Abstract
Background
Vitamin D has pleiotropic effects important for the proper functioning of multiple organ systems. We investigated whether serum 25-hydroxyvitamin D [25(OH)D] levels influenced hospitalization-free survival in patients with chronic kidney disease (CKD).
Methods
In this prospective study, serum levels of 25(OH)D were measured in 210 patients with CKD in the winter of 2009. Data regarding hospitalizations were collected over the subsequent 3 years.
Results
Vitamin D deficiency, as defined by a serum 25(OH)D level below 15 ng/mL, was observed in 76.7% of the patients. The mean 25(OH)D serum level was 13.6 ± 7.8 ng/mL in predialysis patients (n = 62) and 11.3 ± 6.7 ng/mL in dialysis patients (n = 148). During the follow-up, 107 patients (28 predialysis and 79 dialysis) were hospitalized because of infectious (33.6%) or cardiovascular diseases (23.4%). Predialysis and dialysis groups were divided into 2 subgroups based on the median 25(OH)D serum level. Kaplan–Meier analysis revealed that the risk of hospitalization was significantly lower in both predialysis and dialysis patients with above-median serum 25(OH)D levels (log-rank test; P = 0.043 and 0.002, respectively). Multivariate Cox proportional hazards models also demonstrated that the risk of hospitalization was significantly lower for patients with higher serum 25(OH)D levels in both the predialysis (hazard ratio, 0.963; 95% confidence interval, 0.93–0.99) and dialysis groups (hazard ratio, 0.955; 95% confidence interval, 0.91–0.99).
Conclusion
A lower serum 25(OH)D level predicted poorer hospitalization-free survival in both predialysis and dialysis CKD patients.
Keywords: Chronic kidney disease, Hospitalization, 25-Hydroxyvitamin D
Introduction
Vitamin D is a pleiotropic vitamin with roles in bone and mineral metabolism [1], [2] and immunomodulation [3] and has been reported to have antitumoral activity [4] and be involved in renal [5] and cardiovascular protection [6], [7]. Low 25-hydroxyvitamin D [25(OH)D] levels are associated with higher mortality rates in patients with chronic kidney disease (CKD) [8], [9], [10] and the general population [11], [12]. In addition, vitamin D receptor (VDR) activator therapy is associated with lower mortality in CKD patients [8], [9], [10], [13]. This improved prognosis is mainly attributed to the preventive effects of VDR activator against death caused by cardiovascular disease, whereas a few reports have indicated that it can prevent death from infections [14].
Cardiovascular disease and infection are the first and second leading causes of morbidity and mortality in patients with CKD, respectively [14], [15], [16]. Care of patients with CKD is associated with substantial costs to society, much of which is accounted for by a high rate of hospitalization [17].
Patients with chronic renal failure are typically vitamin D deficient [18], [19], [20], [21]. However, few studies have investigated the relationship between vitamin D deficiency and hospitalization in patients with CKD.
The aim of this prospective study was to investigate whether the serum 25(OH)D level influences hospitalization-free survival in predialysis and chronic hemodialysis patients.
Methods
Study population
We recruited adult outpatients from Bundang CHA General Hospital. A total of 210 adult patients with CKD were enrolled in our study. Criteria for inclusion were age 20–90 years and a confirmed diagnosis of CKD [defined as being on dialysis or having 2 previously estimated glomerular filtration rate (eGFR) values <60 mL/min/1.73 m2 calculated according to the equation of the Modification of Diet in Renal Disease Study Group and obtained at an interval of 3–6 months]. Patients were categorized according to the CKD stage and Kidney Disease Outcomes Quality Initiative guidelines [22]. Patients categorized as CKD Stage 5D were on hemodialysis 3 times/wk (>12 h/wk) for at least 3 months without exposure to renal transplantation. Patients with acute infectious diseases, unstable vital signs, or neoplastic illness were excluded. None of the participants were on active vitamin D, vitamin D analogues, warfarin, steroids, or anticonvulsants during the month before study entry. All patients provided written informed consent and participated during the winter period.
Measurements
Body mass index was calculated as body weight in kilograms divided by height in meters squared. Blood pressure was measured after 15 minutes of recumbency. Pulse pressure (PP) was calculated using the formula: PP = SBP – DBP, where SBP and DBP are systolic and diastolic blood pressures, respectively. Arterial stiffness was assessed using a commercially available device (VP-2000; Colin, Komaki, Japan) for brachial-ankle pulse wave velocity (baPWV) measurements. Pulse wave forms were obtained from the brachial and posttibial artery after 15 minutes of recumbency [23]. Pulse wave velocity was calculated as the distance between 2 arterial recording sites divided by transit time, as described previously [23]. The mean baPWV of the right and left sides was obtained in predialysis patients, and 1-side baPWV from 1 arm without arteriovenous access was analyzed in hemodialysis patients. Measurements were obtained before the last hemodialysis of the week in chronic hemodialysis patients. All measurements were performed by a single investigator who was blinded to the clinical data, including the participants' CKD status.
