Abstract
Gallbladder cancer (GBC) is the most common biliary tract malignancy. Incidence varies widely with geographic regions, with northern India being the endemic area for GBC. Curative surgery offers the only chance of cure, but most of patients present with unresectable or metastatic disease and are candidates for palliative treatment only. This study was designed to evaluate efficacy of chemotherapy over best supportive care in unresectable/metastatic GBC. Patients with unresectable/metastatic GBC with proven tissue diagnosis were enrolled for single institution non-randomized prospective cohort study between May 2012 and April 2014. A total of 65 patients received palliative chemotherapy; either combination chemotherapy (n = 59) or single agent chemotherapy (n = 6). Combination chemotherapy regimen were either three weekly Gemcitabine-Cisplatin (n = 45) or Gemcitabine-Oxaliplatin (n = 14) for a maximum of six cycles. Twenty patients, either unfit for chemotherapy or unwilling for the same were advised best supportive care (BSC). The overall response rate to chemotherapy was 34 %. Median survival for chemotherapy group and BSC group were 35.6 and 13 weeks, respectively (p value < 0.001). Median OS for combination chemotherapy (n = 59) and single agent chemotherapy (n = 6) were 37 and 26.7 weeks, respectively (p value- 0.002). Median PFS for combination chemotherapy and single agent chemotherapy were 26 and 15 weeks, respectively (p value-0.012). The results of this study are quite encouraging and support use of chemotherapy for unresectable GBC patients over best supportive care, and that gemcitabine based combination chemotherapy may be a better choice for response rates, OS, and PFS.
Keywords: Best supportive care, Gallbladder cancer, Palliative chemotherapy, Overall survival
Introduction
Gallbladder cancer is an aggressive malignancy occurring predominantly in the elderly with a mean age of 65.2 years reported globally. But in Indian subcontinent the average age at diagnosis of GBC is about 50 years. In this series, the mean age at presentation was 58.5 years (range 28 to 80 years) with no significant difference between female and male. The incidence of gallbladder cancer varies by geographic region and racial-ethnic groups. Gallbladder cancer is up to 25 times more common in some geographical regions compared with others [1]. The highest incidences are reported in Indians, Pakistanis, Chileans, Bolivians, Central Europeans, Israelis and American Indians [2]. The incidence of carcinoma gallbladder in India ranges from 1.01 per 100,000 for males to 10.1 per 100,000 for females but the actual number may be much more in the endemic zones of western Bihar and eastern Uttar Pradesh where it is the third commonest malignancy of the alimentary tract [2]. The reasons for high incidence in this population are not well-understood.
Only 10 % of patients are suitable for curative surgery and the rest of the patients present in advanced and unresectable stage and are candidates for palliative treatment only. Currently, there is no standard chemotherapy for GBC, and the majority of studies have included patients from all subsites of biliary tract cancers. With various chemotherapeutic agents (with or without fluorouracil [FU]) response rates were reported in 0 to 36 % of patients. Median survival for patients presenting with unresectable disease is 2 to 4 months, with fewer than 5 % patients surviving 1 year [3].
There is a paucity of randomized controlled studies on the role of palliative chemotherapy for GBC and most included patients with other biliary tract malignancies. During the 1980s and 90s, a number of studies reported the effect of drugs such as 5-fluorouracil (5 FU) on the management of patients who presented with locally advanced or metastatic biliary tract tumors including GBC. These studies emphasized the poor results in both response and survival. Similar disappointing results were reported with the use of other types of drugs, such as streptozocin, methyl lomustine, amsacrine, and paclitaxel [4, 5]. Only after Gemcitabine was used in the treatment of pancreatic cancer and showed promising results, it began to be incorporated into GBC management. Gemcitabine has been widely evaluated for patients with advanced biliary tract cancers (BTC). A regimen of gemcitabine combined with platinum was recommended as a provisional standard of chemotherapy for patients with advanced BTC, based on a pooled analysis of 104 studies, predominantly phase II clinical trials that investigated the efficacy and toxicity of chemotherapy [6]. A recent phase III trial published in the New England Journal of Medicine compared gemcitabine plus cisplatin to gemcitabine alone in 410 patients with locally advanced or metastatic cholangiocarcinoma, GBC or ampullary cancer [7]. The median overall survival (OS) was 11.7 months among the 204 patients in the cisplatin–gemcitabine group and 8.1 months among the 206 patients in the gemcitabine group (hazard ratio, 0.64; 95 % confidence interval, 0.52 to 0.80; p < 0.001). The median progression-free survival (PFS) was 8.0 months in the cisplatin–gemcitabine group and 5.0 months in the gemcitabine-only group (p < 0.001). In addition, the rate of tumor control among patients in the cisplatin–gemcitabine group was significantly increased (81.4 % vs. 71.8 %, p = 0.049). Cisplatin plus gemcitabine was associated with a significant survival advantage without the addition of substantial toxicity.
