Abstract
Objective
The role of the central nervous system in mediating metabolic effects of Roux-en-Y gastric bypass (RYGB) surgery is poorly understood. Using a rat model of RYGB, we aimed to identify changes in gene expression of key hypothalamic neuropeptides known to be involved in the regulation of energy balance.
Methods
Lean male Sprague-Dawley rats underwent either RYGB or sham surgery. Body weight and food intake were monitored bi-weekly for 60 days post-surgery. In situ hybridization mRNA analysis of hypothalamic AgRP, NPY, CART, POMC and MCH was applied to RYGB and sham animals and compared with ad libitum fed and food-restricted rats. Furthermore, in situ hybridization mRNA analysis of dopaminergic transmission markers (TH and DAT) was applied in the midbrain.
Results
RYGB surgery significantly reduced body weight and intake of a highly palatable diet but increased chow consumption compared with sham operated controls. In the arcuate nucleus, RYGB surgery increased mRNA levels of orexigenic AgRP and NPY, whereas no change was observed in anorexigenic CART and POMC mRNA levels. A similar pattern was seen in food-restricted versus ad libitum fed rats. In contrast to a significant increase of orexigenic MCH mRNA levels in food-restricted animals, RYGB did not change MCH expression in the lateral hypothalamus. In the VTA, RYGB surgery induced a reduction in mRNA levels of TH and DAT, whereas no changes were observed in the substantia nigra relative to sham surgery.
Conclusion
RYGB surgery increases the mRNA levels of hunger-associated signaling markers in the rat arcuate nucleus without concomitantly increasing downstream MCH expression in the lateral hypothalamus, suggesting that RYGB surgery puts a brake on orexigenic hypothalamic output signals. In addition, down-regulation of midbrain TH and DAT expression suggests that altered dopaminergic activity also contributes to the reduced intake of palatable food in RYGB rats.
Keywords: Roux-en-Y gastric bypass, Energy homeostasis, Hypothalamus, Hedonic, Mesolimbic pathway
Highlights
-
•
RYGB induces expression of the orexigenic peptides NPY and AgRP in arcuate nucleus.
-
•
Orexigenic MCH expression in lateral hypothalamus is not altered following RYGB.
-
•
mRNA levels of mesolimbic dopaminergic marker genes are reduced after RYGB.
1. Introduction
Obesity, diabetes and the metabolic syndrome are major health problems worldwide. The World Health Organization estimates that more than 1.9 billion adults and over 42 million children under the age of five are overweight or obese [1]. With today's epidemic proportions of obesity, more deaths are related to overweight than underweight. Lifestyle intervention and pharmacological treatment have had limited success in weight management in obese patients [2], [3]. Hence, to date bariatric surgery procedures, including Roux-en-Y gastric bypass (RYGB), are considered the most effective treatment of obesity and its co-morbidities, such as type-2 diabetes mellitus (T2DM), hypertension and stroke [4], [5].
The exact mechanisms underlying the weight loss and anti-diabetic effects of gastric bypass are not fully understood, and substantial efforts are being made to identify novel targets for more efficacious anti-obesity drug therapies. One of the consistent findings in animal models as well as in humans after RYGB is elevated plasma levels of gut-derived hormones such as glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), and it is believed that these hormones are involved in the beneficial effects of surgery [6], [7], [8], [9]. However, despite the intense research into the underlying mechanisms of RYGB-induced weight loss, currently, there is a lack of studies specifically investigating the changes in central signaling pathways involved in metabolic control. It is well-known that long-term energy balance is regulated by a complex network of central signaling pathways that regulate food intake, energy expenditure and metabolic efficacy [10]. Essential for weight homeostasis is the integration of orexigenic and anorexigenic signaling by the leptin and melanocortin dependent pathways within the central nervous system (CNS) [11]. The hypothalamic arcuate nucleus (Arc) is of special importance, as this region contains two distinct leptin responsive neuronal populations, i.e. orexigenic neuropeptide Y (NPY) and agouti-related peptide (AgRP) expressing neurons as well as anorexigenic cocaine and amphetamine related transcript (CART) and proopiomelanocortin (POMC) expressing neurons [12], [13], [14]. These first order neurons in the Arc project to the paraventricular nucleus (PVN) and the lateral hypothalamus (LHA), which, in combination, determine the balance of behavioral and autonomic outputs involved in eating behavior and metabolism (For review see [14]). Present information on transcriptional changes in the Arc following bariatric surgery is limited to a few studies assessing the effects of biliopancreatic and duodenal-jejunal bypass in rats. Interestingly, both procedures were shown to significantly increase the arcuate NPY expression, indicating that orexigenic pathways were stimulated without neutralizing the surgically induced reductions in food intake and body weight [15], [16]. This counterintuitive response indicates that other downstream signaling cascades may be blocked, potentially MCH expression in the LHA, which is known to be strongly modulated by POMC (inhibition) and NPY (activation) application [12].
Besides the homeostatic regulation of energy balance, central hedonic signaling is also able to influence whole body energy homeostasis [17]. In accordance, the mesolimbic dopaminergic pathway is closely associated with the regulation of food intake where elevated levels of dopamine in the ventral tegmental area (VTA) causes increased motivation to consume particularly energy-dense food [18]. This may also be relevant for the weight lowering effect of gastric bypass procedures, as rodent RYGB models are reported to change food preference from high to low sweet and fat food stimuli [19], [20], [21], indicating that RYGB modifies the hedonic value of energy-dense food. To date, however, very few studies have investigated the central component of this altered behavior on the transcriptional level.
The present study was undertaken to get further insight into the central signaling pathways potentially influenced by RYGB surgery. A special emphasis was put on key relay structures conveying orexigenic and anorexigenic signaling in the hypothalamus, including the Arc and LHA. In addition, to investigate neural circuitries underlying the interaction between nutritional status and reward, the impact of RYGB on transcriptional markers of midbrain dopaminergic neurotransmission was assessed.