Blood chemistry
All blood chemistry measurements were determined with samples drawn before the hemodynamic study and included serum albumin, serum high-sensitive C-reactive protein, blood lipid, serum phosphate, and calcium levels. Serum calcium was adjusted for serum albumin. The total intact parathyroid (iPTH) hormone level was evaluated by an electrochemiluminescence immunoassay. Serum levels of 25(OH)D2 + D3 were measured using a chemiluminescent immunoassay (LIAISON; DiaSorin, Saluggia, Italy). Levels of 1,25(OH)2D3 were determined using an 125I radioimmunoassay (DiaSorin). On the basis of previously published studies, vitamin D deficiency was considered at serum 25(OH)D levels <15 ng/mL, vitamin D insufficiency at levels of 15–30 ng/mL, and vitamin D adequacy at serum levels >30 ng/mL [21].
Clinical outcome: hospitalization-free survival
We prospectively observed all hospitalization events, mortality, and kidney transplantation over a 3-year follow-up period. Hospitalization was defined as any hospitalization event, regardless of cause of admission, with more than 1 overnight stay. The causes of hospitalization were classified as infectious and/or inflammatory, cardiovascular, cerebrovascular, gastrointestinal bleeding, or other by medical record review or telephone contact. The outcome for this analysis was time to hospitalization from any cause.
Statistical analysis
Continuous variables are expressed as means ± standard deviation and categorical variables as frequencies. For univariate analysis, the unpaired Student's t test, analysis of variance, or the χ2 test was used, depending on the characteristics of the variables. Pearson's correlation coefficients were used to summarize the cross-sectional relationship among biochemical, clinical, and 25(OH)D levels. Predialysis and dialysis groups were divided into 2 subgroups based on the median 25(OH)D serum level. 25(OH)D was analyzed as a categorical variable to characterize the pattern of the relationship between each exposure and clinical outcome. The Kaplan–Meier method and Cox proportional hazard models were also used to investigate the influence of each factor on hospitalization-free survival in predialysis and dialysis patients. A value of P < 0.05 was considered significant. Statistical analyses were performed with SPSS for Windows (version 17; SPSS, Chicago, IL, USA).
Results
Patient characteristics and prevalence of vitamin D deficiency
Baseline clinical and biochemical characteristics are shown in Table 1. Of the patients studied, 29.5% were predialysis patients and their eGFR was 29.7 ± 15.4 mL/min/1.73 m2. Of these patients, 20.3% were Stage 3, 35.9% were Stage 4, and 17.2% were Stage 5. Chronic hemodialysis patients comprised 70.5% of the patients studied, and their mean duration of dialysis was 52.9 ± 47.7 months. The mean Kt/V of chronic hemodialysis patients was 1.4 ± 0.4. The mean 25(OH)D serum level was 13.6 ± 7.8 ng/mL in predialysis patients and 11.3 ± 6.7 ng/mL in dialysis patients. Most of the patients had vitamin D deficiency (67.6% and 80.4% of predialysis and dialysis patients, respectively), and only a few patients had an adequate vitamin D level (3.2% and 2.0% of predialysis and dialysis patients, respectively; Table 1).
Table 1.
Baseline characteristics of the study cohort
| Characteristics | Predialysis | Dialysis |
|---|---|---|
| Patients | 62 (29.5) | 148 (70.5) |
| Age (y) | 61.7 ± 12.3 | 57.0 ± 12.7 |
| Men | 62.5 | 48.0 |
| Diabetes | 50.0 | 48.6 |
| BMI (kg/m2) | 24.2 ± 3.3 | 24.4 ± 3.6 |
| Previous CVD | 21.9 | 39.9 |
| Coronary | 4.3 | 18.2 |
| Cerebrovascular | 17.2 | 27.1 |
| Peripheral VD | 4.3 | 12.2 |
| MDRD-4 (mL/min/1.73 m2) | 29.7 ± 15.4 | 6.3 ± 6.3 |
| SBP (mmHg) | 135.6 ± 20.2 | 145.3 ± 25.2 |
| PP (mmHg) | 55.8 ± 13.4 | 62.6 ± 16.6 |
| BaPWV (m/s) | 17.3 ± 4.5 | 18.4 ± 4.3 |
| WBC (103/μL) | 6.3 ± 2.0 | 6.3 ± 2.0 |
| Hemoglobin (g/dL) | 11.5 ± 1.6 | 10.5 ± 2.7 |
| Albumin (g/dL) | 4.2 ± 0.4 | 3.9 ± 0.4 |
| BUN (mg/dL) | 37.8 ± 19.8 | 63.9 ± 21.1 |
| Calcium (mg/dL) | 9.1 ± 0.6 | 9.2 ± 0.9 |
| Phosphate (mg/dL) | 3.5 ± 0.7 | 4.8 ± 1.7 |
| iPTH (pg/dL) | 96.8 ± 123.9 | 178.6 ± 252.3 |
| CRP (mg/dL) | 0.2 ± 0.4 | 0.5 ± 1.5 |
| 1,25(OH)2D (ng/mL) | 31.1 ± 13.9 | 22.5 ± 8.5 |
| 25(OH)D (ng/mL) | 13.6 ± 7.8 | 11.3 ± 6.7 |
| Normal (>30 ng/mL) | 3.2 | 2.0 |
| Insufficiency (15–30 ng/mL) | 29.0 | 17.6 |
| Deficiency (<15 ng/mL) | 67.6 | 80.4 |
| Medications | ||
| ACEi/ARBs | 85.2 | 84.0 |
| Statin | 48.4 | 45.3 |
| Ca-containing P-binder | 14.1 | 73.0 |
Data are presented as means ± SD or number of observations (%).
