggested to be approximately 1.5 per 100,000 population per year. [2] GISTs can occur at any age with median occurrence at 60–65 years, and it is slightly more common in males then females. GISTs commonly arise from the stomach (>50 % of GISTs), small bowel (25–30 %), large bowel (10 %), and esophagus (5 %), or elsewhere in the abdominal cavity (omentum, mesentery) (5 %) [3]. GISTs typically express the receptor tyrosine kinase c-kit, also known as CD117 [4]. The prognosis of patients with a GIST is based on tumor size, mitotic rate, and organ of origin. The published literature on GISTs in patients who present with an abdominal or pelvic mass is limited to isolated case reports and single case series [5–13]. The goal of this retrospective study was to report on a series of patients who presented with a presumptive diagnosis of ovarian cancer in gynaecologic oncology department and all were finally diagnosed to have a GIST. We describe the clinical presentation, surgical management, adjuvant treatment, and overall outcome in these patients. This retrospective study also discusses the published literature on GISTs presenting as ovarian cancer.
Materials and Methods
Retrospective data was collected from hospital data base of the patients who presented with an abdominal or pelvic mass/pain to the department of gynaecologic oncology, Kidwai Memorial Institute of Oncology, Bangalore from January 2010 to July 2015. The departmental review board approval was obtained prior to study. Data were collected from the patient’s hospital files on age, presenting symptoms, tumor size on physical examination, findings on ultrasonography/CT abdomen and pelvis, serum CA-125 levels, intraoperative findings, primary site of disease, pathologic diagnosis, mitotic rate, adjuvant therapy, recurrence and disease status at last follow-up. The survival analysis was done from the date of hospital registration. The patients inclusion criteria in this study were