Abstract
Research on the prognostic value of lymph node ratio (LNR) in gastric cancer (GC) remains limited and controversial results were obtained. In this study, we aimed to evaluate whether LNR was an independent prognostic factor for gastric carcinoma. A retrospective review of a database of gastric cancer patients was performed to determine the effect of the LNR on the overall survival (OS) and the disease-free survival (DFS). Of the total 135 patients with gastric cancer who underwent resection between March 2012 and December 2013, 44 patients with non metastatic gastric cancer were eligible for analysis. Survival curves were estimated using the Kaplan-Meier method. Cox regression analyses, after adjustments for potential confounders, were used to evaluate the relationship between the LNR and survival. According to the cutoff point 0.37 (37 %), the one-year OS rate for LNR ≤ 37 % was significantly better than that for LNR > 37 % (91.3 % and 61.9 %, respectively, P = 0.02). The one-year DFS for LNR ≤ 37 % was significantly better than that for LNR > 37 % (91.3 % and 66.7 %, respectively, P = 0.027). In stratified and multivariate analyses adjusted for age, gender, histology and tumor status, a higher LNR was associated with high pN stage and so associated with worse OS and DFS. Thus, the LNR 37 % as a cutoff point was found not to be an independent factor for predicting the one-year OS or DFS in patients with non-metastatic GC. The LNR is a prognostic factor in GC. However, no single cut-off value was determined as an independent prognostic factor.
Keywords: Gastric cancer, Lymph node metastasis, Lymph node ratio, Prognosis
Introduction
Gastric cancer (GC) is the fourth most common malignancy with ~1 million patients diagnosed with GC worldwide per year, and the second leading cause of cancer-related mortality worldwide with 800,000 fatalities per year [1]. Although incidence and mortality rates are decreasing, survival remains poor with a 5-year survival rate of only ~20–25 % [2]. Accurate prediction of the prognosis of patients with GC is crucial, as surgery is the most important therapeutic approach [3]. Among the known prognostic factors of gastric carcinoma, depth of invasion and lymph node metastasis (nodal status) are considered to be the most important [4]. The most commonly used staging system of GC is proposed by the American Joint Committee on Cancer (AJCC) and is known as the AJCC tumor-node-metastases (TNM) staging system. According to the current UICC (the International Union Against Cancer)/AJCC (American Joint Committee on Cancer) staging system, which is the most widely used, nodal status is categorized based on the number of metastatic lymph nodes (pN0, no metastasis; pN1, 1–6 lymph nodes positive; pN2, 7–15 and pN3, >15) [5, 6]. However, the difficulty of the UICC/AJCC TNM classification is that for adequate N staging at least 15 lymph nodes should be retrieved. Literature expresses that in some countries, this amount of lymph nodes is not met by surgeons or pathologists, which can lead to understaging [7].
The metastatic lymph node ratio (LNR) is defined as the ratio of metastatic lymph nodes to the number of total lymph nodes pathologically examined. This figure seems to be superior to the absolute number of metastatic lymph nodes in predicting the prognosis and to be useful in reducing stage migration in types of solid cancers such as cancers of the stomach [8–10], breast [11], bladder [12], pancreas [13], and lung [14].
In a previous study we showed that LNR is a better prognostic factor than the absolute number of metastatic lymph nodes in cases of stage III rectal cancer. Also, we found a cutoff value of the LNR for predicting a prognosis for patients with rectal cancer [15].
In the present study, we aimed to evaluate whether LNR was an independent prognostic factor for gastric carcinoma. In addition, we tried to find a cutoff value of the LNR for predicting the prognosis for patients with gastric cancer.
Patients and Methods
Patient Selection
A retrospective review of a prospectively-collected database of 135 patients with GC who underwent resection between March 2012 and December 2013 was performed to determine the effect of the LNR on overall survival (OS) and disease-free survival (DFS).