1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; ACEi, angiotensin-converting enzyme inhibitor; ARBs, angiotensin II receptor blockers; BaPWV, brachial-ankle pulse wave velocity; BMI, body mass index; BUN, blood urea nitrogen; Ca-containing P-binder, calcium-containing phosphate binder; CRP, C-reactive protein; CVD, cardiovascular disease; iPTH, intact parathyroid hormone; MDRD-4, estimated glomerular filtration rate estimated by Modification of Diet in Renal Disease formula; PP, pulse pressure; SBP, systolic blood pressure; VD, vascular disease; WBC, white blood cell.
Differences in patient characteristics according to the serum 25(OH)D level
Analysis using Pearson's correlation coefficient revealed an inverse correlation between the serum 25(OH)D level and blood urea nitrogen (BUN; r = –0.27, P = 0.037), serum creatinine (r = –0.256, P = 0.045), phosphate (r = –0.33, P = 0.008), and alkaline phosphatase (r = –0.40, P = 0.028), whereas there were direct correlations between the serum 25(OH)D level and hemoglobin (r = 0.33, P = 0.012) and eGFR (r = 0.29, P = 0.023) in predialysis patients. However, only the total CO2 level (r = 0.20, P = 0.014) was positively correlated with the serum 25(OH)D level in dialysis patients.
Predialysis and dialysis groups were divided into 2 subgroups based on the median 25(OH)D serum level (Table 2). There were more women than men with 25(OH)D levels below the median among both predialysis (P = 0.008) and dialysis patients (P < 0.001). Predialysis patients with a 25(OH)D serum level below the median had a lower eGFR, higher levels of BUN and iPTH, and lower hemoglobin and phosphate levels than patients with a higher 25(OH)D serum level (P < 0.001). Among dialysis patients, there was a greater percentage of diabetes patients in the group with 25(OH)D levels below the median than the group with 25(OH)D levels above the median. However, in contrast to predialysis patients, there were no significant differences in the level of phosphate, iPTH, BUN, hemoglobin, or eGFR between the groups with serum 25(OH)D levels above or below the median in dialysis patients.
Table 2.
Comparison of clinical laboratory variables between subgroups based on median 25(OH)D serum levels
| Characteristics | Predialysis patients |
Dialysis patients |
||||
|---|---|---|---|---|---|---|
| 25(OH)D ≥11.8 | 25(OH)D <11.8 | P | 25(OH)D ≥9.6 | 25(OH)D <9.6 | P | |
| Patients | 31 (50.0) | 31 (50.0) | – | 73 (49.3) | 75 (50.7) | – |
| Age (y) | 62.6 ± 11.9 | 60.8 ± 12.8 | 0.581 | 57.0 ± 12.2 | 57.1 ± 13.2 | 0.780 |
| Men | 80.6 | 48.4 | 0.008 | 69.9 | 34.7 | < 0.001 |
| Diabetes | 41.9 | 61.3 | 0.130 | 37.0 | 60.0 | 0.005 |
| BMI (kg/m2) | 24.5 ± 2.8 | 23.9 ± 3.7 | 0.485 | 22.6 ± 3.0 | 21.9 ± 3.4 | 0.203 |
| Previous CVD | 22.6 | 22.6 | 1.000 | 42.5 | 37.3 | 0.525 |
| Coronary | 3.2 | 3.2 | 1.000 | 13.7 | 22.7 | 0.159 |
| Cerebrovascular | 19.4 | 16.1 | 0.742 | 32.9 | 21.3 | 0.115 |
| Peripheral VD | 3.2 | 6.5 | 0.557 | 8.3 | 16.0 | 0.158 |