those, who presented with abdominopelvic mass/pelvic pain, radiologic imaging showing a pelvic or abdominopelvic mass, and intraoperative/final histopathology revealing GIST. CA-125 normal values were defined as <35 U/mL postmenopausal and <90 U/ml in premenopausal women. The routine fine needle aspiration cytology (FNAC) is contraindicated in patients with suspected ovarian tumors to prevent upstaging of disease, and therefore FNAC was not done. Pathologic diagnosis was confirmed by conventional methods and through immunohistochemistry using the following markers: c-Kit (CD117), CD34, and DOG-1 protein. We also performed a literature review on PubMed database, using the following terms: “gastrointestinal stromal tumor,” “adnexal mass,” and “incidental finding.”
The event free survival and overall survival was calculated for all patients using Kaplan Meier curve (SPSS19-SPSS Inc. USA).
Results
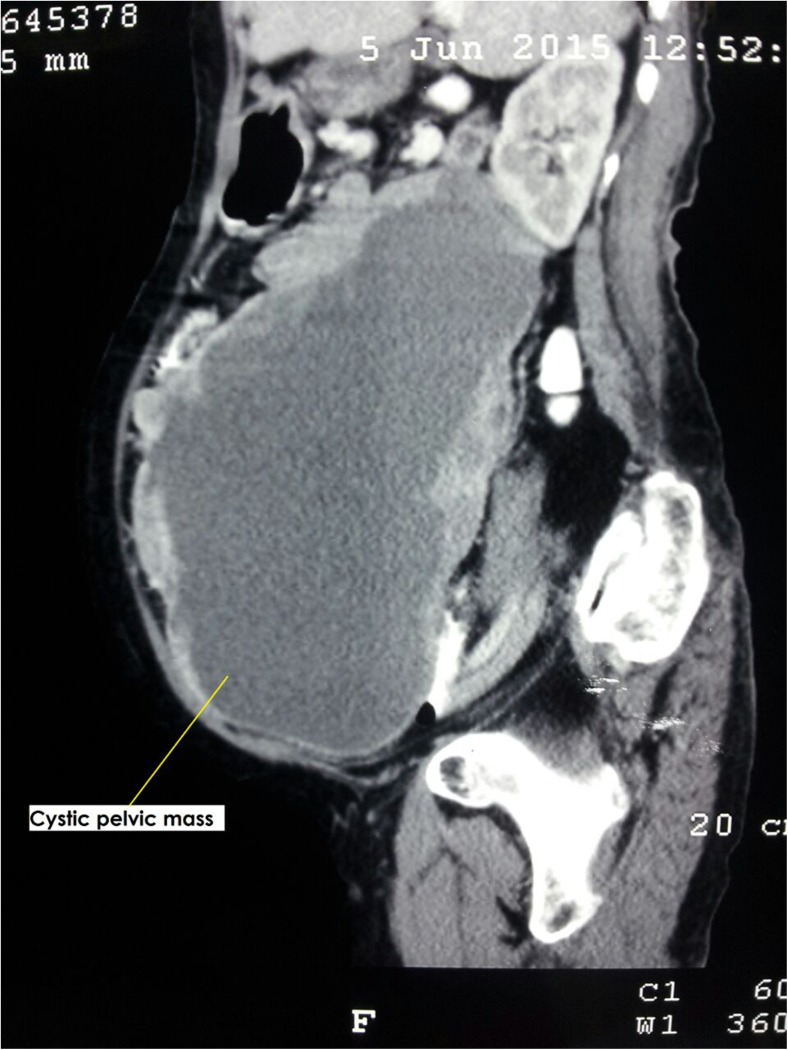
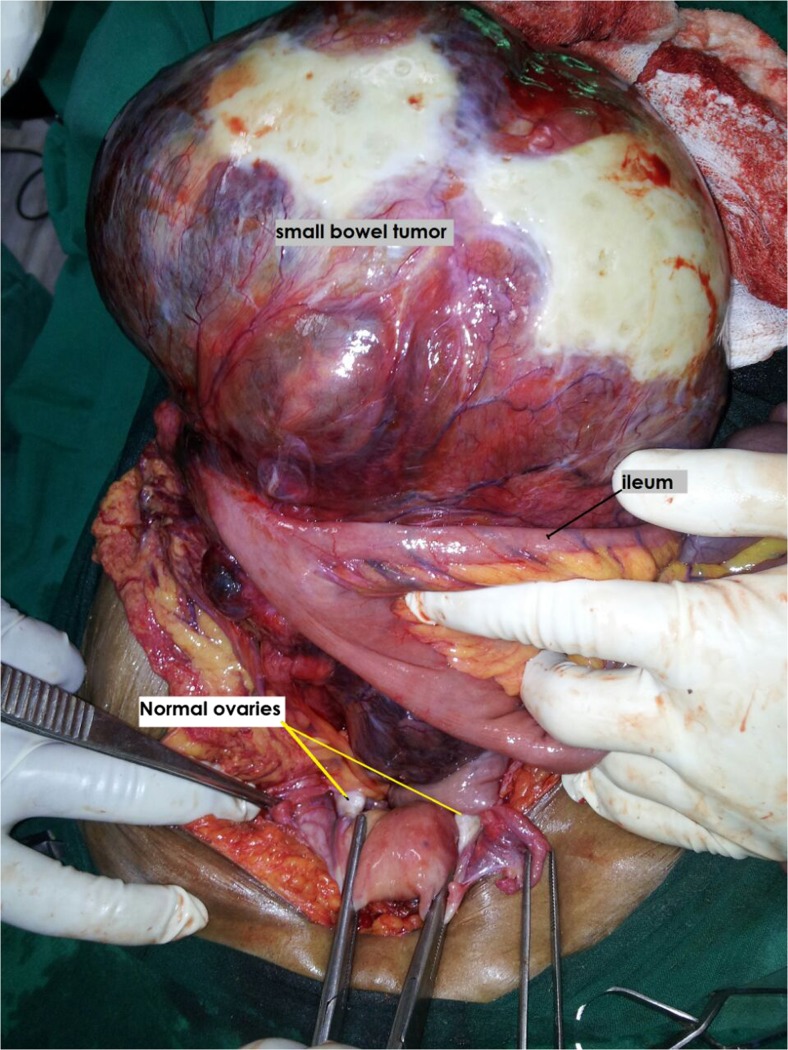
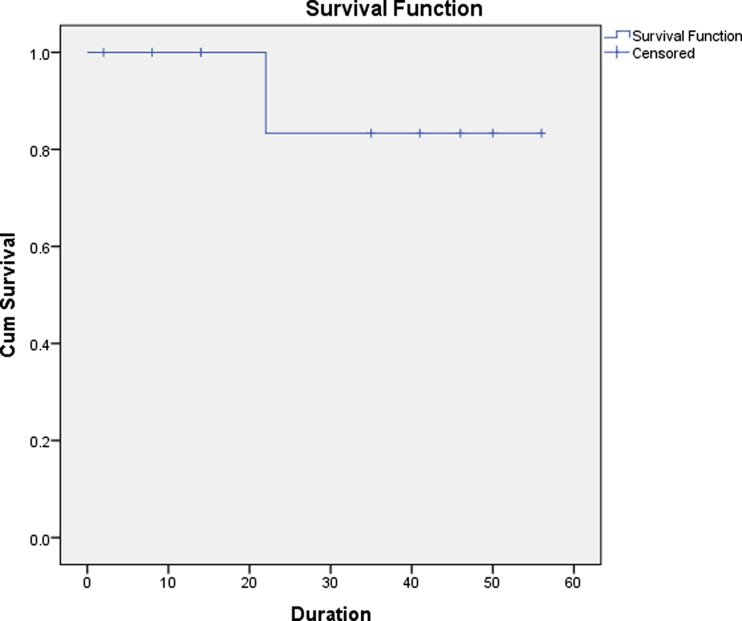
A total of 10 patients were diagnosed to have GIST during the study period. The mean age was 52 years (range, 41 to 70 years). All patients were referred to our institute with a presumptive diagnosis of ovarian tumor. Five patients presented with lower abdominal mass and associated pain ranging from 15 days to 3 months duration, four presented with lower abdominal mass and one patient with lower abdominal pain. On physical examination, nine of 10 patients had a palpable lower abdominal mass, whereas, all patients revealed palpable pelvic mass during per vaginal and per rectal examination. The mean size of the masses was 13.9 cm (range, 3.9 to 24 cm). All patients were suspected to have ovarian mass on ultrasonography/and or computed tomography (Fig. 1). The mean serum CA-125 level for all patients was 35.5 U/mL (range, 6.5 to 161 U/mL) (Table 1). All patients underwent an exploratory laparotomy with a preoperative diagnosis of