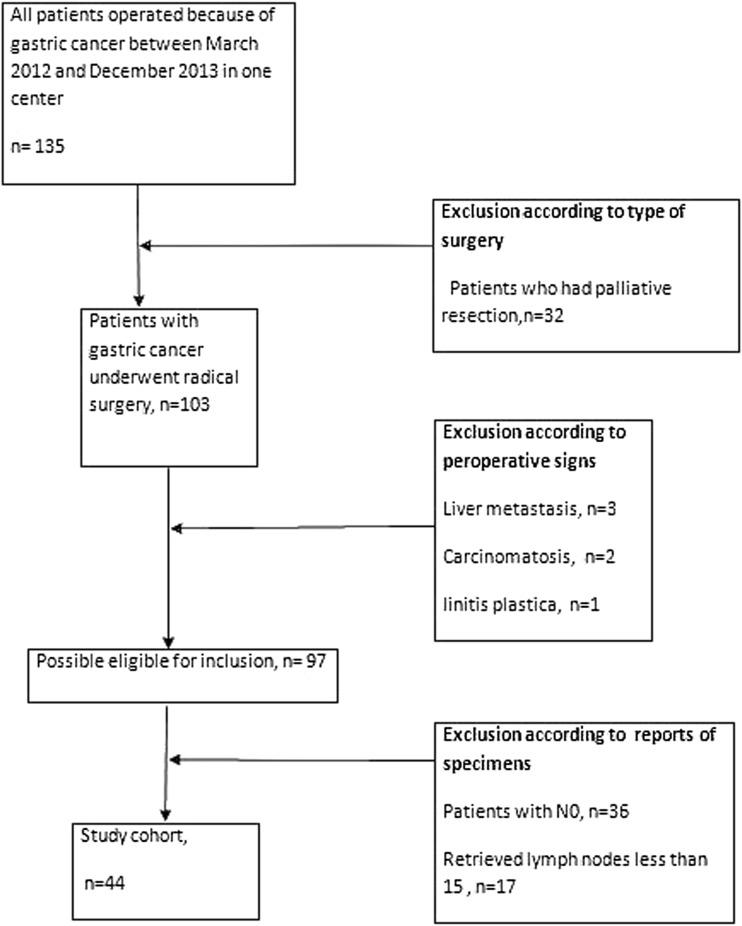
Among these patients, only those who had a radical resection (R0) and >15 LNs retrieved were enrolled into the final study. Patients who only had palliative resection, retrieved lymph nodes less than 15, pN0 stage, metastatic patients and who received neoadjuvant treatment were excluded from the study. Based on the above criteria, a total number of 44 gastric cancer patients were included in the study (Fig. 1).
Fig. 1.
Flow-chart for inclusion in the study
Clinicopathological Analysis
Demographic data with patients’ age, sex, type of surgery, lab and image study information, pre- and postoperative therapies, and follow-up information as well as pathological findings including tumor size, location, depth of tumor invasion, presence of lymphovascular invasion, Lauren’s classification and tumor grading were reviewed. All patients were staged preoperatively using computed tomographic (CT) scans. When needed, magnetic resonance imaging or positron emission tomography-CT scans were used in the preoperative evaluation. All patients underwent R0 resection with D1 lymphadenectomy, the standard surgical technique in western countries. A combination of chemotherapy and radiotherapy was provided postoperatively to all patients. Recurrence, whether loco-regional or distant, was confirmed histologically or clinically (tumor that may be associated with clinical deterioration identified on imaging studies).
Staging
Tumors were staged according to the current American Joint Commission on Cancer (AJCC) TNM staging system. The LNR was calculated as the ratio of the number of metastatic lymph nodes to the total number of lymph nodes dissected. The LNR cut-off point was determined as the median LNR value of all cohort. The study population was divided into 2 groups according to the cutoff value determined.
Statistical Analysis
All data were analyzed using SPSS 17.0 statistical software package (SPSS, Inc., Chicago, IL, USA). Survival was calculated using the Kaplan-Meier method and compared by log-rank test. Univariate and multivariate analyses of prognostic factors were performed using the Cox proportional hazard model. Background clinical data were analyzed using the t-test or Mann-Whitney U test for continuous data and Fisher’s exact test or the Chi-squared test for categorical data. All tests were two-sided and p-values below 0.05 were considered statistically significant.