| MDRD-4 (mL/min/1.73 m2) | 37.5 ± 15.2 | 23.8 ± 13.2 | 0.002 | 6.4 ± 5.9 | 6.1 ± 6.7 | 0.819 |
| SBP (mmHg) | 131.2 ± 20.1 | 139.9 ± 19.6 | 0.100 | 145.8 ± 26.7 | 144.9 ± 23.7 | 0.843 |
| PP (mmHg) | 52.5 ± 13.0 | 58.9 ± 13.2 | 0.067 | 62.1 ± 18.5 | 63.2 ± 14.6 | 0.688 |
| BaPWV (m/s) | 16.8 ± 4.0 | 17.9 ± 4.8 | 0.342 | 17.9 ± 4.0 | 19.0 ± 4.6 | 0.144 |
| WBC (103/μL) | 6.2 ± 1.6 | 6.5 ± 2.4 | 0.659 | 6.1 ± 1.9 | 6.5 ± 2.1 | 0.182 |
| Hemoglobin (g/dL) | 12.0 ± 1.7 | 11.0 ± 1.3 | 0.011 | 10.1 ± 0.9 | 10.9 ± 3.4 | 0.081 |
| Albumin (g/dL) | 4.4 ± 0.3 | 4.1 ± 0.5 | 0.057 | 4.0 ± 0.3 | 3.9 ± 0.4 | 0.215 |
| BUN (mg/dL) | 31.1 ± 14.7 | 44.4 ± 22.0 | 0.007 | 65.7 ± 18.1 | 62.3 ± 23.6 | 0.346 |
| Calcium (mg/dL) | 9.1 ± 0.7 | 9.2 ± 0.4 | 0.503 | 9.2 ± 0.7 | 9.2 ± 1.1 | 0.939 |
| Phosphate (mg/dL) | 4.0 ± 0.9 | 3.4 ± 0.8 | 0.007 | 4.8 ± 1.7 | 4.9 ± 1.8 | 0.902 |
| iPTH (pg/dL) | 61.9 ± 57.4 | 131.7 ± 159.4 | 0.027 | 170.6 ± 215.5 | 186.5 ± 284.9 | 0.693 |
| CRP (mg/dL) | 0.2 ± 0.3 | 0.2 ± 0.5 | 0.540 | 0.4 ± 0.9 | 0.6 ± 2.0 | 0.487 |
| 1,25(OH)2D (ng/mL) | 36.0 ± 16.1 | 26.1 ± 9.0 | 0.004 | 23.7 ± 8.6 | 21.4 ± 8.3 | 0.107 |
| 25(OH)D (ng/mL) | 19.4 ± 7.1 | 7.8 ± 2.2 | < 0.001 | 15.9 ± 6.8 | 6.9 ± 1.7 | < 0.001 |
| Medications | ||||||
| ACEi/ARBs | 80.4 | 100.0 | 0.789 | 67.4 | 73.4 | 0.560 |
| Statin | 51.6 | 48.4 | 0.801 | 40.8 | 51.4 | 0.206 |
| Ca-containing P-binder | 12.9 | 16.1 | 0.721 | 77.5 | 71.6 | 0.421 |
Data are presented as means ± SD or number of observations (%).
1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; ACEi, angiotensin-converting enzyme inhibitor; ARBs, angiotensin II receptor blockers; BaPWV, brachial-ankle pulse wave velocity; BMI, body mass index; BUN, blood urea nitrogen; Ca-containing P-binder, calcium-containing phosphate binder; CRP, C-reactive protein; CVD, cardiovascular disease; iPTH, intact parathyroid hormone; MDRD-4, estimated glomerular filtration rate estimated by Modification of Diet in Renal Disease formula; PP, pulse pressure; SBP, systolic blood pressure; VD, vascular disease; WBC, white blood cell.
Serum 25(OH)D level and risk of hospitalization
One hundred seven patients (28 predialysis and 79 dialysis) were hospitalized, 24 patients died (2 predialysis and 22 dialysis), and 4 patients underwent kidney transplantation during the 3-year observational period. Mean observational period was 1,048.8 ± 331.0 days. Causes of hospitalization events included infection-related disease (33.6%), cardiovascular disease (23.4%), and cerebrovascular disease (7.5%; Table 3). Most common causes of death were cardiovascular disease (37.5%) and infection-related disease (29.2%).
Table 3.
Causes of hospitalization during the follow-up period
| Characteristics | Predialysis patients (n) | Dialysis patients (n) |
|---|---|---|
| Infectious/inflammatory | 8 | 28 |
| Cardiovascular | 7 | 18 |
| Cerebrovascular | 0 | 8 |
| GI bleeding | 1 | 3 |
| Other | 12 | 22 |
| Total | 28 | 79 |
GI, gastrointestinal.