2. Materials and methods
2.1. Experimental animals
All animal experiments were conducted in accordance with internationally accepted principles for the care and use of laboratory animals, and in compliance with personal animal license (2013-15-2934-00784) issued by the Danish Committee for Animal Research.
Male Sprague Dawley rats 12 weeks of age underwent either RYGB (n = 9; body weight 409 ± 16 g) or sham surgery (n = 9; body weight 360 ± 7 g). Four weeks prior to surgery, animals had ad libitum access to a two-choice diet consisting of chow (altromin 1324, Brogaarden, Denmark) and the Gubra high palatable high fat diet (HPHF diet; Nutella (Ferrero, Italy), peanut butter (pcd, Netherlands) and powdered chow [22]). The rats were single-housed throughout the study under controlled environmental conditions (12 h light/12 h dark cycle; 22 ± 1 °C; 50 ± 10% relative humidity). Body weight, food and water intake were monitored bi-weekly during the study.
In addition, male Sprague Dawley rats were included as food restricted (n = 8) and free-fed (n = 8) controls for the analysis of hunger vs. satiety associated hypothalamic gene expression. Free-fed animals had ad libitum access to the two-choice diet as described above, whereas food restricted animals had access to approximately 50% of the food ingested by free-fed animals. Animals were terminated by decapitation under CO2/O2 anesthesia after 48 h, and brains were quickly removed and snap frozen on crushed dry ice and stored at −80 °C until further processing.
2.2. Surgery
Three days prior to surgery, animals were put on liquid diet (Osmolite 1 Cal.; Abbott Nutrition). On the day of surgery, animals underwent whole body composition analysis by non-invasive EchoMRI scanning (EchoMRI-900 Analyzer, EchoMRI, USA), and a wire grate was placed in the bottom of the cage to prevent coprophagia. Prior to surgery, animals were subcutaneously administered with warm saline (20 ml/kg), buprenorphine (0.03 mg/kg), enrofloxacin (0.5 mg/kg) and carprofen (0.5 mg/kg) to prevent post-operative infection, lethargy and pain relief.
The RYGB surgical procedure was performed according to Chambers et al. [23]. In brief, the abdomen was exposed using a midline laparotomy after induction of surgical anesthesia with an isoflurane/O2 mixture. The jejunum was transected 30 cm distal to the ligament of Treitz. A longitudinal anti-mesenteric incision was made 10 cm distal to the transected bowel and connected to the afferent limb of the jejunum with a running 8-0 Vicryl absorbable suture (Ethicon, Somerville, NJ, USA). The stomach was exposed and the fundus was excised by making a vertical cut along the line separating the corpus from the fundus with an ETS-FLEX 35-mm staple gun (Ethicon). A second staple line was placed across the waist of the stomach, creating a gastric pouch approximately 10% the size of the normal stomach. The distal remnant was returned to the peritoneal cavity and an incision was made on one side of the gastric pouch considering the vascular architecture. The efferent limb of the transected jejunum was connected to the gastric pouch with a running 8-0 nylon non-absorbable suture (Ethicon). After repositioning the gastric pouch into the peritoneal cavity, the abdominal wall was closed.
The sham procedure followed the steps of the RYGB but gastrointestinal surgery only included a transection of the jejunum 30 cm distal to the ligament of Treitz, which was immediately re-sutured.
2.3. Post-surgery care
The wire grate was kept in place for five days post-operatively, and only liquid diet was offered in this period. Subcutaneous injections of warm saline BID (20 ml/kg), carprofen QD (5 mg/kg) and enrofloxacin QD (5 mg/kg) were administered for five days after surgery. On day six, the wire grate was removed and ad libitum access to the two-choice diet was re-introduced.
2.4. Termination
60 days post-surgery, animals were euthanized by decapitation under CO2/O2 anesthesia. All animals were euthanized in the morning, 2–3 h after lights on. RYGB and sham operated animals were terminated in a randomized order. Prior to termination, animals underwent an additional whole-body echo-MRI scan. Following termination, brains were quickly removed, snap frozen on crushed dry ice, and stored at −80 °C until further processing.
2.5. Tissue processing
12 μm thick coronal sections of the hypothalamus and midbrain were cut on a cryostat (CM 1850, Leica, Germany) using systematic uniform random sampling. Serial sections were sampled at every 180 μm (i.e. every 15th section) and mounted on super-frost plus slides. Sections from the hypothalamus were sampled from the rostral part of the hypothalamic PVN to the caudal part of the Arc. Sections from the midbrain spanned the full rostro-caudal extension of the VTA and the substantia nigra (SN). Sections were allowed to dry at room temperature for an hour and then kept at −80 °C until hybridization was carried out.
2.6. In situ hybridization
In situ hybridizations (ISH) were performed using 33P-labeled RNA probes according to previously described procedures [24], [25]. Riboprobes were directed against rat cDNAs of neuropeptide Y (NPY, bp57-404, GenBank accession number M20373), agouti-related peptide (AgRP, alternative splice variant, GenBank accession number U89486), proopiomelanocortin (POMC, bp162-626, GenBank accession number J00759), cocaine-and-amphetamine-regulated transcript (CART, bp226–411; GenBank accession number U10071), melanin-concentrating hormone (MCH, bp45-440, GenBank accession number M29712), tyrosine hydroxylase (TH, bp14-1165, GenBank accession number M10244) and dopamine transporter (DAT, bp1406-1805, GenBank accession number M80570). Anti-sense probes were generated by in vitro transcription from the linearized plasmid DNA containing the cDNA clones mentioned above.
Prior to ISH, sections were fixated in 4% PFA for 5 min, rinsed 2 times in phosphate buffered saline and acetylated in triethanolamine (0.1 M), rinsed again and rehydrated through an ethanol gradient from absolute ethanol to water. A viscous hybridization mixture containing the RNA probe was added to the dry sections (80 μl per slide) after which the sections were cover-slipped. Hybridization was carried out in a sealed polypropylene box at 47 °C and 100% relative humidity over-night. Post-hybridization washes were performed at 62 °C and 67 °C in 50% formamide (1 h at each temperature). Finally, single stranded RNA was removed by RNAse digestion (30 min at 45 °C). After hybridization, sections were exposed to autoradiographic films, exposed for 1–7 days depending on the individual probe and subsequently developed in Kodak D19 developer.