Results
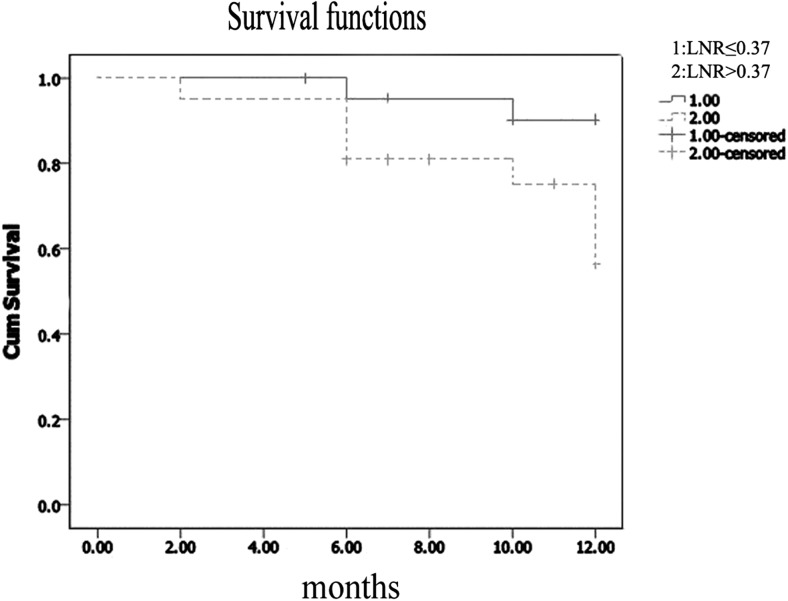
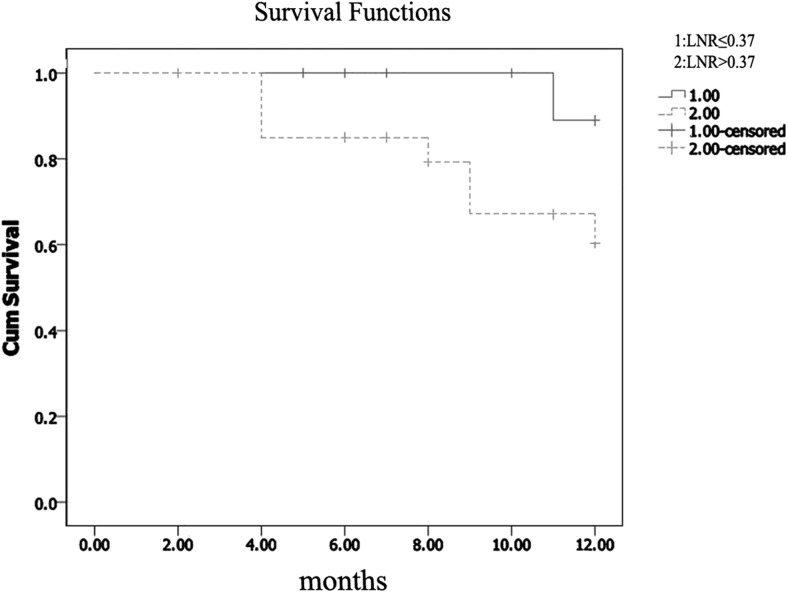
Among 135 GC patients who had undergone resection in the General Surgery Clinic at Marmara University School of Medicine Pendik Training & Research Hospital between March 2012 and December 2013, 36 patients had pN0, 32 had palliative resection, 17 had less than 15 lymph nodes retrieved, three had liver metastasis identified preoperatively, two had carcinomatosis and one patient had linitis plastica. Thus, 44 patients were enrolled into the final study. The median age was 59 years (range 31–83 years). Demographics, tumor location, type of the surgical procedure and histopathological characteristics of the patients are listed in Table 1 (Table 1). The median number of removed LNs was 21 (range 15–55). 32 (73 %) were male patients. The median LNR was 0.37 (0.04–1) and thus the cutoff value was considered to be 37 %. The study population was divided into 2 groups: lower than or equal to 37 % (n = 23) and greater than 37 % (n = 21). In Kaplan-Meier analysis the one-year overall survival (OS) rate for LNR ≤ 37 % was significantly better than that for LNR > 37 % (91.3 % and 61.9 %, respectively, P = 0.02) (Fig. 2). The one-year disease free survival (DFS) for LNR ≤ 37 % was significantly better than that for LNR > 37 % (91.3 % and 66.7 %, respectively, P = 0.027) (Fig. 3). Multivariate analysis showed that neither 1 year OS nor 1 year DFS were significantly different between the two groups (p = 0.95 and p = 0.74 respectively). Thus, the LNR 37 % as a cutoff point was found not to be an independent factor for predicting the one-year OS or DFS in patients with non-metastatic GC. No significant difference was observed between the two groups in terms of age, gender, type of surgery, number of lymph nodes retrieved, histologic grade and classification, vascular invasion, lymphatic invasion, perineural invasion, Her-2 expression, postoperative chemotherapy and postoperative radiotherapy. However, the LNR > 37 % group mainly consisted of pN3 stage (95 %) while out of 23 patients with LNR ≤ 37 % only 5 patients (22 %) had pN3 (p = <0.0001). In addition, the LNR > 37 % group mainly consisted of stage pT4 (71 %) while out of 23 patients with LNR ≤ 37 % only 7 patients (30 %) had stage pT4 (p = <0.0001) (Table 2).