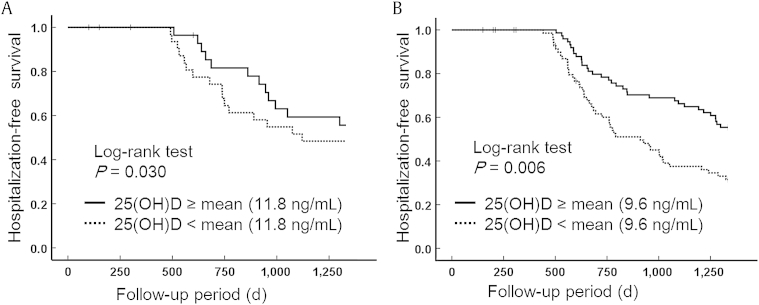
Hospitalization-free survival according to the level of serum 25(OH)D in predialysis and dialysis patients is shown in Fig. 1. Kaplan–Meier analysis revealed that the risk of hospitalization was significantly lower in both predialysis and dialysis patients with above-median serum 25(OH)D levels (log-rank test; P = 0.030 and 0.006, respectively). In univariate Cox proportional analysis, serum 25(OH)D level, diabetes, coronary disease, eGFR, hemoglobin level, and phosphate level were significant predictors of all-cause hospitalization in predialysis patients. In contrast, low serum 25(OH)D level, age, sex, and diabetes were significant predictors of hospitalization in dialysis patients (Table 4). After adjustment for all related covariables (sex, diabetes, coronary disease, eGFR, hemoglobin level, phosphate level, and iPTH level for predialysis patients and age, sex, and diabetes for dialysis patients), multivariate Cox proportional hazards models demonstrated that the risk of hospitalization was significantly lower for patients with above-median serum 25(OH)D levels in both the predialysis (hazard ratio, 0.963; 95% confidence interval, 0.93–0.99) and dialysis groups (hazard ratio, 0.955; 95% confidence interval, 0.91–0.99; Table 5).
Figure 1.
Serum 25(OH)D level associated with hospitalization-free survival. (A) Kaplan–Meier estimates of hospitalization-free survival probability of predialysis patients in relation to serum 25(OH)D (mean) ≥11.8 ng/mL or <11.8 ng/mL. (B) Kaplan–Meier estimates of hospitalization-free survival probability of dialysis patients in relation to serum 25(OH)D ≥9.6 ng/mL (mean) or <9.6 ng/mL.
25(OH)D, 25-hydroxyvitamin D.
Table 4.
Univariate Cox proportional models for hospitalization
| Characteristics | Predialysis patients |
Dialysis patients |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (y) | 1.000 (0.98–1.02) | 0.991 | 1.024 (1.01–1.04) | 0.007 |
| Sex (female/male) | 0.761 (0.49–1.18) | 0.222 | 0.592 (0.38–0.92) | 0.021 |
| Diabetes (absent/present) | 3.217 (2.00–5.18) | < 0.001 | 2.374 (1.50–3.75) | < 0.001 |
| Coronary (absent/present) | 13.631 (5.47–34.00) | < 0.001 | 1.358 (0.78–2.35) | 0.275 |
| MDRD-4 (mL/min/1.73 m2) | 0.982 (0.97–0.98) | 0.014 | – | – |
| Albumin (g/dL) | 0.579 (0.27–1.24) | 0.160 | 0.677 (0.37–1.24) | 0.677 |
| Hemoglobin (g/dL) | 0.776 (0.68–0.89) | < 0.001 | 1.036 (0.97–1.11) | 0.316 |
| Calcium (mg/dL) | 0.703 (0.47–1.06) | 0.091 | 1.131 (0.86–1.49) | 0.386 |
| Phosphate (mg/dL) | 1.485 (1.17–1.88) | 0.001 | 0.995 (0.88–1.13) | 0.944 |
| iPTH (pg/dL) | 1.001 (0.99–1.00) | 0.227 | 0.999 (0.99–1.00) | 0.108 |
| CRP (mg/dL) | 1.548 (0.95–2.51) | 0.780 | 0.928 (0.76–1.13) | 0.458 |
| 25(OH)D (ng/mL) | 0.940 (0.91–0.97) | 0.001 | 0.934 (0.89–0.97) | 0.004 |
| 25(OH)D (below/above median) | 0.624 (0.41–0.96) | 0.033 | 0.536 (0.34–0.84) | 0.007 |
25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; iPTH, intact parathyroid hormone; MDRD-4, estimated glomerular filtration rate estimated by Modification of Diet in Renal Disease formula.
Table 5.
Multivariate Cox proportional hazard models for hospitalization-free survival in predialysis and dialysis patients
| Characteristics | Predialysis patients |
Dialysis patients |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (y) | – | – | 1.023 (1.00–1.04) | 0.014 |
| Sex (female/male) | 0.808 (0.47–1.38) | 0.440 | 0.767 (0.48–1.22) | 0.260 |
| Diabetes (absent/present) | 3.579 (2.00–6.22) | < 0.001 | 2.017 (1.27–3.21) | 0.003 |
| Coronary (absent/present) | 31.719 (11.57–86.98) | < 0.001 | – | – |
| MDRD-4 (mL/min/1.73 m2) | 0.993 (0.96–1.02) | 0.682 | – | – |
| Hemoglobin (g/dL) | 0.945 (0.80–1.12) | 0.519 | – | – |
| Phosphate (mg/dL) | 1.090 (0.76–1.58) | 0.625 | – | – |
| PTH | 1.003 (1.01–1.05) | 0.005 | – | – |
| 25(OH)D (ng/mL) | 0.963 (0.93–0.99) | 0.039 | 0.955 (0.91–0.99) | 0.040 |
25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; HR, hazard ratio; MDRD-4, estimated glomerular filtration rate estimated by Modification of Diet in Renal Disease formula; PTH, parathyroid hormone.