There have been very few studies exclusively enrolling GBC patients. In one trial, 26 patients with metastatic or unresectable GBC and no prior chemotherapy received single-agent gemcitabine. Of the 25 evaluable patients, an overall response rate of 36 % (95 % CI, 17.1 %–57.9 %) and median survival time of 30 weeks were observed [8]. Gemcitabine and Cisplatin were evaluated in 30 patients with unresectable GBC [9]. Complete and partial response rates were 13.3 and 23.3 % respectively, while 1- year survival rate was 18.6 %. These findings indicate that gemcitabine based regimens are also active in GBC. A randomized controlled trial evaluating the efficacy of modified gemcitabine and oxaliplatin (mGEMOX) (group C) over best supportive care (BSC) (group A) or fluorouracil (FU) and folinic acid (FA) (group B) in unresectable GC was recently reported [10]. Complete response plus partial response in the three groups was 0 (0 %), four (14.3 %), and eight (30.8 %) respectively (p = 0.001). Median OS was 4.5, 4.6, and 9.5 months for the BSC, FUFA, and mGEMOX arms (p = 0.039), respectively. PFS was 2.8, 3.5, and 8.5 months for the three groups (p = 0.001) supporting the use of mGEMOX for patients with advanced GBC.
Patients and Methods
Study Design
This was a single centre prospective non-randomized cohort study conducted at Malignant Disease Treatment Centre of Army Hospital (Research & Referral), New Delhi. This study was conducted during May 2012 to April 2014. Study data and informed consent were gathered in accordance with the Declaration of Helsinki and approved by the institute’s ethics committee.
Inclusion Criteria and Treatment
Patients with unresectable/metastatic disease at presentation were evaluated for palliative chemotherapy. Patients with proven tissue diagnosis of GBC; adequate major organ and bone marrow function; hemoglobin higher than 10 gm%; absolute neutrophil count higher than 1.5 × 109/L; platelets higher than 100 × 109/L; serum creatinine lower than 1.8 mg%; serum bilirubin ≤3 mg%; liver enzymes (AST and ALT) within 3 times the normal limit and Eastern Cooperative Oncology Group performance status of ≤2 were considered for chemotherapy; either combination chemotherapy (Gemcitabine-Cisplatin or Gemcitabine-Oxaliplatin) or Single agent chemotherapy (Inj. Gemcitabine or Cap. Capecitabine). Treatment for patients with grade 3 or 4 toxicity was either delayed until resolution of toxicity or return of toxicity to lower than grade 2. Chemotherapy dose was reduced by 25 % and rounded off in cases of grade 4 neutropenia or thrombocytopenia.
Patients with relatively poor PS who were not a candidate for combination chemotherapy were considered for single agent chemotherapy, or subjected to best supportive care on discretion of patient’s decision. In BSC patients received only symptomatic treatment. Palliation of surgical obstructive jaundice and gastric outlet obstruction were done by interventional approach wherever feasible.
Outcome
Primary end points were to measure overall survival (OS), to investigate response rates with chemotherapeutic drugs in unresectable adenocarcinoma of gall bladder. Progression free survival (PFS) was calculated as secondary end point for those who underwent chemotherapy. Response Evaluation Criteria in Solid Tumors (RECIST) was used for assessment of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Response assessment was done by computed tomography (CT) scan after three cycles and six cycles and thereafter every 3 months for 1 year and thereafter every six monthly. Patients who developed progressively increasing jaundice during the follow-up were considered to have progressive disease. All patients were actively followed-up by means of out-patient review, postal and/or telephonic communication as applicable and feasible.