ovarian tumor. At the time of surgery, six patients were found to have a mass arising from the ileum (Fig. 2), two patients were found to have a mass arising from the jejunum, one patient had sigmoid mass and another patient revealed mass in the base of small bowel mesentry. None of the patients had distant metastasis. Eight patients underwent small intestinal resection with end-to end anastomosis, one patient underwent sigmoid resection with colorectal anastomosis, and one of the patient with the small bowel mesentry mass, which was adherent to uterus needed complete excision of tumor with hysterectomy and bilateral salphingo oophorectomy to achieve no residual disease (Table 2). None of the patients had residual disease. There were no intraoperative complications. One patient developed postoperative intestinal fistula and was managed conservatively and was discharged after 19 days from hospital with complete recovery. The mean hospital stay for all patients was 6 days (range, 5 to 19 days). The final pathology/immunohistochemical analysis in all patients revealed GIST with positive stain for CD117, four were positive for CD-34, and DOG1 was positive in five patients (Table 2). The majority, eight of 10 were high risk and two patients intermediate risk GISTs. The clinical oncologist reviewed all cases. All patients received adjuvant therapy with imatinib (400 mg once daily). One of the patient who was on imatinib for 11 months developed recurrence at multiple sites in abdomen and was started on sunitinib. After three days, patient died because of advanced disease. At a median follow-up of 18 months (range, 2 to 45 months), seven patients are alive without any evidence of disease. Two patients were lost to follow up. One patient died of recurrent disease (Fig. 3). A total of approximately 2150 patients with ovarian tumor were operated at our institute during the time span of the study with GIST accounting for 10/2150 (0.45 %) of the cases.