Table 1.
Demographics and histopathological characteristics of patients (n = 44)
| Variable | N = 44(100 %) | Median (Min-Max) |
|---|---|---|
| Age | 59 (31–83) | |
| Gender | ||
| Male | 32(73 %) | |
| Female | 12 (27 %) | |
| Tumor location | ||
| Cardia Corpus Antrum |
13 (30 %) 8 (18 %) 23 (52 %) |
|
| Surgical procedure | ||
| Total gastrectomy Subtotal gastrectomy |
24 (55 %) 20 (45 %) |
|
| Grade of the tumor | ||
| Grade 1 Grade 2 Grade 3 |
6 (14 %) 23 (52 %) 15 (34 %) |
|
| Number of retrieved lymph nodes | 21 (15–55) | |
| Number of metastatic lymph nodes | 7 (1–24) | |
| Lymph node ratio | 0.37 (0.04–1) | |
| pT stage | ||
| T1 T2 T3 T4 |
2 (5 %) 2 (5 %) 18 (41 %) 22 (50 %) |
|
| pN stage | ||
| N1 N2 N3 |
10 (22.7 %) 9 (20.5 %) 24 (56.8 %) |
|
| Stage | ||
| 1B | 1 (2.3 %) | |
| 2 A | 2 (4.5 %) | |
| 2B | 6 (13.6 %) | |
| 3 A | 7 (15.9 %) | |
| 3B | 11 (25 %) | |
| 3C | 17 (38.6 %) | |
| Lauren classification | ||
| Intestinal | 27 (61.4 %) | |
| Diffuse Mixed |
12 (27.3 %) 5 (11.4 %) |
|
| WHO classification | ||
| Tubular | 24 (54.5 %) | |
| Signet ring | 4 (9.1 %) | |
| Mucinous | 5 (11.4 %) | |
| Mixed | 7 (15.9 %) | |
| Poorly cohesive | 4 (9.1 %) | |
| Ming classification | ||
| Infiltrative | 40 (91 %) | |
| Ekspanding | 4 (9 %) | |
| Vascular invasion | 21 (48 %) | |
| Lymphatic invasion | 40 (91 %) | |
| Perineural invasion | 25 (57 %) | |
| Her 2 expression | 5 (11 %) | |
| Adjuvant chemotherapy | 32 (73 %) | |
| Follow -up (month) | 13 (2–24) | |
Fig. 2.
The 1 year overall survival in gastric cancer was significantly better in the lymph node ratio (LNR) ≤ 0.37 group than it was in the LNR > 0.37 group
Fig. 3.
The 1 year diseasefree survival in gastric cancer was significantly better in the lymph node ratio (LNR) ≤ 0.37 group than it was in the LNR > 0.37 group
Table 2.
Comparing characteristics of patients according to the outcome of study treatment
| Variable | LNR ≤ 0.37 group (n = 23) | LNR > 0.37 group (n = 21) | P value |
|---|---|---|---|
| Age (years)a | 63 (34–83) | 60 (31–80) | 1.0b |
| Gender: | 0.9c | ||
| Male Female |
17 (74 %) 6 (26 %) |
15 (71 %) 6 (29 %) |
|
| Surgical procedure: | 0.7c | ||
| Total gastrectomy Subtotal gastrectomy |
12 (52 %) 11 (48 %) |
12 (57 %) 9 (43 %) |
|
| Number of retrieved lymph nodes | 22 (15–55) | 21 (15–48) | 0.18b |
| Number of metastatic lymph nodes | 4 (1–9) | 12 (6–24) | <0.0001b |
| Histologic grade | 0.2b | ||
| 1 2 3 |
4 (17.4) 13(56.5) 6(26.1) |
2 (9.5 %) 10 (47.6) 9 (42.9) |
|
| pTstage | 0.004b | ||
| T1 T2 T3 T4 |
2(9 %) 2(9 %) 12(52 %) 7(30 %) |
0(0 %) 0(0 %) 6(29) 15(71 %) |
|
| pNstage | <0.0001b | ||
| 1 2 3 |
10(43.5 %) 8(34.8 %) 5(21.7) |
0(0 %) 1(5 %) 20(95 %) |
|
| Lauren’s classification | 0.10b | ||
| Intestinal Diffuse Mixed |
17 4 2 |
10 8 3 |
|
| Vascular invasion | 8 (35 %) | 13 (62 %) | 0.07c |
| Lymphatic invasion | 20 (87 %) | 20 (95 %) | 0.61d |
| Perineural invasion | 10 (43 %) | 15 (71 %) | 0.06c |
| Her 2 expression | 2 (8 %) | 3 (14 %) | 0.66d |
| Postoperative chemotherapy | 0.12c | ||
| Yes No |
19 (83 %) 4 (17 %) |
13 (62 %) 8 (38 %) |
|
| Postoperative radiotherapy | 0.07c | ||
| Yes No |
15 (62 %) 8 (28 %) |
8 (38 %) 13 (62 %) |
aValues are medians (range)
bMann-Whitney U test
cChi squared test
dFisher’s exact test
Discussion
In this study, which included 44 patients with non-metastatic GC, the study population was divided into 2 groups according to the LNR cutoff value of 37 %. This study, showed that the one-year OS rate and one-year DFS for LNR ≤ 37 % was significantly better than that for LNR > 37 %. However, multivariate analysis showed that the LNR with cutoff of 37 % was not an independent prognostic factor for neither OS nor DFS.