Overall survival and progression free survival for chemotherapy group (CT) was calculated; subgroup comparison between those who received double agent chemotherapy (DCT) with those who received single agent chemotherapy was done. Overall survivals of BSC and CT groups were compared. Subgroup analysis of OS was done between BSC and CT after excluding ECOG-3 cases from BSC group as this parameter was statistically different between BSC and CT group.
Data Analysis
The descriptive statistics is presented using mean (with SD) and median (with range) for quantitative variables and categorical variables are presented in frequencies along with respective percentages. The statistical comparisons for quantitative variables were done by using Student’s t-test. For categorical variables Chi-square test or Fisher’s exact test was used according to the nature of data. OS was calculated from date of entry to date of death or censoring at the date last known for being alive for all the patients. PFS was calculated from date of enrollment to documented tumor progression (clinical or radiologic). For survival analysis, Kaplan-Meier survival curve were plotted to see the survival pattern in different subgroups and Log-rank test was used for comparison of survival. Data were entered and coded in MS Excel (Version, 2007) and all statistical analyses were performed by using SPSS software (Version 22, SPSS Inc, Chicago, IL, USA). The p value less than 0.05 were considered statistically significant.
Results
A total of 85 patients with unresectable/metastatic gallbladder cancer were enrolled from May 2012 to April 2014 and evaluated for palliative chemotherapy. Cutoff date for survival analysis was April 30, 2014. Baseline characteristics of enrolled patients are presented in Table 1. A total of 65 patients received palliative chemotherapy; either combination chemotherapy (n = 59) or single agent chemotherapy (n = 6). Combination chemotherapy regimen were either three weekly Gemcitabine-Cisplatin (n = 45) or Gemcitabine-Oxaliplatin (n = 14) for six cycles. Single agent chemotherapy (Inj. Gemcitabine or Cap. Capecitabine) was given to patients with relatively poor performance status.
Table 1.
Baseline characteristics of patients (N = 85)
| Variables | BSC (n = 20) | CT (n = 65) | P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| <=50 | 1 | 5 | 16 | 25 | 0.062 |
| >50 | 19 | 20 | 49 | 75 | |
| Sex | |||||
| Male | 8 | 40 | 10 | 15 | 0.018 |
| Female | 12 | 60 | 55 | 85 | |
| ECOG score | |||||
| 1 | 0 | 0 | 41 | 63 | 0.001 |
| 2 | 16 | 80 | 24 | 37 | |
| 3 | 4 | 20 | 0 | 0 | |
| Hyperbilirubinemia | 10 | 50 | 17 | 26 | 0.045 |
| Raised AST/ALT | 5 | 25 | 6 | 9 | 0.066 |
| Raised SAP | 10 | 50 | 16 | 24 | 0.064 |
| Albumin <3.5 | 11 | 55 | 21 | 32 | 0.067 |
| HB < 10 gm% | 10 | 50 | 11 | 17 | 0.003 |
| Gall stones | 13 | 65 | 54 | 83 | 0.084 |
| Liver metastasis | 6 | 30 | 34 | 52 | 0.080 |
| Prior surgery | 5 | 25 | 12 | 18 | 0.523 |
| Stage | |||||
| III | 2 | 10 | 1 | 1.5 | 0.137 |
| IV | 18 | 90 | 64 | 98.5 | |
Abbreviations BSC best supportive care, CT Chemotherapy SAP serum alkaline phosphatase
Response Rate to Chemotherapy
RECIST criteria were used for assessment of CR, PR, stable disease, and progressive disease. Response assessment was done by computed tomography (CT) scan after three cycles and six cycles and thereafter every 3 months for 1 year. Patients who developed progressively increasing jaundice during the follow-up were considered to have progressive disease.
Chemotherapy dropout rate was found to be 21 % (14/65). The major causes of discontinuation of chemotherapy were rapid progression of disease (10 cases) and severe toxicity (4 cases).