Fig. 1.
CT scan image of abdominopelvic mass
Table 1.
Patient’s baseline characteristics
| Case no | Age in years | Month/Year | Symptoms | Imaging | Ca-125 in u/ml |
|---|---|---|---|---|---|
| 1 | 60 | Nov/2010 | Mass abdomen | Large necrotic lobulated pelvic abdominal mass of 24*12.9*21.5 cms – CT scan | 10.9 |
| 2 | 55 | June/2011 | Mass abdomen | 16*14*9.5 cms complex mass in pelvis – Ultrasound | 161 |
| 3 | 50 | Sep/2011 | Pain abdomen | Heterogeneous mass lesion in rt. adnexa of 3.9*3 cms – CT scan | 6.5 |
| 4 | 45 | Mar/2012 | Pelvic pain | 19*24*15 cms solid cystic mass. Omental caking present – CT scan | 59.8 |
| 5 | 41 | Aug/2012 | Mass abdomen | Solid cystic mass of 8*6*10 cms present in pelvis – Ultrasound | 32.2 |
| 6 | 39 | Oct/2013 | Pelvic pain | 7*12*10 cms pelvic mass – Ultrasound | 27.4 |
| 7 | 41 | May/2014 | Pelvic pain | Complex cystic mass of 20x20x18 cms in pelvis – Ultrasound | 6.8 |
| 8 | 55 | June/2014 | Pain abdomen | Cystic mass of 13x13x14 cms in pelvis – Ultrasound | 16.4 |
| 9 | 63 | Dec/2014 | Pelvic pain | 10x8x8 cms solid and cystic mass in pelvis – Ultrasound | 25 |
| 10 | 70 | May/2015 | Mass abdomen | Solid enhancing area, heterogeneous mass of 18x11x20 cms in pelvis, omental deposits present – CT scan | 8.5 |
cms Centimeters; CT Computerised tomography
Fig. 2.
Intraoperative photograph of ileal GIST
Table 2.
Surgical treatment, histopathology and immunohistochemistry (IHC) details
| Case no | Surgical procedure | Histopathology/mitosis per HPF | IHC CD-117 CD-34 DOG-1 | ||
|---|---|---|---|---|---|
| 1 | Tumor debulking with resection anastomosis of jejunum | Ileal GIST, mitotic rate > 5/50 | + | + | − |
| 2 | Laparotomy with segmental resection of ileum with end to end anastomosis | Ileal GIST, mitotic rate > 5/50 | + | − | − |
| 3 | Laparotomy with removal of ileal tumor and end to end anastomosis | Ileal GIST, mitotic rate > 5/50 | + | − | − |
| 4 | Laparotomy with tumor debulking with THA and BSO | Mesentric GIST, mitotic rate > 5/50 | + | + | − |
| 5 | Ileal mass excision with anastomosis | Ileal GIST, mitotic rate > 5/50 | + | − | + |
| 6 | Resection of ileal tumor with anastomosis | Ileal GIST, mitotic rate > 5/50 | + | + | + |
| 7 | Laparotomy, resection of jejunal tumor and end to end anastomosis | jejunal GIST, mitotic rate > 5/50 | + | − | − |
| 8 | Laparotomy with resection of ileal tumor, with anastomosis of ileum | Ileal GIST, mitotic rate < 5/50 | + | − | + |
| 9 | Sigmoidectomy with colorectal anastomosis | Sigmoid GIST, mitotic rate > 5/50 | + | + | + |
| 10 | Resection of ileal mass with End to end anastomosis | Ileal GIST, mitotic rate < 5/50 | + | − | + |
GIST Gastrointestinal stromal tumor; HPF high power field; TAH Total abdominal hysterectomy; BSO Bilateral salphingo oopharectomy; IHC Immunohistochemistry; CD Cluster differentiation; DOG Detected on GIST
Fig. 3.