Research on the prognostic value of LNR in GC remains limited and controversial results were obtained. Compared with previous studies, our study used a single cutoff point which was determined to be the median LNR of the whole cohort. However, some previous studies on the LNR in GC selected cutoff points arbitrarily and compared the prognostic value of the LNR by separating the patients into many groups with many cutoff points which produced unclear results. However, in this study we have divided the cohort into two groups according to the single determined cutoff point.
Major limitations of this study are the small sample size, the short follow up time and the retrospective design of the study.
As more than 15 lymph nodes should be examined for correct assessment of N staging according to TNM classification system [4], in this study, only patients with >15 LN resections were included and the median number of removed LNs was 21 (range 15–55). Thus, we evaluated whether LNR is an accurately independent prognostic factor from the pN stage.
Previous studies on LNR confirmed the superiority of the LNR staging system compared with the AJCC TNM staging system through univariate and multivariate analysis and Kaplan-Meier survival curves [16]. However, there is still controversy regarding the appropriate classification of nodal status, as well as the significance of positive lymph node number vs. metastatic lymph node ratio, and no definite consensus has yet been reached on either issue [4]. The current UICC/AJCC staging system, which is based on the number of metastatic lymph nodes, is a simple and reproducible method. However, when the AJCC/UICC staging system is used, the phenomenon of stage migration has been observed in about 10–15 % of cases [10, 17]; Moreover, more than 15 lymph nodes should be examined for correct assessment of N staging. Some studies have recently proposed the LNR as an alternative prognostic factor to supplement the limitations of the conventional N staging system [18–20], particularly when a limited number of lymph nodes is obtained. In the present study, we aimed to evaluate whether LNR is a better to predict the survival in comparison with known prognostic factors in gastric carcinoma. In addition, we tried to determine a cutoff point to get a meaningful separation of survival.
Previous studies gave more than one cutoff points and divided patients into many groups according to LNR intervals to determine the survival of the patients. Therefore, methods used in pervious studies were not simple and not reproducible. For example, in one study for defining the Tumor-node-ratio-metastases (TRM) staging system, two recognized methods were used to determine the best cut-off points for LNR. One was the commonly used cut-off approach using the log-rank test, the other was X-tile [21]. In an other study, the LNR cut-off points were based on the most commonly used cut-off points for the LNR found in the literature [22]. However, these previous studies, proved that MLR (metastatic lymph node ratio) was a better prognostic factor than the conventional pN staging system, no consensus has been made on the optimal categorization of MLR, for each study was carried out by different standards. In addition, in these studies patients were divided into many groups and even up to ten cutoff points in one study with different many intervals determined which made the results unclear and not reproducible. However, in this study we simply determined one single cut-off value as this value may be more practical and reproducible than using intervals with multiple cut-off values.
Through survival analysis, we showed that the cut-off value which we determined as 37 % is a prognostic factor for GC. However, multivariate analysis showed that it was dependent on pN stage. It was also clear in this study that the high LNR was associated with high pN stage. In this study we confirmed the well known fact that the most important prognostic factors of GC are depth of invasion and lymph node metastasis (nodal status) [4]. However, in contrast to our previous study on rectal cancer [15], this study could not determine a single cut-off value of LNR as an independent prognostic factor for GC.