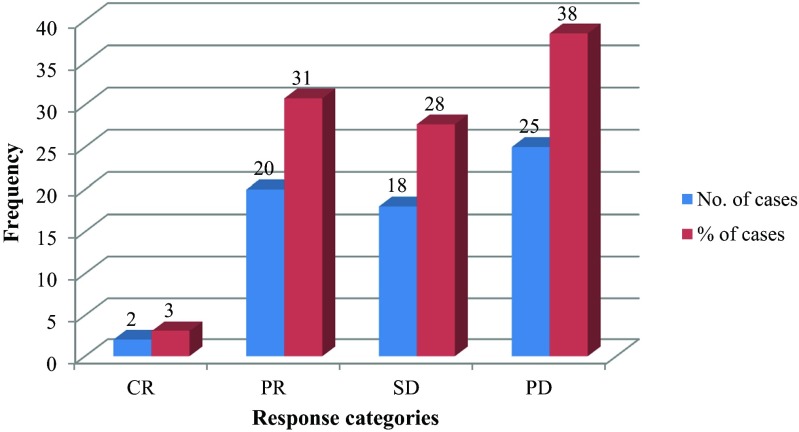
Two patients (3 %) had CR, 20 patients (31 %) had PR, and 18 patients (28 %) had stable disease. The overall (CR + PR) response rate to chemotherapy was 34 %. Abrogation of PD (CR + PR + SD) was observed in 62 % (Fig. 1). Twenty two patients received second line chemotherapy (5-FU, 5-FU–Cisplatin, Cap Capecitabine, and Inj Docetaxel etc.). The response rate to second line chemotherapy was only 9 % (2 cases).
Fig. 1.
Response rates to palliative chemotherapy for GBC (n = 65). CR-Complete Response; PR-Partial Response; SD-Stable Disease; PD-Progressive Disease
Twenty patients, either unfit for chemotherapy or unwilling for the same were advised best supportive care. Base line characteristics of enrolled patients in chemotherapy and BSC groups are presented in table 1.
Survival Analysis
Overall survival and progression free survival for chemotherapy group (CT) was calculated. Subgroup comparison between those who received double agent chemotherapy (DCT) with those who received single agent chemotherapy was also done. Overall survivals of BSC and CT groups were compared. Subgroup analysis of OS was done between BSC and CT groups after excluding ECOG-3 cases from BSC group as this parameter was highly statistically significant between BSC and CT group. Overall survival of chemotherapy group (n = 65) at 6 and 12 months were 72.5 and 16.1 %. Median survival was 35.6 weeks (SE, 2.6; 95 % C.I.) for CT group. Median OS for DCT (n = 59) and SCT (n = 6) were 37 and 26.7 weeks respectively (p = 0.002).
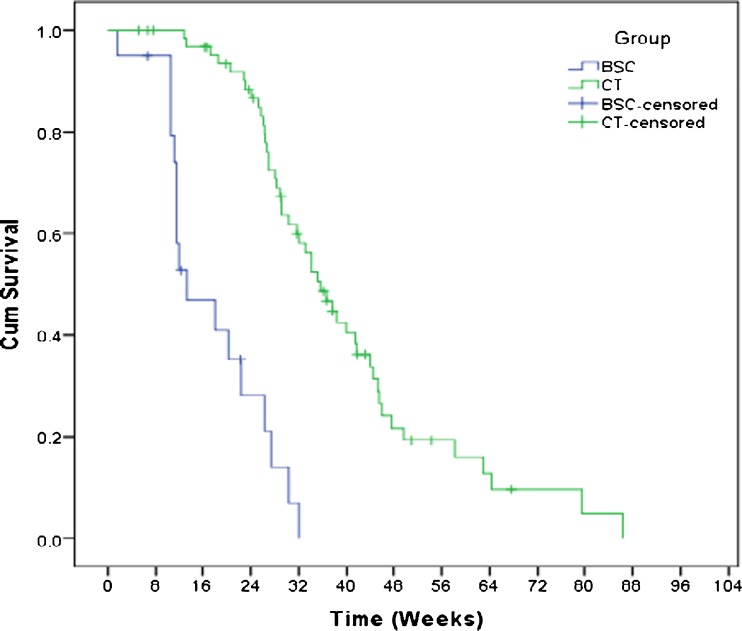
The estimated overall survival of BSC group at 3 and 6 months were 46 and 14 %. Median OS was 13 weeks for BSC group (SE, 2.02; 95 % C.I.). Log Rank test was used to compare OS between BSC and CT group (Fig. 2). Statistically significant difference in OS was seen (p value < 0.001).
Fig. 2.
Kaplan-Meier estimation of overall survival of patients who received best supportive care (n = 20) and palliative chemotherapy (n = 65). BSC, best supportive care; CT, chemotherapy
Overall survival was compared between BSC and CT group after excluding ECOG-3 patients (4) from BSC group. There was still a significant difference of OS between these groups (p value <0.001).