The hybridization signals were evaluated using VIS image analysis software (Visiopharm, Denmark) on high-resolution scanned films. The area under examination was delineated by a region of interest (ROI) in the software, and the signals were quantified as the product of intensified area and mean pixel intensity. Local background subtraction method was applied.
2.7. Statistics
Graphical presentations, calculations and statistical analyses were carried out using GraphPad software (GraphPad Prism version 5, San Diego, California, USA). Statistical analysis was performed using either two-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test, or student's unpaired t-test (p < 0.05 was considered significant). Results are presented as mean ± standard error of the mean (SEM).
3. Results
3.1. RYGB reduces body weight and adiposity
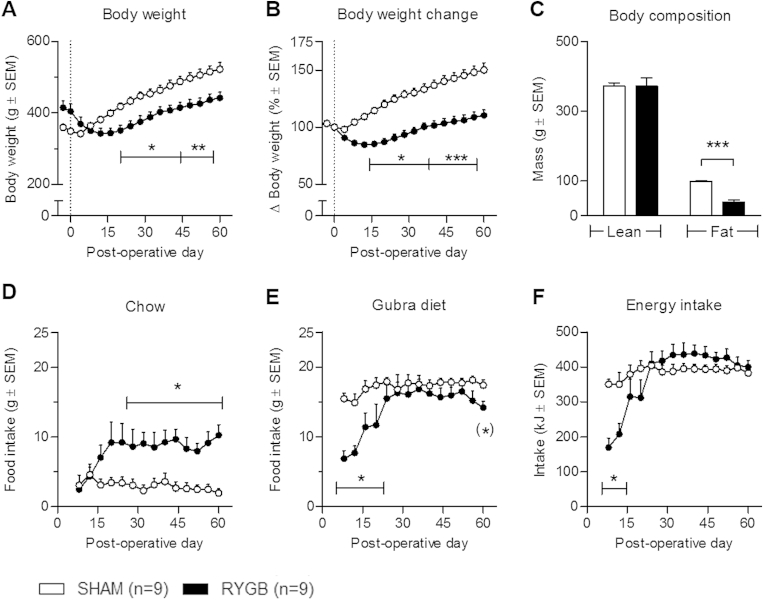
After a short initial period of post-operative weight loss, sham rats returned to normal weight gain for the remainder of the study period (Figure 1A). In contrast, animals undergoing gastric bypass surgery showed a significant reduction in terminal body weight (441.6 ± 16.0 g) compared with sham controls (522.2 ± 18.9 g, p < 0.01) (Figure 1A). When expressed as body weight change, RYGB animals exhibited a maximal body weight loss of 16 ± 2% (on post-surgery day 16) compared to pre-surgical body weight, followed by progressive weight regain resulting in a terminal body weight of 110 ± 5% relative to day −1. In comparison, sham-operated rats showed a terminal body weight of 153 ± 5% relative to their pre-surgical body weight (Figure 1B).
Figure 1.
Body weight and food intake in RYGB and SHAM animals. RYGB surgery led to a sustained decreased in body weight relative to sham (A + B) as well as a significant reduction in body fat mass (C). RYGB induced a persistent significant increase in chow intake (D) whereas Gubra diet intake (E) and total energy intake (F) were similar between sham and RYGB after an initial recovery period. Data are presented as mean ± SEM; two-way ANOVA with Bonferroni post hoc test; *p < 0.05, **p < 0.01, ***p < 0.001. On the day of euthanization (day 60) the intake of Gubra diet was significantly reduced in the RYGB group (E); student's unpaired t-test (*)p < 0.05.
Body composition analysis by echo-MRI demonstrated a significant reduction in adiposity in RYGB animals compared with sham controls. Hence, RYGB animals had a significant lower terminal fat mass than sham operated animals (RYGB: 39.7 ± 5.4 g; sham: 98.2 ± 3.5 g, p < 0.001). Lean mass was similar in the two experimental groups (Figure 1C).
3.2. RYGB induces a change in food preference
Throughout the study, sham animals had a stable intake of both chow and HPHF diet exhibiting a strong preference for the HPHF diet (Figure 1D,E). After an initial post-operative recovery period, RYGB animals increased their food intake of both chow and HPHF diet, but with an augmented preference for chow relative to sham throughout the study period (Figure 1D). On day of termination (post-surgery day 60), RYGB animals showed a significantly lowered intake of HPHF diet relative to sham animals (p < 0.05, student's unpaired t-test). Except for the initial post-surgery period (day 1–16), the total energy intake was comparable between sham and gastric bypass operated animals (Figure 1F).
3.3. Upregulation of orexigenic signals in Arc but not lateral hypothalamus after RYGB
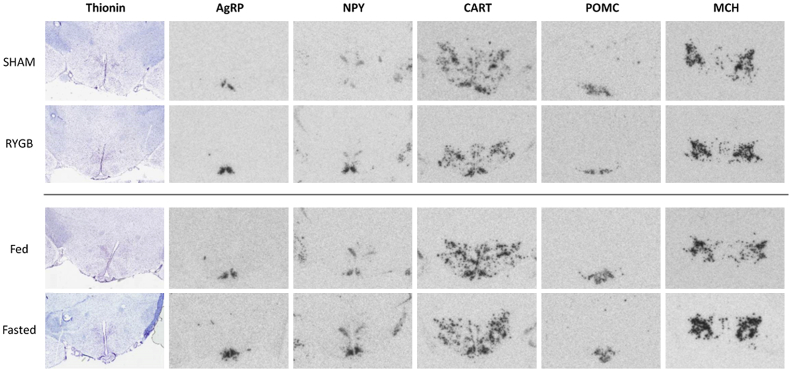
To investigate potential changes in homeostatic control of energy balance, we performed semi-quantitative mRNA in situ hybridizations of selected neuropeptides in the hypothalamus. Representative images of these hybridizations are shown in Figure 2.