Survival curve
Discussion
Gastrointestinal stromal tumors are rare tumors and may present a diagnostic dilemma in a women with abdominopelvic mass. Complete surgical resection is considered the standard treatment for localized tumors. Segmental intestinal resection is often necessary. When segmental resection is not possible, a wide, en bloc resection should be performed. Approximately 95 % of GISTs express the marker c-kit (CD117) [14]. The tyrosine kinase inhibitors such as imatinib (Gleevec) are the drug of choice for gastrointestinal stromal tumor, hence detection of receptor tyrosine kinase c-kit status in these group of patients plays critical role. The use of such tyrosine kinase inhibitors is considered the standard therapy in patients with metastatic or unresectable disease [15]. The preferred imaging modality in the evaluation of patients with a suspected GIST is enhanced computed tomography. The predominant pattern is a heterogeneously enhancing exophytic mass. Gastric lesions are characterized by a homogeneous enhancement, while small bowel lesions are more heterogeneous. Distal small bowel tumors and those arising from the sigmoid colon and rectum may be erroneously identified as gynecologic cancers [16–20]. Gross histopathologic findings typically include a fish-flesh appearance of soft consistency. Tumors can be necrotic or present with cystic degeneration. Morphologically, gastrointestinal stromal tumors can be made up of spindle cells (in more than two- third of cases), epithelioid cells, or both [21]. Other markers expressed by these tumors are CD34 (70 % of tumors), smooth muscle actin (30 %), h-caldesmon (60 %), and S-100 (5 %). The DOG1 (discovered on GIST) marker is a fragment of cDNA encoding a protein whose function is unknown. This marker may be helpful when this tumor is negative for c-kit; DOG1 has high sensitivity (94.4 %) and is positive in 30 % of those that are negative for c-kit [22, 23]. The prognosis of patients with a GIST is based on tumor size and number of mitoses. Tumors smaller than 2 to 5 cms with fewer than 5 mitoses per 50 HPF are considered very low risk for recurrence. Tumors smaller than 5 cm with 6 to 10 mitoses per 50 HPF and tumors measuring 5 to 10 cm with fewer than 5 mitoses per 50 HPF are considered intermediate risk. High-risk tumors are those measuring 5 to 10 cm with 6 to 10 mitoses per 50 HPF/and or tumor larger than 10 cm and any tumor with more than 10 mitoses per 50 HPF [24, 25]. GIST with intermediate risk/and or high risk needs adjuvant treatment with a tyrosine kinase inhibitor. The per oral Imatinib 400 mg once daily is drug of choice for three years [26]. These regimens have been shown to improve disease-free survival and overall survival. In patients treated with tyrosine kinase inhibitors, studies have documented a relapse free survival rate of 97 % at 1 year, a 5-year disease-free survival rate of 65 %, and a 5-year disease-specific survival rate of 68 % [27]. In patients with low risk GIST, the complete surgical resection is curative and postoperatively only regular clinical follow up is recommended. The lymph nodal metastasis is rare in GIST and the lymphadenectomy does not improve survival [28]. A review of literature revealed nine individual case reports and one case series study with six cases by Monoz et al. (4). The Table 3 details the comparison of published case series study with the current ten patients study. The mean age of the patients was 59 years in their study and 52 years in our study. In their study, mean CA-125 level was 41.2 U/ml and 35.5 U/ml in our study. In published case series study three patients had tumor in jejunum and three patients had tumor in ileum. In our study six patients had ileal GISTs, two had jejunal, one in sigmoid and one in small bowel mesentry. Two out of six patients (33.3 %) received Imatinib in Munoz study and nine of 10 patients (90 %) received Imatinib in present study as all patients belonged to intermediate and high risk GISTs. One patient defaulted for adjuvant treatment. In six patient series four are disease free, in our series seven patients are disease free, two lost follow up and one patient died because of disease..
Table 3.