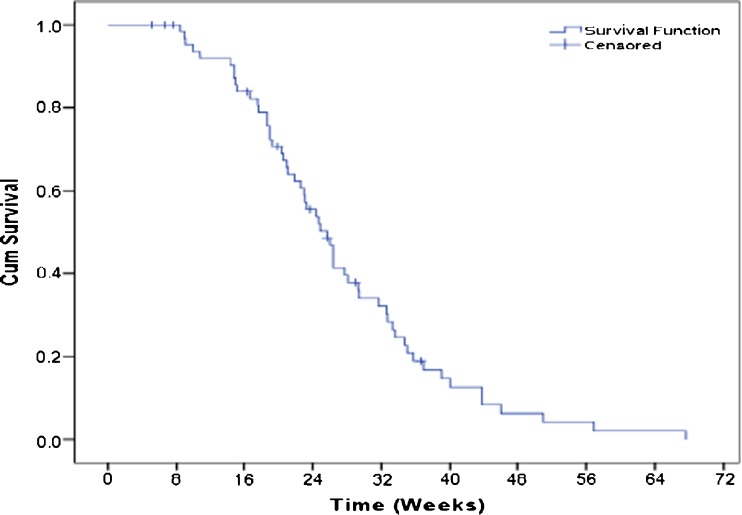
Progression free survival of patients who received chemotherapy was 39 % at 6 and 12.6 % at 18 months. Estimated median PFS was 25.7 weeks (Fig. 3). PFS was compared between two subgroups; combination chemotherapy (DCT) and single agent chemotherapy (SCT). Median PFS for DCT and SCT were 26 and 15 weeks respectively. Significant difference in PFS was observed (p value-0.012).
Fig 3.
Kaplan-Meier estimation of Progression free survival (PFS) in weeks who received palliative chemotherapy (n = 65)
Discussion
Gallbladder cancer is the most common malignant lesion of the biliary tract and the fifth most common among malignancy of the digestive tract [11]. It is a highly fatal disease with poor prognosis. This is largely attributed to advanced stage of disease at presentation, which frequently precludes a curative resection. Currently, there is no standard chemotherapy protocol for unresectable GBC, and median survival for patients presenting with unresectable disease is 2 to 4 months, with 1-year survival lower than 5 % [3]. Small number of patients, inclusion of bile duct and periampullary cancers in the studies, and the lack of randomized control trials are the main drawbacks of the published literature in this field. There were only two randomized trials comparing BSC and chemotherapy in biliary tract cancers (not limited to GBC only) using FU-based chemotherapy. In the study by Glimelius et al., thirty-seven patients were randomly assigned to FU-based chemotherapy or BSC. Median OS was 6.5 months in the chemotherapy group and 2.5 months in BSC group (p value = 0.1) [12]. It was possible that because of small sample size, statistical significance could not be achieved. In another study by Takada et al., chemotherapy was compared to BSC. Patient population was heterogeneous including pancreatic, GBC, and biliary tract cancers. No significant improvement was seen with use of chemotherapy [13].
Gemcitabine and platinum compounds are emerging as commonly used drugs, either as a single agent or in combination. The study by DC Doval et al. using gemcitabine and cisplatin reported 38 % response rates and 4.8 months of median survival [9].
In the present study, a total of 65 patients received palliative chemotherapy (CT); either combination chemotherapy (n = 59) or single agent chemotherapy (n = 6). Combination chemotherapy regimen were either three weekly Gemcitabine-Cisplatin (n = 45) or Gemcitabine-Oxaliplatin (n = 14) for six cycles. Oxaliplatin is a third-generation platinum compound has much less emetic and renal toxicity and was used in relatively elderly patients. Twenty unresectable patients were treated with best supportive care (BSC).
Overall median survival in our study with palliative chemotherapy was 35.6 weeks, significantly superior to BSC (p value < 0.001). Sharma et al. (2010) has reported the only randomized control trial comparing best supportive care with palliative chemotherapy for unresectable GBC [10]. They reported overall median survival in chemotherapy (mGEMOX) arm to be 9.5 and 4.5 months for BSC arm (p value = 0.01). The results of our study in terms of OS are comparable to Sharma et al. Relatively high statistical difference between these treatment arms in our study compared to Sharma et al. (p value < 0.001 vs 0.039) may be explained by the fact that our patients did not had matched baseline characteristics as was there in Sharma et al. report. BSC arm of our series contained several patients with poor ECOG score (p value = 0.001) and anemia (p value = 0.003) leading to poor OS.