Figure 2.
Hypothalamic in situ hybridization micrographs. Representative photomicrographs of radioactive in situ mRNA hybridization in sham and RYGB operated animals as well as in fed and fasted animals.
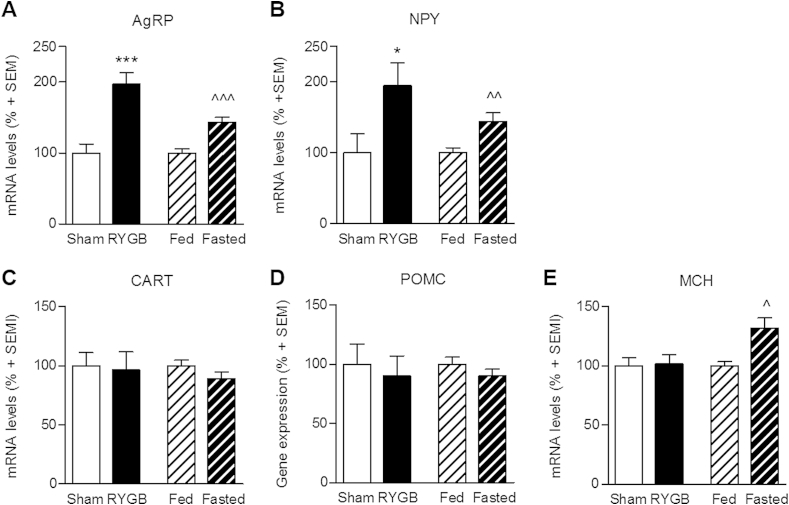
As expected, 48 h food restriction in DIO rats led to a significant upregulation in mRNA expression of the orexigenic AgRP (43 ± 7%, p < 0.001 vs. free-fed animals) and NPY (44 ± 12%, p < 0.01 vs. free-fed animals) (Figure 3A,B). An even stronger response was observed in RYGB animals showing an almost doubling in mRNA levels of both AgRP (97 ± 16%, p < 0.001 vs. sham-operated controls) and NPY (95 ± 32%, p < 0.05 vs. sham-operated controls). In contrast, expression of the anorexigenic POMC and CART were unregulated in both RYGB and food restricted animals as compared to their respective controls (Figure 3C,D). POMC is expressed in both Arc and median eminence (see Figure 2), however, mRNA levels were only quantified in the Arc.
Figure 3.
Quantification of hypothalamic gene expression. RYGB surgery led to a significant increase in mRNA levels of the orexigenic peptides AgRP (A) and NPY (B); a similar increase was seen in fasted animals compared with fed animals. No regulation in expression of the anorectic peptides CART and POMC was observed in neither RYGB surgery nor in mildly fasted animals (C + D). MCH was significantly upregulated in the lateral hypothalamus in fasted animals, but no change was observed in RYGB animals (E). Data are presented as mean ± SEM; student's unpaired t-test; * compares RYGB to sham, ˆcompares fasted to fed; *p < 0.05, **p < 0.01, ***p < 0.001.
To further assess downstream signaling from the Arc, we quantified mRNA levels of the orexigenic MCH in the LHA. MCH mRNA expression was significantly higher in food restricted animals as compared to free-fed animals (32 ± 9%, p < 0.05), whereas MCH expression was unaltered following RYGB surgery (p > 0.05 vs. sham-operated controls) (Figure 3E).
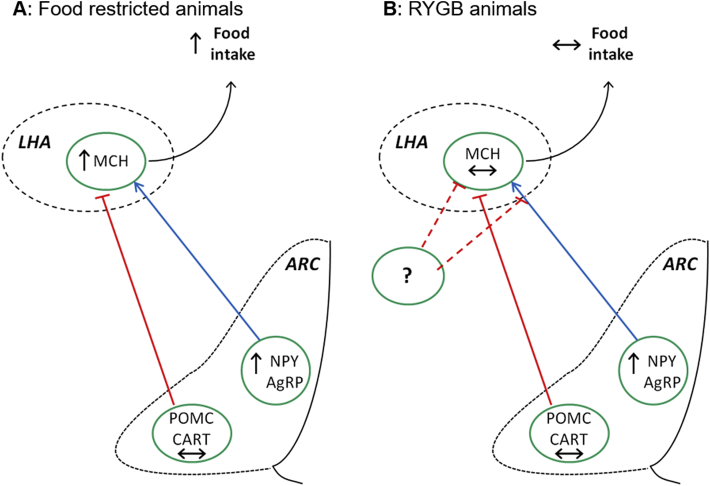
The overall findings of hypothalamic gene expression in both food-restricted and RYGB operated animals are illustrated in Figure 4. Orexigenic signaling cascades are stimulated in animals following food restriction (Figure 4A) but blunted in RYGB animals at the level of the LHA (Figure 4B).
Figure 4.
Hypothalamic orexigenic signals in food restricted and RYGB animals. In 48 h mildly food restricted rats, elevated NPY and AgRP expression in the Arc leads to an increase in MCH expression in the LHA compared with as lib fed rats (A). In RYGB operated animals the orexigenic signal from the Arc is blunted leading to unregulated MCH expression relative to sham animals (B).