However, based on these retrospective results, more comprehensively planned, prospective, randomized controlled studies need to be conducted.
In conclusion, the LNR is a prognostic factor in GC. However, no single cut-off value was determined as an independent prognostic factor.
Compliance with Ethical Standards
Conflict of Interest
We hereby declare explicitly that we have no relationship with any third party of conflict of interest or a fund that support our study.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hartgrink HH, Jansen EP, van Grieken NC, Van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang XN, Liang H. Some problems in the surgical treatment of gastric cancer. Chin J Cancer. 2010;29:369–373. doi: 10.5732/cjc.009.10629. [DOI] [PubMed] [Google Scholar]
- 4.Lee SY, Hwang I, Park YS, Gardner J, Ro JY. Metastatic lymph node ratio in advanced gastric carcinoma: a better prognostic factor than number of metastatic lymph nodes? Int J Oncol. 2010;36(6):1461–1467. doi: 10.3892/ijo_00000632. [DOI] [PubMed] [Google Scholar]
- 5.Fleming ID, Cooper JS, Henson DE, et al. (1997) American joint committee on cancer, cancer staging Manuel. Lippincott-Raven, Philadelphia, pp 71-76
- 6.Sobin LH, Wittekind CH. International union against cancer (UICC): TMN classification of malignant tumors. 5th. New York: John Wiley & Sons; 1997. [Google Scholar]
- 7.Lemmens VE, Dassen A. E, van der wurff a a, coebergh JW and bosscha K: lymph node examination among patients with gastric cancer: variation between departments of pathology and prognostic impact of lymph node ratio. Eur J Surg Oncol. 2011;37:488–496. doi: 10.1016/j.ejso.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Persiani R, Rausei S, Biondi A, Boccia S, Cananzi F, D’Ugo D. Ratio of metastatic lymph nodes: impact on staging and survival of gastric cancer. Eur J Surg Oncol. 2008;34:519–524. doi: 10.1016/j.ejso.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Inoue K, Nakane Y, Iiyama H, Sato M, Kanbara T, Nakai K, et al. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9:27–34. doi: 10.1245/aso.2002.9.1.27. [DOI] [PubMed] [Google Scholar]
- 10.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 11.Weir L, Speers C, D’yachkova Y, Olivotto IA. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol. 2002;20:1793–1799. doi: 10.1200/JCO.2002.07.112. [DOI] [PubMed] [Google Scholar]
- 12.Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167:1295–1298. doi: 10.1016/S0022-5347(05)65284-6. [DOI] [PubMed] [Google Scholar]
- 13.Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70:235–240. [PubMed] [Google Scholar]
- 14.Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21:1029–1034. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Attaallah W, Gunal O, Manukyan M. Ozden G and yegen C: prognostic impact of the metastatic lymph node ratio on survival in rectal cancer. Ann Coloproctol. 2013;29(3):100–105. doi: 10.3393/ac.2013.29.3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu HL, Tian Q, Peng CW, Liu SP, Li Y. Multivariate survival and outcome analysis of 154 patients with gastric cancer at a single Chinese institution. Asian Pac J Cancer Prev. 2011;12:3341–3345. [PubMed] [Google Scholar]
- 17.Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, Lise M. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077–1085. doi: 10.1245/ASO.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 18.Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy. Ann Surg. 2007;245:543–552. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Lu P, Lu Y, Xu H, Wang S, Chen J. Clinical implications of metastatic lymph node ratio in gastric cancer. BMC Cancer. 2007;7:200. doi: 10.1186/1471-2407-7-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celen O, Yildirim E, Berberoglu U. Prognostic impact of positive lymph node ratio in gastric carcinoma. J Surg Oncol. 2007;96:95–101. doi: 10.1002/jso.20797. [DOI] [PubMed] [Google Scholar]
- 21.Zeng WJ, Hu WQ, Wang LW, et al. Lymph node ratio is a better prognosticator than lymph node status for gastric cancer: a retrospective study of 138 cases. Oncology Letters. 2013;6(6):1693–1700. doi: 10.3892/ol.2013.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelen SD, van Steenbergen LN, Dassen AE, van der Wurff AA, Lemmens VE, Bosscha K. The lymph node ratio as a prognostic factor for gastric cancer. Acta Oncol. 2013;52(8):1751–1759. doi: 10.3109/0284186X.2012.754991. [DOI] [PubMed] [Google Scholar]