Comparison of patient’s characteristics with published case series
| author | year | cases | Age(yrs) | Size(cms) | ca-125 (U/ml) |
mitotic rate (per HPF) |
residual disease |
adjuvant therapy |
follow up(mon) |
outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Munoz | 2010 | 6 | 42 50 79 54 46 65 |
6 × 6 × 4 6 × 4.5 × 3.5 27 × 34 12 × 10 × 7 33 × 32 10 |
23.6 15 1.62 16.5 34.4 156 |
<5/50 3/50 28/50 none none >12/50 |
no no no no yes no |
no no imatinib no imatinib no |
40 36 34 30 18 died |
disease free disease free disease free disease free disease control died |
| Present study | 2015 | 10 | 60 55 50 45 41 39 41 55 63 70 |
24 × 12.9 × 21.5 16x14x9.5 3.9 × 3 19x24x15 8x6x10 7x12x10 20x20x18 13x13x14 10x8x8 18x11x20 |
10.9 161 6.5 59.8 32.2 27.4 6.8 16.4 25 8.5 |
>5/50 >5/50 >5/50 >5/50 >5/50 >5/50 >5/50 <5/50 >5/50 <5/50 |
no no no no no no no no no no |
- imatinib imatinib imatinib imatinib imatinib/ sunitinib imatinib imatinib imatinib imatinib |
Lost Lost 47 40 36 Died 14 13 7 2 |
- - Disease free Disease free Disease free Died Disease free Disease free Disease free Disease free |
In summary, the gastrointestinal stromal tumor of small bowel and recto-sigmoid region presents as a heterogeneous complex pelvic tumor, the clinical and imaging features mimics like an ovarian tumor due to the absence of specific symptoms/and or imaging characters of GIST. The female patients with abdominopelvic mass presenting to the Gynecologic Oncology services, the GIST needs be considered as one of the differential diagnosis to the ovarian tumors. The intra-operative findings and frozen section study guides the treating surgeons in such circumstances. The complete resection of tumor with resection anastomosis of involved bowel and appropriate adjuvant therapy with imatinib results in better outcomes. Therefore, Gynaecologic Oncologists needs to be aware of this rare disease in a common clinical scenario of patients with a presumptive diagnosis of ovarian tumor.
Conclusion
Gastrointestinal stromal tumors should be considered in the differential diagnosis of patients presenting with an abdominal/pelvic mass or pelvic pain in Gynaecologic oncology department. In such unusual circumstances the complete resection and appropriate adjuvant treatment results in complete durable remission.
Compliance with Ethical Standards
Conflict of Interest
Authors declare no conflict of interest.
References
- 1.Kindblom L-G, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (gipact): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cell of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence clinical course, and prognostication in the pre-imatinib mesylate era – a populationbased study in Western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 3.Quek R, George S. Gastrointestinal stromal tumor: a clinical overview. Hematol Oncol Clin North Am. 2009;23:69–78. doi: 10.1016/j.hoc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Barnett CM, Corless CL, Heinrich MC. Gastrointestinal stromal tumors: molecular markers and genetic subtypes. Hematol Oncol Clin North Am. 2013;27:871–888. doi: 10.1016/j.hoc.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz M, Ramirez PT, Echeverri C, Alvarez LG, Palomino MA, Pareja LR. Gastrointestinal stromal tumors as an incidental finding in patients with a presumptivediagnosis of ovarian cancer. J Gynecol Oncol. 2012;23(1):48–52. doi: 10.3802/jgo.2012.23.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell JL, Kotwall CA, Wright BD, Temple RH, Jr, Ross SC, White WC. Gastrointestinal stromal tumor mimicking ovarian neoplasia. J Pelvic Surg. 2002;8:117–119. [Google Scholar]