3.4. RYGB causes selective reduction in dopaminergic turnover
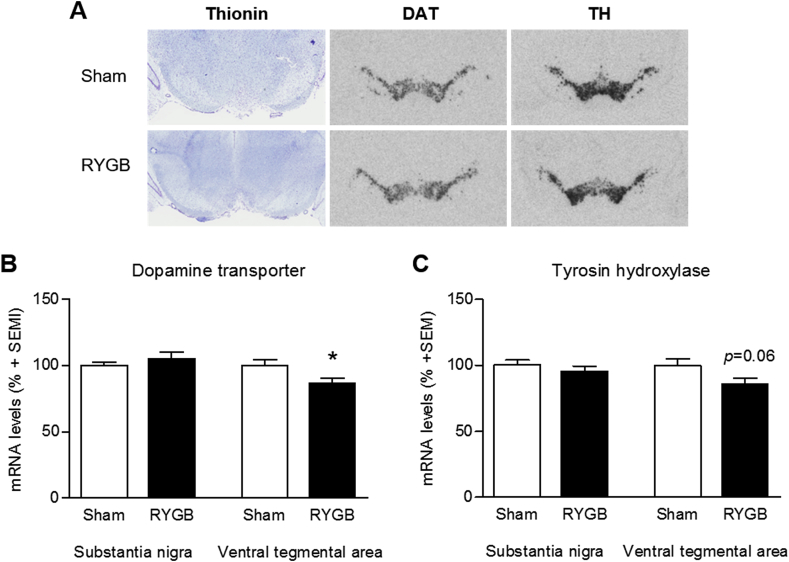
Due to the change in food preference repeatedly reported in RYGB studies, we looked at marker genes for dopaminergic signaling in the midbrain. Representative images of dopamine transporter (DAT) and tyrosine hydroxylase (TH) mRNA in situ hybridization are shown in Figure 5A. Quantification of the mRNA levels of DAT and TH demonstrated a downregulation in the VTA whereas no regulation was observed in the SN. In RYGB operated animals in the VTA, DAT mRNA was significantly downregulated by 14 ± 4% (p < 0.05) and TH expression showed a tendency for downregulation (15 ± 4%, p = 0.06) (Figure 5B,C).
Figure 5.
Quantification of midbrain gene expression. Representative photomicrographs of radioactive in situ mRNA hybridization in sham and RYGB operated animals in the midbrain (A). RYGB surgery led to a reduction in DAT and TH mRNA levels in the ventral tegmental area, whereas no changes were observed in the substantia nigra (B + C). Data are presented as mean ± SEM; student's unpaired t-test; *p < 0.05.
4. Discussion
In a rat model of RYGB surgery, we have examined whether changes in body weight and food preference may be related to transcriptional changes in key anorexigenic and orexigenic neuronal populations in the hypothalamus. Our results show that arcuate levels of orexigenic NPY and AgRP mRNAs are significantly upregulated in RYGB rats compared with sham animals to a similar degree as is observed in food-restricted animals. In contrast, second-order downstream orexigenic MCH mRNA expression in the LHA is blunted in RYGB rats, potentially related to a reduced mesolimbic dopaminergic neurotransmission.
4.1. Increased hunger signaling in the Arc following RYGB
Due to the specific interest in central appetite regulation, primary focus was directed towards the well-characterized hypothalamic Arc, which is known to harbor specific cell groups responsive to metabolic restrictions [26]. Both NPY/AgRP and POMC/CART expressing neurons are metabolic sensors whose activity is altered by feeding status, glucose, long chain fatty acids, leptin and insulin (for review see [27]). Interestingly, our data demonstrate that orexigenic NPY and AgRP mRNAs were significantly upregulated in rats at 60 days after RYGB surgery. In contrast, POMC/CART expression was unaltered in the Arc, indicating a selective activation in hunger associated circuits. An upregulation of arcuate AgRP and NPY transcription with unchanged POMC expression has also been reported in rat models of biliopancreatic and duodenal-jejunal bypass surgery [15], [16]. However, Romanova et al. demonstrated that immunohistochemically detectable NPY was decreased in the Arc and in the PVN 10 days after RYGB surgery in rats, as well as in pair fed controls compared with sham surgery [28]. These data partly contradict our findings but may be related to differences in study periods (i.e. an acute vs. a chronic setting) or differences in transcriptional processing. Accordingly, the reduction in NPY peptide levels in both RYGB and pair fed animals may be a result of NPY depletion supporting increased activity of NPY signaling.
In addition, Grayson et al. recently reported that vertical sleeve gastrectomy induces hypothalamic AgRP but not POMC expression in the rat, implying that different experimental bariatric surgery procedures may result in similar transcriptional regulations of orexigenic signaling markers in the Arc [29]. In comparison to vertical sleeve gastrectomy, a rat model of RYGB did not show altered AgRP expression [29]. In contrast to the unbiased and systematic section sampling and in situ hybridization procedure employed in the present study, the study by Grayson et al. analyzed gene expression in hypothalamic tissue homogenates, which could have precluded sufficient structural resolution and assay sensitivity. Furthermore, it should be noted that AgRP levels cycles diurnally, and that the time of euthanization might influence the AgRP mRNA levels [30], [31]. In the present study, however, all animals were euthanized 2–3 h after light onset ensuring consistency between groups.
4.2. Downstream signaling
The observation of elevated levels of Arc orexigenic transcription markers is at odds with the finding that the RYGB rats showed no overall increase in energy intake, compared to sham controls. We therefore investigated MCH mRNA expression in the LHA, as MCH-positive neurons in the LHA are strategically positioned to directly or indirectly receive input (like leptin) that relate peripheral energy balance to the CNS. Indeed, MCH neurons in the LHA receive innervation from NPY/AgRP and POMC neurons supporting that the MCH system is downstream of the leptin responsive arcuate neurons [32]. Furthermore, LHA MCH expression is sensitive to changes in nutritional status, as acute food deprivation, acute pharmacological glucose deprivation and acute insulin-induced hypoglycemia all increase MCH mRNA expression [33], [34]. Taken together these data supports that MCH release is stimulated by NPY whereas POMC inhibits its release (as indicated in Figure 4A). The upregulated expression of MCH mRNA in food restricted rats in the present study is in agreement with the current understanding that MCH in the LHA acts as a downstream mediator of NPY signaling [12]. Interestingly, however, MCH expression was unregulated in RYGB animals compared to sham, suggesting that another extra-hypothalamic component is able to overrule the hunger signals from the Arc.