Discussion
We demonstrated a high prevalence of 25(OH)D deficiency and insufficiency in predialysis and dialysis patients. Most importantly, a low serum 25(OH)D level predicted poor hospitalization-free survival in CKD patients with or without hemodialysis.
CKD is an increasing global health problem because of the high risk of total and cardiovascular morbidity and mortality. In the United States, the prevalence estimate of CKD increased from 12.3% to 14.0% over the past 20 years [24]. Similarly, in Korea, the prevalence of end stage renal disease requiring dialysis increased by approximately ∼8–9% per year during the period 2005–2013 [25]. Along with the increase in CKD prevalence, the economic costs resulting from the care of these patients have increased. Medicare data from the United States showed that in 2012, the annual expenditure spending for patients with CKD was 24% of the total budget, a number greater than that associated with chronic heart failure or diabetes [24]. Therefore, proper management of CKD patients is required to improve clinical outcomes and reduce medical costs. In this regard, our results indicating a significant relationship between the serum level of 25(OH)D and hospitalization in CKD patients could provide a basis and goal for vitamin D supplement therapy among these patients.
It is well known that vitamin D deficiency is highly prevalent among CKD patients [18], [19], [20], [21]. The prevalence of hypovitaminosis, including insufficient and deficient serum 25(OH)D levels, has been reported to be as high as 40% among CKD patients, although there are wide variations in reported prevalence depending on race, season, latitude, residual renal function, and type of dialysis treatment [18], [19], [20]. Many factors may contribute to low 25(OH)D levels in CKD, including loss of vitamin D–binding proteins in the urine, ineffective vitamin D synthesis in the skin on exposure to UV-B radiation, and possibly reduced sun exposure and nutritional intake [21]. Moreover, an increase in the level of fibroblast growth factor 23, which can directly suppress the activity and expression of 1α-hydroxylase, decreases the vitamin D level during the course of the disease [26]. Decreased expression of renal megalin, which mediates the active process of endocytosis of 25(OH)D filtered from the glomerular ultrafiltrate, may further reduce serum 25(OH)D concentrations in CKD patients [27]. It can be inferred that an insufficient serum vitamin D level and low renal megalin expression result in a vicious negative feedback cycle based on the function of 1,25(OH)2D3 as an inducer of renal megalin [28].
In the hemodialysis (HEMO) study, which was designed to examine the effect of dialysis dose and membrane flux in 1,846 patients undergoing maintenance hemodialysis in the United States, participants with higher serum 25(OH)D levels were younger, more frequently male, less likely to have diabetes, and more likely to have higher serum albumin and calcium and lower serum iPTH and high-sensitive C-reactive protein levels than participants with lower serum 25(OH)D levels [15]. However, in our study, only male gender and diabetes were significantly different between dialysis patients with 25(OH)D levels above or below the median, possibly due to the small size of our study cohort. In addition, arterial stiffness parameters (PP and baPWV) had a tendency to be higher in the lower 25(OH)D groups among both predialysis and dialysis patients. A larger study may be better able to elucidate 25(OH)D-associated variables in Asian CKD patients.
Insight into the nonhormonal, intracrine, and paracrine actions of vitamin D over the past few decades has resulted in a new perspective on this vitamin [29]. In addition to being a circulating regulator of mineral and skeletal homeostasis, vitamin D has multifunctional roles in regulating innate immunity, blood pressure, cell proliferation, erythropoiesis, and insulin resistance [28], [30], [31], [32]. Actually, most tissues and cells in the body have a VDR, and several contain 1α-hydroxylase, which converts 25(OH)D into the active form 1,25(OH)2D3.
When toll-like receptors on monocyte–macrophages are activated by pathogen-associated membrane patterns, such as lipopolysaccharide, 1α-hydroxylase and VDR are upregulated, and 25(OH)D is converted to 1,25(OH)2D3. Then, the 1,25(OH)2D3–VDR–retinoid X receptor complex promotes the expression of the cathelicidin antimicrobial gene in the nucleus [29]. Recently, the association of upper respiratory infection and serum 25(OH)D has been demonstrated in both the general population and CKD patients [14], [15], [33]. Interestingly, 1,25(OH)2D3-induced expression of cathelicidin antimicrobial peptide has been reported in other tissues such as the gut, placenta, and skin [34], [35], [36], which means vitamin D deficiency could contribute to various infectious diseases.