Progression free survival of patients who received chemotherapy was 39 % at 6 months and 12.6 % at 18 months. Estimated median PFS was 25.7 weeks. Andre et al. [14] and Sharma et al. reported a median PFS of 5.7 and 8.5 months, respectively with gemcitabine based combination chemotherapy which is quite comparable to this study.
In this study we also tried compare outcome of single agent chemotherapy (n = 6) with gemcitabine-based combination chemotherapy (n = 59) for GBC. Median PFS for combination chemotherapy and single-agent chemotherapy were 26 and 15 weeks, respectively. Significant difference in PFS was observed (p value-0.012). No patients in single agent chemotherapy arm (SCT) showed objective CR or PR. Though the number of patients and baseline characteristics in these two groups were not comparable, the results are well in accordance with published literature. Cassier et al. (2010) [15], in their study showed superiority of gemcitabine based combination chemotherapy over single agent gemcitabine or 5 FU based therapies for biliary tract malignancies. The overall survival with chemotherapy in their study was 7.5 months, comparable to our present study which showed median OS of 35.6 weeks. Median OS for DCT (n = 59) and SCT (n = 6) were 37 and 26.7 weeks respectively (p = 0.002). These findings are again consistent with Cassier et al. study which had shown significant difference in OS with combination chemotherapy and single agent chemotherapy (p value < 0.0001).
In our study, two patients (3 %) had CR, 20 patients (31 %) had PR, and 18 patients (28 %) had stable disease. The overall (CR + PR) response rate to chemotherapy was 34 %. Abrogation of PD (CR + PR + SD) was observed in 62 %. Sharma et al. [10], Cassier et al. [15], DC Doval et al. [9], and several other authors have reported comparable response rates up to 60 % and abrogation of PD (CR + PR + SD) in 50–93 % with gemcitabine based chemotherapy for biliary tract cancers. Of all these reports, Sharma et al. (2010) and DC Doval et al. (2004) needs special mention as they have included only gallbladder cancer patients similar to our series. Sharma et al. reported overall response rate (CR + PR) 30.7 % and disease stabilization (CR + PR + SD) in approximately 69 %. Median OS, PFS and 1-year survival with gemcitabine based chemotherapy were 9.5, 8.5 months, and 22 %. Similarly DC Doval et al. achieved a RR (CR + PR) of 36.6 % and abrogation of progression in 60 % of GBC patients. The median overall survival was 20 weeks, and 1-year overall survival was 18.6 %. Median 1-year survival of 16.1 % in our study is comparable to these contemporary reports and seems to be better than historical control of 5 % [3]. These results are quite encouraging and support use of chemotherapy for unresectable GBC patients over best supportive care.
As such, currently gemcitabine in combination with cisplatin can be considered as the standard of care in patients with advanced GBC, though the reported response rates have been in the range of 30 to 40 % only with median survival of less than 1 year. We need to explore other potential therapeutic strategies to improve the outcome of this deadly disease. In this context, advanced gallbladder cancer may be an ideal setting for regional chemotherapy either for primary treatment or as an adjuvant to resection. In one study, a 48 % overall response rate and a prolongation of median survival from 5 to 14 months compared with historical controls was reported with intra-arterial mitomycin C [16]. Because of propensity for intraperitoneal spread, an intraperitoneal chemotherapeutic approach may be ideal in an adjuvant setting, especially where prior simple cholecystectomy may have resulted in tumor cell spillage.
For palliation of obstructive jaundice, multimodality approaches combining EBRT with 5FU-based therapy have been supported by consensus guidelines from European Society of Medical Oncology (ESMO) and the National Comprehensive Cancer Network [17]. Although the benefit of EBRT is minimal, it does appear to be well tolerated and may improve symptoms and survival in selected patients. Finally, molecular profiling of these cancers may result in paradigm shift, allowing for individualized treatment of patients based on single-agent/combination therapy predicted on the perturbation of aberrant pathways.