4.3. Changes in mesolimbic dopaminergic gene expression
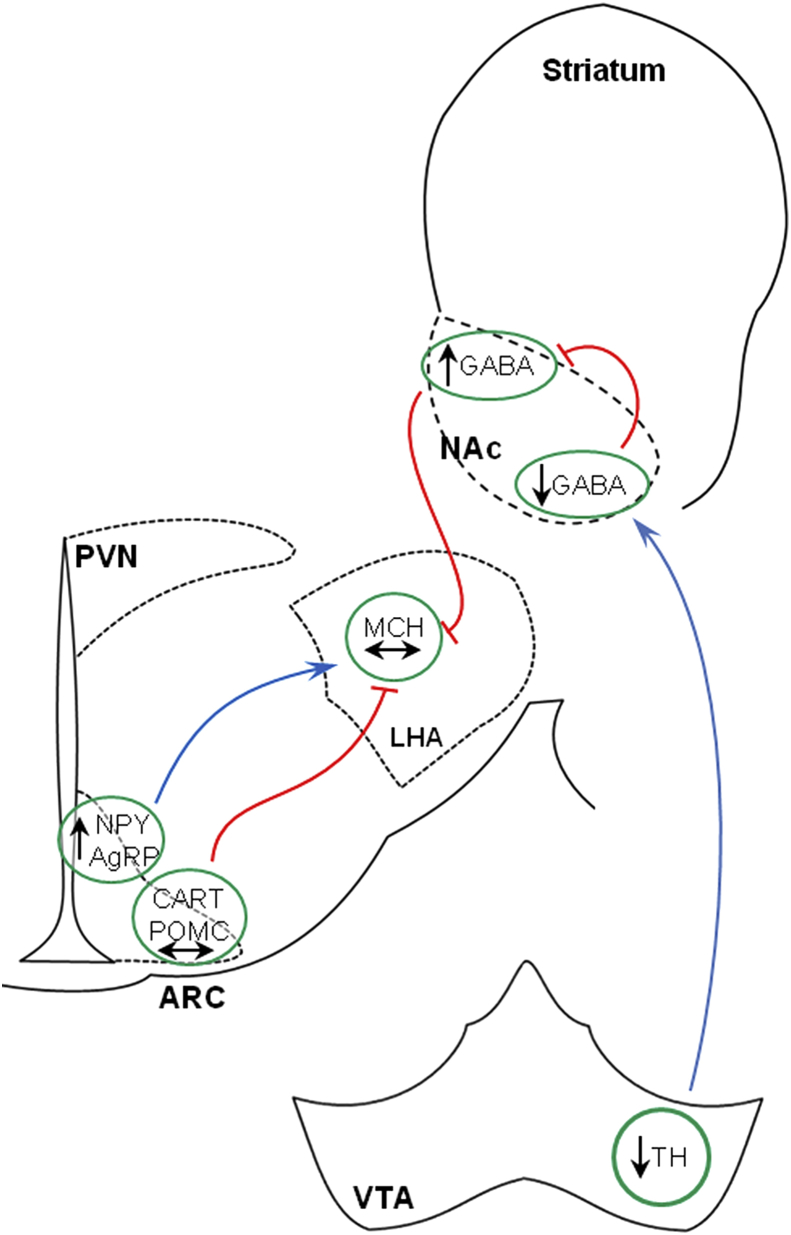
Besides a role in the homeostatic regulation of feeding behavior, the LHA is also known as an integrative center of hedonic signaling. It has been shown previously that dopaminergic signaling through the mesolimbic pathway elevates the motivation to work for food, particularly energy dense food, and thereby increases food intake despite no obvious homeostatic requirement [18], [35]. Our in situ hybridization data for TH (the rate limiting enzyme in dopamine biosynthesis) and DAT (the enzyme responsible for dopamine reuptake) demonstrate a reduction in mRNA levels in the VTA but not in the SN, indicating that RYGB could lead to changes in dopaminergic turnover in the mesolimbic pathway. Based on these findings we hypothesized, that downregulated signaling through this pathway in rats following RYGB surgery may lead to an increased inhibitory GABAergic output from the NAc shell which consequently overrule the stimulated orexigenic pathway from the Arc to the LHA (see Figure 6). This hypothesis is supported by the reciprocal and functional connections documented between the NAc shell and MCH neurons in the LHA [36], [37] and that temporary inactivation of the LHA is able to block the increased food intake induced by a GABA agonist [38]. Even though additional studies are needed in order to support our hypothesis, potential alterations in dopaminergic signaling after surgery may explain not only the blunted NPY induced hyperphagia but also the mechanisms behind the observed changes in food preference with an increased preference for chow. Alterations in dopaminergic signaling after RYGB have been reported by others, with reduced neural responsivity to food cues in the mesolimbic pathway [39] and alteration in dopamine receptor availability in the striatum [40]. Similarly, a RYGB induced shift in food preference towards low-calorie foods is now consistently reported in studies from both animal models and man [19], [20], [41] supporting further an involvement of the hedonic system in the beneficial effects of the RYGB surgery.
Figure 6.
Reduced dopaminergic transmission in the mesolimbic pathway may overrule orexigenic signals from the arcuate nucleus. We hypothesize that the downregulated dopaminergic signaling in the mesolimbic pathway in rats following RYGB surgery may lead to an increased inhibitory GABAergic output from the NAc shell, which consequently overrules the stimulated orexigenic pathway from the Arc to the LHA.