- 7.Zighelboim I, Henao G, Kunda A, Gutierrez C, Edwards C. Gastrointestinal stromal tumor presenting as a pelvic mass. Gynecol Oncol. 2003;91:630–635. doi: 10.1016/j.ygyno.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Hsu S, Chen SS, Chen YZ. Gastrointestinal Stromal Tumors Presenting as Gynecological Tumors. Eur J Obstet Gynecol Reprod Biol. 2006;125:139–140. doi: 10.1016/j.ejogrb.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Carlomagno G, Beneduce P. A gastrointestinal stromal tumor (gist) masquerading as an ovarian mass. World J Surg Oncol. 2004;2:15. doi: 10.1186/1477-7819-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belics Z, Csapo Z, Szabo I, Papay J, Szabo J, Papp Z. Large gastrointestinal stromal tumor presenting as an ovarian tumor: a case report. J Reprod Med. 2003;48:655–658. [PubMed] [Google Scholar]
- 11.Pinto V, Ingravallo G, Cicinelli E, Pintucci A, Sambati GS, Marinaccio M, et al. Gastrointestinal stromal tumors mimicking gynecological masses on ultrasound: a report of two cases. Ultrasound Obstet Gynecol. 2007;30:359–361. doi: 10.1002/uog.4097. [DOI] [PubMed] [Google Scholar]
- 12.Matteo D, Dandolu V, Lembert L, Thomas RM, Chatwani AJ. Unusually large extraintestinal gist presenting as an abdomino- pelvic tumor. Arch Gynecol Obstet. 2008;278:89–92. doi: 10.1007/s00404-007-0528-9. [DOI] [PubMed] [Google Scholar]
- 13.Angioli R, Battista C, Muzii L, Terracina GM, Cafa EV, Sereni MI, et al. A gastrointestinal stromal tumor presenting as a pelvic mass: a case report. Oncol Rep. 2009;21:899–902. doi: 10.3892/or_00000301. [DOI] [PubMed] [Google Scholar]
- 14.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728–734. [PubMed] [Google Scholar]
- 15.Kubota T. Gastrointestinal stromal tumor (gist) and imatinib. Int J Clin Oncol. 2006;11:184–189. doi: 10.1007/s10147-006-0579-0. [DOI] [PubMed] [Google Scholar]
- 16.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283–304. doi: 10.1148/rg.232025146. [DOI] [PubMed] [Google Scholar]
- 17.Sandrasegaran K, Rajesh A, Rydberg J, Rushing DA, Akisik FM, Henley JD. Gastrointestinal stromal tumors: clinical, radiologic, and pathologic features. AJR Am J Roentgenol. 2005;184:803–811. doi: 10.2214/ajr.184.3.01840803. [DOI] [PubMed] [Google Scholar]
- 18.Gong JS, Zuo M, Yang P, Zang D, Zhang Y, Xia L, et al. Value of CT in the diagnosis and follow-up of gastrointestinal stromal tumors. Clin Imaging. 2008;32:172–177. doi: 10.1016/j.clinimag.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Anorectal gastrointestinal stromal tumors: ct and mr imaging features with clinical and pathologic correlation. AJR Am J Roentgenol. 2003;180:1607–1612. doi: 10.2214/ajr.180.6.1801607. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen MM, Corless CL, Debiec-Rychter M, et al. Gastrointestinal stromal tumors. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, et al., editors. Mertens F, eds. Lyon, France: IARC; 2013. pp. 164–167. [Google Scholar]
- 22.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 23.Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than kit in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437–446. doi: 10.1097/PAS.0b013e318186b158. [DOI] [PubMed] [Google Scholar]
- 24.Raut CP, DeMatteo RP. Prognostic factors for primary gist: prime time for personalized therapy? Ann Surg Oncol. 2008;15:4–6. doi: 10.1245/s10434-007-9634-y. [DOI] [PubMed] [Google Scholar]
- 25.Hassan I, You YN, Shyyan R, Dozois EJ, Smyrk TC, Okuno SH, et al. Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Ann Surg Oncol. 2008;15:52–59. doi: 10.1245/s10434-007-9633-z. [DOI] [PubMed] [Google Scholar]
- 26.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 27.Trent JC, Benjamin RS. New developments in gastrointestinal stromal tumor. Curr Opin Oncol. 2006;18:386–395. doi: 10.1097/01.cco.0000228747.02660.e2. [DOI] [PubMed] [Google Scholar]
- 28.Schnadig ID, Blanke CD. Gastrointestinal stromal tumors: imatinib and beyond. Curr Treat Options in Oncol. 2006;7:427–437. doi: 10.1007/s11864-006-0018-5. [DOI] [PubMed] [Google Scholar]