In summary, we can say that palliative chemotherapy is superior to BSC, and that gemcitabine based combination chemotherapy may be a better choice for response rates, OS, and PFS. New chemotherapy and biologic therapy should be studied in patients with advanced GBC to help improve the dismal prognosis. Instead of analyzing individual institutional data, high volume institutions with the necessary expertise for treating GBC should collaborate with a view to generating strong evidence to formulate clinical practice guidelines.
Contributor Information
Santosh Kumar Singh, Phone: +918826272421, Email: mlnsantosh@yahoo.co.in.
Rajnish Talwar, Email: rajnish_onco@hotmail.com.
Narayanan Kannan, Email: majkannan@gmail.com.
Arvind Kumar Tyagi, Email: oncocdosurgeon@gmail.com.
Pradeep Jaiswal, Email: drjaiswalpradeep@yahoo.co.in.
References
- 1.Randi G, Franceschi S, La VC. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 2.Shukla VK, Roy SK, Vaidya MP. Primary carcinoma gallbladder: a review of a 16 year period at the university hospital. J Surg Oncol. 1985;28:32–35. doi: 10.1002/jso.2930280109. [DOI] [PubMed] [Google Scholar]
- 3.Perpetuo MO, Valdivieso M, Heilbrun LK, et al. Natural history study of gallbladder cancer: a review of 36 years experience at M.D. Anderson Cancer Center. Cancer. 1978;42:330–335. doi: 10.1002/1097-0142(197807)42:1<330::AID-CNCR2820420150>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Jones DV, Jr, Lozano R, Hoque A, Markowitz A, Patt YZ. Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol. 1996;14:2306–10. doi: 10.1200/JCO.1996.14.8.2306. [DOI] [PubMed] [Google Scholar]
- 5.Harvey JH, Smith FP, Schein PS. 5-Fluorouracil, mitomycin, and doxorubicin (FAM) in carcinoma of the biliary tract. J Clin Oncol. 1984;2:1245–8. doi: 10.1200/JCO.1984.2.11.1245. [DOI] [PubMed] [Google Scholar]
- 6.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med;362:1273–81 [DOI] [PubMed]
- 8.Gallardo JO, Rubio B, Fodor M, Orlandi L, Yanez M, Gamargo C, Ahumada M. A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol. 2001;12:1403–6. doi: 10.1023/A:1012543223020. [DOI] [PubMed] [Google Scholar]
- 9.Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, Awasthy BS. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004;90:1516–20. doi: 10.1038/sj.bjc.6601736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P, Chaudhary SP. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 28:4581–6 [DOI] [PubMed]
- 11.Mekeel KL, Hemming AW. Surgical management of gallbladder carcinoma: a review. J Gastrointest Surg. 2007;11:1188–93. doi: 10.1007/s11605-007-0115-1. [DOI] [PubMed] [Google Scholar]
- 12.Glimelius B, Hoffman K, Sjo¨de’n PO, et al. Chemotherapy improves survival and quality of life in pancreatic and biliary tract cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 13.Takada T, Nimura Y, Katoh H, et al. Prospective randomized trial of 5 fluorouracil, doxorubicin, and mitomycin C for non resectable pancreatic and biliary carcinoma: multicenter randomized trial. Hepatogastroenterology. 1998;45:2020–2026. [PubMed] [Google Scholar]
- 14.Andre’ T, Tournigand C, Rosmorduc O, GERCOR Group et al. Gemcitabine combined with Oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 15.Cassier PA, Thevenet C, Walterb T, et al. Outcome of patients receiving chemotherapy for advanced biliary tract or gallbladder carcinoma. Eur J Gastroenterol Hepatol. 2010;22:1111–1117. doi: 10.1097/MEG.0b013e3283396dde. [DOI] [PubMed] [Google Scholar]
- 16.Makela JT, Kairaluorna MI. Superselective intra-arterial chemotherapy with mitomycin for gallbladder cancer. Br J Surg. 1993;159:415–20. doi: 10.1002/bjs.1800800739. [DOI] [PubMed] [Google Scholar]
- 17.Eckel F, Brunner T, Jelic S, Grp EGW Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and followup. Ann Oncol. 2011;22:vi40–vi44. doi: 10.1093/annonc/mdr375. [DOI] [PubMed] [Google Scholar]