4.4. Gut–brain interaction
The observation that RYGB also induces resolution of T2DM prior to the occurrence of weight loss indicates that the mechanism of action might involve endocrine effects [42]. The re-routing of salivary secretions and meals directly into the jejunum stimulate the release of gut hormones which may affect glucose homeostasis and provide signal to central appetite systems [43]. A series of papers have conclusively validated that RYGB increases the secretion of gut-derived peptides such as GLP-1 and PYY, while e.g. ghrelin is reduced [6], [7], [8], [9]. These peptides are all part of the gut–brain axis with receptors located in the periphery as well as in the CNS [44], [45], [46] and all play important roles in satiety signaling in the brain [47], [48]. Recently, it was shown that peripheral administration of the GLP-1 receptor analog, liraglutide, gains access to the hypothalamus with the body weight lowering effects being, at least partly, dependent on increased CART expression in the Arc [49]. Moreover, both liraglutide and the DPP-IV inhibitor, linagliptin, have been shown to alter food preference from a highly palatable diet towards chow [22], [50]. In the present study, RYGB did not alter the expression of satiety-associated POMC/CART mRNAs in the hypothalamus suggesting that the body weight lowering effects of RYGB surgery is not directly related to GLP-1 effects in the Arc. Actually, the orexigenic tone in the Arc observed in the present study indicates that this specific area is not primarily responsible for the effects on food intake and body weight. On the contrary, several of the gut-derived hormones that are altered after RYGB (e.g. ghrelin, GLP-1 and PYY) have receptors in the LHA and may reach these through circulation and blood barrier transport, or by neural pathways such as vagal afferent connections from the brainstem nucleus of the solitary tract [51], [52]. Whether these gut-derived hormones may be able to blunt the NPY-MCH signaling pathway and prevent overeating in RYGB operated animals is still not known. More data are also needed to address whether increased gut–brain signaling could be involved in modulating the expression of mesolimbic dopaminergic marker genes after RYGB.
4.5. Conclusion
In conclusion, we show that arcuate levels of orexigenic NPY and AgRP mRNAs are significantly upregulated in RYGB rats indicating that classical hunger signaling pathways are activated. In contrast, second-order downstream orexigenic signals in the LHA (MCH neurons) are blunted in RYGB rats suggesting that the hunger-signals arising from the Arc do not translate into sensations of hunger nor into food-seeking behavior. Simultaneously, RYGB leads to alterations in mid- and forebrain hedonic signaling that can potentially be involved in the modulation of appetite and body-weight in this model.
Acknowledgments
The authors would like to thank Lisbeth Pedersen, Farida Shahebzadeh and Sarah Kampfeldt for excellent technical assistance. The research was sponsored by the Danish Innovation Fund and Gubra.
Conflict of interest
The authors declare that they have no conflicts of interest relevant to this manuscript.
References
- 1.WHO . 2014. Global status report on noncommunicable diseases. [DOI] [PubMed] [Google Scholar]
- 2.Bray G.A. Medications for weight reduction. Endocrinology and Metabolism Clinics of North America. 2008;37:923–942. doi: 10.1016/j.ecl.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Wing R.R. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Archives of Internal Medicine. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer P.R., Kashyap S.R., Wolski K., Brethauer S.A., Kirwan J.P., Porthier C.E. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New England Journal of Medicine. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mingrone G., Panunzi S., De Gaetano A., Guidone C., Iaconelli A., Leccesi L. Bariatric surgery versus conventional medical therapy for type 2 diabetes. New England Journal of Medicine. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 6.le Roux C.W., Welbourn R., Werling M., Osborne A., Kokkinos A., Laurenius A. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Annals of Surgery. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 7.Beckman L.M., Beckman T.R., Sibley S.D., Thomas W., Ikramuddin S., Kellogg T.A. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN Journal of Parenteral and Enteral Nutrition. 2011;35:169–180. doi: 10.1177/0148607110381403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen N.B., Jacobsen S.H., Dirksen C., Bojsen-Moller K.N., Naver L., Hvolris L. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. American Journal of Physiology, Endocrinology and Metabolism. 2012;303:E122–E131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 9.Hansen C.F., Bueter M., Theis N., Lutz T., Paulsen S., Dalboge L.S. Hypertrophy dependent doubling of L-cells in Roux-en-Y gastric bypass operated rats. PLoS One. 2013;8:e65696. doi: 10.1371/journal.pone.0065696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 11.Spiegelman B.M., Flier J.S. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 12.Elias C.F., Aschkenasi C., Lee C., Kelly J., Ahima R.S., Bjoerbaek C. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 13.Elmquist J.K., Elias C.F., Saper C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz M.W., Woods S.C., Porte D., Jr., Seeley R.J., Baskin D.G. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 15.Nadreau E., Baraboi E.D., Samson P., Blouin A., Hould F.S., Marceau P. Effects of the biliopancreatic diversion on energy balance in the rat. International Journal of Obesity (London) 2006;30:419–429. doi: 10.1038/sj.ijo.0803166. [DOI] [PubMed] [Google Scholar]
- 16.Warne J.P., Padilla B.E., Horneman H.F., Ginsberg A.B., Pecoraro N.C., Akana S.F. Metabolic and neuroendocrine consequences of a duodenal-jejunal bypass in rats on a choice diet. Annals of Surgery. 2009;249:269–276. doi: 10.1097/SLA.0b013e3181961d5d. [DOI] [PubMed] [Google Scholar]