The exact mechanism of the protective role of the 25(OH)D level against cardiovascular disease is still under investigation. However, clinical data have demonstrated that hypovitaminosis D is associated with hypertension, coronary heart disease, congestive heart failure, and peripheral artery disease [2], [16], [30], [37]. London et al [38] reported that 25(OH)D and 1,25(OH)2D3 were negatively correlated with aortic pulse wave velocity and positively correlated with brachial artery distensibility and flow-mediated dilatation in a cross-sectional study of chronic hemodialysis patients. They proposed that vitamin D deficiency could be associated with arteriosclerosis and endothelial dysfunction in those patients [38]. The renin–angiotensin–aldosterone system (RAS) is a hormone system that regulates blood pressure and fluid balance. Angiotensin II, the main peptide of the RAS, is not only a vasoactive hormone but also a regulator of cell proliferation and/or apoptosis, fibrosis, and a proinflammatory mediator [39]. One mechanism by which vitamin D is cardiovascular protective is through suppression of RAS because 1,25(OH)2D3 can inhibit renin synthesis in the kidney [2]. In our study, infectious (33.6%) and cardiovascular (23.4%) diseases were the most common causes of hospitalization (Table 3), which is consistent with previous reports [9], [14], [15], [16]. Among the various extrarenal functions of vitamin D, its antimicrobial and cardiovascular protective effects may lower hospitalization events and promote longer hospitalization-free survival.
Although 1,25(OH)2D3 is the active form of vitamin D, 25(OH)D in the serum is the most plentiful and stable metabolite of vitamin D, so it is the best indicator of vitamin D status. Compared with 25(OH)D, which has a half-life of 2–3 weeks, circulating 1,25(OH)2D3 only has a half-life of 8–12 hours. Furthermore, circulating 1,25(OH)2D3 concentration is only one-thousandth of the 25(OH)D concentration and responds rapidly to changes in calcium and phosphate metabolism [33]. Multiple observational studies have shown that a low 25(OH)D level is associated with poor clinical outcomes in CKD and end stage renal disease patients [9], [14], [15], [20], [40]. Several studies have reported that 1,25(OH)2D3 is an independent predictor of clinical outcome [9], [40]. In our study, the 25(OH)D level was significantly correlated with hospitalization-free survival (Table 5, Fig. 1), but the 1,25(OH)2D3 level was not (data not shown). This finding may be because of the low concentration and short half-life of 1,25(OH)2D3 in blood and the small size of our study.
This study had several limitations. Only the baseline level of serum 25(OH)D was measured, and changes in serum 25(OH)D levels, which may affect hospitalization, were not recorded. Fibroblast growth factor 23, cathelicidin, and rennin levels, which can all affect vitamin D status and clinical outcomes, were not examined. We were also not able to determine the association between serum 25(OH)D levels and mortality because of the low number of deaths during the follow-up period.
Nevertheless, our study had several strengths. Serum 25(OH)D levels are affected by seasonal variations and the assessment method. Here, we measured 25(OH)D levels of all the participants during winter. Furthermore, patients who received treatment with anticonvulsants or steroids, which affect serum vitamin D levels, were excluded. The homogeneity of the participants in each group was attributed to the single-center nature of the study. Patients on hemodialysis for >3 months at a frequency of 3 times/wk (>12 h/wk) who did not undergo renal transplantation were selectively enrolled.
In conclusion, our findings suggest that the serum 25(OH)D level is a predictor of hospitalization-free survival in CKD patients; vitamin D supplementation in these patients may therefore improve clinical outcome.
Acknowledgments
This study was supported by a grant from the Korea Society of Hypertension in 2011.
Contributor Information
Dong Ho Yang, Email: dhyang@cha.ac.kr.
So-Young Lee, Email: ysy0119@cha.ac.kr.
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;76(Suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 2.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Veldman C.M., Cantorna M.T., DeLuca H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 4.Holick M.F. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R., Acharya M., Tian J., Hippensteel R.L., Melnick J.Z., Qiu P., Williams L., Batlle D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 6.Zittermann A., Koerfer R. Protective and toxic effects of vitamin D on vascular calcification: clinical implications. Mol Aspects Med. 2008;29:423–432. doi: 10.1016/j.mam.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Pilz S., Marz W., Wellnitz B., Seelhorst U., Fahrleitner-Pammer A., Dimai H.P., Boehm B.O., Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy C.P., Ahmadzadeh S., Anderson J.E., Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 9.Wolf M., Shah A., Gutierrez O., Ankers E., Monroy M., Tamez H., Steele D., Chang Y., Camargo C.A., Jr., Tonelli M., Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 10.Duranton F., Rodriguez-Ortiz M.E., Duny Y., Rodriguez M., Daurès J.P., Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013;37:239–248. doi: 10.1159/000346846. [DOI] [PubMed] [Google Scholar]