- 17.Kelley A.E., Baldo B.A., Pratt W.E. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. Journal of Comparative Neurology. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 18.Richard J.M., Castro D.C., Difeliceantonio A.G., Robinson M.J., Berridge K.C. Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley. Neuroscience and Biobehavioral Reviews. 2013;37:1919–1931. doi: 10.1016/j.neubiorev.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin A.C., Zheng H., Pistell P.J., Berthoud H.R. Roux-en-Y gastric bypass surgery changes food reward in rats. International Journal of Obesity (London) 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.le Roux C.W., Bueter M., Theis N., Werling M., Ashrafian H., Lowenstein C. Gastric bypass reduces fat intake and preference. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2011;301:R1057–R1066. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeidi N., Nestoridi E., Kucharczyk J., Uygun M.K., Yarmush M.L., Stylopoulos N. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. International Journal of Obesity (London) 2012;36:1396–1402. doi: 10.1038/ijo.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen G., Jelsing J., Vrang N. Effects of liraglutide and sibutramine on food intake, palatability, body weight and glucose tolerance in the gubra DIO-rats. Acta Pharmacologica Sinica. 2012;33:194–200. doi: 10.1038/aps.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers A.P., Jessen L., Ryan K.K., Sisley S., Wilson-Perez H.E., Stefater M.A. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jelsing J., Larsen P.J., Vrang N. Identification of cannabinoid type 1 receptor expressing cocaine amphetamine-regulated transcript neurons in the rat hypothalamus and brainstem using in situ hybridization and immunohistochemistry. Neuroscience. 2008;154:641–652. doi: 10.1016/j.neuroscience.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Vrang N., Larsen P.J., Clausen J.T., Kristensen P. Neurochemical characterization of hypothalamic cocaine- amphetamine-regulated transcript neurons. Journal of Neurosciences. 1999;19:RC5. doi: 10.1523/JNEUROSCI.19-10-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods S.C., Seeley R.J., Porte D., Jr., Schwartz M.W. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 27.Levin B.E., Magnan C., Dunn-Meynell A., Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology. 2011;152:2552–2557. doi: 10.1210/en.2011-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanova I.V., Ramos E.J., Xu Y., Quinn R., Chen C., George Z.M. Neurobiologic changes in the hypothalamus associated with weight loss after gastric bypass. Journal of American College of Surgeons. 2004;199:887–895. doi: 10.1016/j.jamcollsurg.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Grayson B.E., Hakala-Finch A.P., Kekulawala M., Laub H., Egan A.E., Ressler I.B. Weight loss by calorie restriction versus bariatric surgery differentially regulates the hypothalamo-pituitary-adrenocortical axis in male rats. Stress. 2014:1–36. doi: 10.3109/10253890.2014.967677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui H., Kohsaka A., Waki H., Bhuiyan M.E., Gouraud S.S., Maeda M. Metabolic cycles are linked to the cardiovascular diurnal rhythm in rats with essential hypertension. PLoS One. 2011;6:e17339. doi: 10.1371/journal.pone.0017339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X.Y., Shieh K.R., Kabbaj M., Barsh G.S., Akil H., Watson S.J. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- 32.Elias C.F., Saper C.B., Maratos-Flier E., Tritos N.A., Lee C., Kelly J. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. Journal of Comparative Neurology. 1998;402:442–459. [PubMed] [Google Scholar]
- 33.Presse F., Sorokovsky I., Max J.P., Nicolaidis S., Nahon J.L. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71:735–745. doi: 10.1016/0306-4522(95)00481-5. [DOI] [PubMed] [Google Scholar]
- 34.Sergeyev V., Broberger C., Gorbatyuk O., Hokfelt T. Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport. 2000;11:117–121. doi: 10.1097/00001756-200001170-00023. [DOI] [PubMed] [Google Scholar]
- 35.Kelley A.E., Delfs J.M. Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology (Berl) 1991;103:187–196. doi: 10.1007/BF02244202. [DOI] [PubMed] [Google Scholar]
- 36.Heimer L., Zahm D.S., Churchill L., Kalivas P.W., Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 37.Kirouac G.J., Ganguly P.K. Topographical organization in the nucleus accumbens of afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neuroscience. 1995;67:625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 38.Stratford T.R., Kelley A.E. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. Journal of Neuroscience. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochner C.N., Stice E., Hutchins E., Afifi L., Geliebter A., Hirsch J. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience. 2012;209:128–135. doi: 10.1016/j.neuroscience.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steele K.E., Prokopowicz G.P., Schweitzer M.A., Magunsuon T.H., Lidor A.O., Kuwabawa H. Alterations of central dopamine receptors before and after gastric bypass surgery. Obesity Surgery. 2010;20:369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- 41.Pepino M.Y., Bradley D., Eagon J.C., Sullivan S., Abumrad N.A., Klein S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 2014;22:E13–E20. doi: 10.1002/oby.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hickey M.S., Pories W.J., MacDonald K.G., Jr., Cory K.A., Dohm G.L., Swanson M.S. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Annals of Surgery. 1998;227:637–643. doi: 10.1097/00000658-199805000-00004. [discussion 643–634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fobi M.A., Lee H., Holness R., Cabinda D. Gastric bypass operation for obesity. World Journal of Surgery. 1998;22:925–935. doi: 10.1007/s002689900496. [DOI] [PubMed] [Google Scholar]
- 44.Vrang N., Larsen P.J. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Progress in Neurobiology. 2010;92:442–462. doi: 10.1016/j.pneurobio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Babilon S., Morl K., Beck-Sickinger A.G. Towards improved receptor targeting: anterograde transport, internalization and postendocytic trafficking of neuropeptide Y receptors. Biological Chemistry. 2013;394:921–936. doi: 10.1515/hsz-2013-0123. [DOI] [PubMed] [Google Scholar]
- 46.Sivertsen B., Holliday N., Madsen A.N., Holst B. Functionally biased signalling properties of 7TM receptors – opportunities for drug development for the ghrelin receptor. British Journal of Pharmacology. 2013;170:1349–1362. doi: 10.1111/bph.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turton M.D., O'Shea D., Gunn I., Beak S.A., Edwards C.M., Meeran K. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 48.Tang-Christensen M., Larsen P.J., Goke R., Fink-Jensen A., Jessop D.S., Moller M. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. American Journal of Physiology. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 49.Secher A., Jelsing J., Baquero A.F., Hecksher-Sorensen J., Cowley M.A., Dalboge L.S. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. Journal of Clinical Investigation. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen H.H., Hansen G., Paulsen S., Vrang N., Mark M., Jelsing J. The DPP-IV inhibitor linagliptin and GLP-1 induce synergistic effects on body weight loss and appetite suppression in the diet-induced obese rat. European Journal of Pharmacology. 2014;741:254–263. doi: 10.1016/j.ejphar.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Tang-Christensen M., Vrang N., Larsen P.J. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. International Journal of Obesity and Related Metabolic Disorders. 2001;25(Suppl. 5):S42–S47. doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y.P., Zhu J.N., Chen K., Li H.Z., Wang J.J. Neurons in the rat lateral hypothalamic area integrate information from the gastric vagal nerves and the cerebellar interpositus nucleus. Neurosignals. 2005;14:234–243. doi: 10.1159/000088639. [DOI] [PubMed] [Google Scholar]