- 11.Melamed M.L., Michos E.D., Post W., Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schottker B., Jorde R., Peasey A., Thorand B., Jansen E.H., Groot L., Steppel M., Gardiner J., Ordóñez-Mena J.M., Perna L., Wilsgaard T., Rathmann W., Feskens E., Kampman E., Signos G., Njølstad, Mathiesen E.B., Kubínová R., Pająk A., Topor-Madry R., Tamosiunas A., Hughes M., Kee F., Bobak M., Trichopoulou A., Boffetta P., Brenner H., Consortium on Health and Ageing: Network of Cohorts in Europe and the United States Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. doi: 10.1136/bmj.g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teng M., Wolf M., Ofsthun M.N., Lazarus J.M., Hernán M.A., Camargo C.A., Jr., Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 14.Tsujimoto Y., Tahara H., Shoji T., Emoto M., Koyama H., Ishimura E., Tabata T., Nishizawa Y., Inaba M. Active vitamin D and acute respiratory infections in dialysis patients. Clin J Am Soc Nephrol. 2011;6:1361–1367. doi: 10.2215/CJN.08871010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chonchol M., Greene T., Zhang Y., Hoofnagle A.N., Cheung A.K. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol. 2016;27:227–237. doi: 10.1681/ASN.2014101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T.J., Pencina M.J., Booth S.L., Jacques P.F., Ingelsson E., Lanier K., Benjamin E.J., D'Agostino R.B., Wolf M., Vasan R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St Peter W.L., Khan S.S., Ebben J.P., Pereira B.J., Collins A.J. Chronic kidney disease: the distribution of health care dollars. Kidney Int. 2004;66:313–321. doi: 10.1111/j.1523-1755.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.Y., Kim H.Y., Gu S.W., Kim H.J., Yang D.H. 25-Hydroxyvitamin D levels and vascular calcification in predialysis and dialysis patients with chronic kidney disease. Kidney Blood Pressure Res. 2012;35:349–354. doi: 10.1159/000335952. [DOI] [PubMed] [Google Scholar]
- 19.Barreto D.V., Barreto F.C., Liabeuf S., Temmar M., Boitte F., Choukroun G., Fournier A., Massy Z.A. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1128–1135. doi: 10.2215/CJN.00260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravani P., Malberti F., Tripepi G., Pecchini P., Cutrupi S., Pizzini P., Mallamaci F., Zoccali C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 22.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 23.Andrade J., Er L., Ignaszewski A., Levin A. Exploration of association of 1,25-OH2D3 with augmentation index, a composite measure of arterial stiffness. Clin J Am Soc Nephrol. 2008;3:1800–1806. doi: 10.2215/CJN.00900208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins A.J., Foley R.N., Herzog C., Chavers B., Gilbertson D., Herzog C., Ishani A., Johansen K., Kasiske B., Kutner N., Liu J., St Peter W., Ding S., Guo H., Kats A., Lamb K., Li S., Li S., Roberts T., Skeans M., Snyder J., Solid C., Thompson B., Weinhandl E., Xiong H., Yusuf A., Zaun D., Arko C., Chen S.C., Daniels F., Ebben J., Frazier E., Hanzlik C., Johnson R., Sheets D., Wang X., Forrest B., Constantini E., Everson S., Eggers P., Agodoa L. US renal data system 2012 annual data report. Am J Kidney Dis. 2013;61(Suppl 1) doi: 10.1053/j.ajkd.2012.11.031. A7, e1–e476, doi:10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Jin D.C. Dialysis registries in the world: Korean Dialysis Registry. Kidney Int Suppl. 2015;5:8–11. doi: 10.1038/kisup.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perwad F., Azam N., Zhang M.Y., Yamashita T., Tenenhouse H.S., Portale A.A. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 27.Dusso A.S. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int Suppl. 2011;1:136–141. doi: 10.1038/kisup.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W., Yu W.R., Carling T., Juhlin C., Rastad J., Ridefelt P., Akerström G., Hellman P. Regulation of gp330/megalin expression by vitamins A and D. Eur J Clin Invest. 1998;28:100–107. doi: 10.1046/j.1365-2362.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 29.Adams J.S., Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ke L., Mason R.S., Kariuki M., Mpofu E., Brock K.E. Vitamin D status and hypertension: a review. Integr Blood Press Control. 2015;8:13–35. doi: 10.2147/IBPC.S49958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams J.S., Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucisano S., Di Mauro E., Montalto G., Cernaro V., Buemi M., Santoro D. Vitamin D and anemia. J Ren Nutr. 2014;24:61–62. doi: 10.1053/j.jrn.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Ginde A.A., Mansbach J.M., Camargo C.A., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu N., Kaplan A.T., Low J., Nguyen L., Liu G.Y., Equils O., Hewison M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod. 2009;80:398–406. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu N., Nguyen L., Chun R.F., Lagishetty V., Ren S., Wu S., Hollis B., DeLuca H.F., Adams J.S., Hewison M. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–4808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schauber J., Oda Y., Buchau A.S., Yun Q.C., Steinmeyer A., Zugel U., Bikle D.D., Gallo R.L. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 37.Kim D.H., Sabour S., Sagar U.N., Adams S., Whellan D.J. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–1544. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 38.London G.M., Guerin A.P., Verbeke F.H., Pannier B., Boutouyrie P., Marchais S.J., Mëtivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Ortega M., Lorenzo O., Ruperez M., Esteban V., Suzuki Y., Mezzano S., Plaza J.J., Egido J. Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 40.Dobnig H., Pilz S., Scharnagl H., Renner W., Seelhorst U., Wellnitz B., Kinkeldei J., Boehm B.O., Weihrauch G., Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]