Abstract
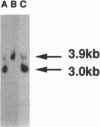
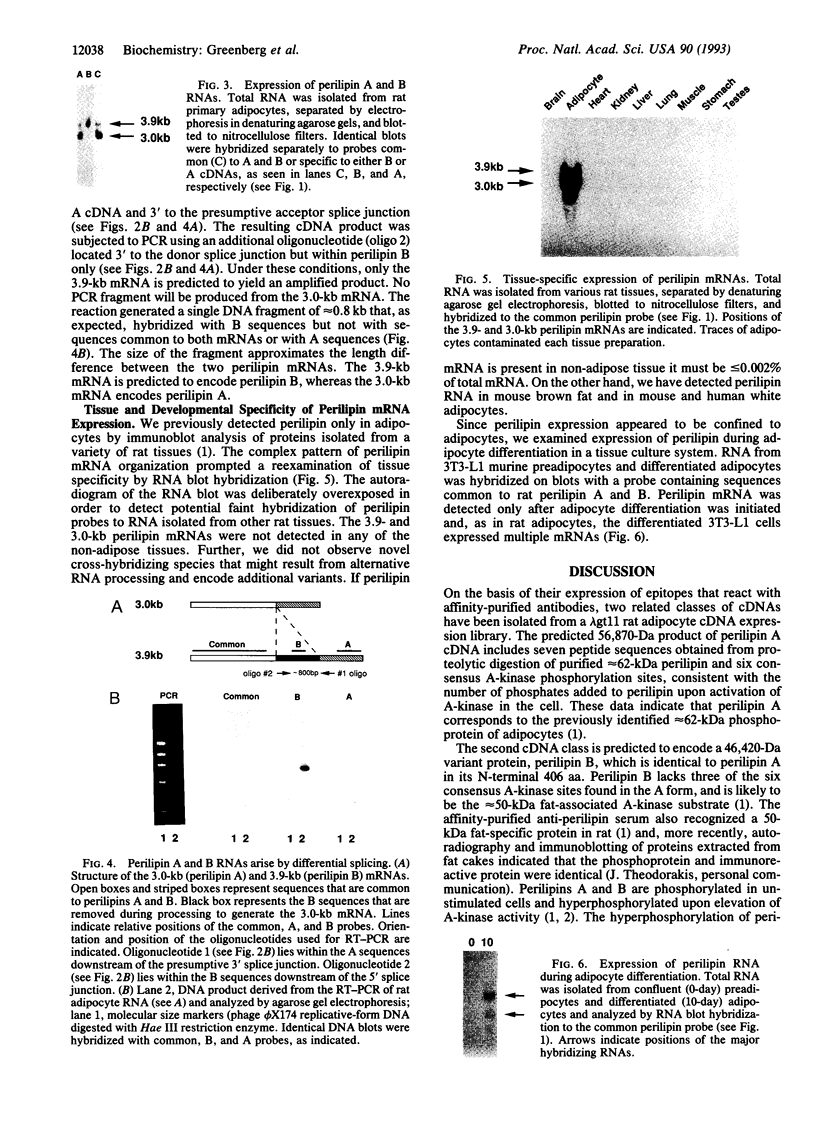
The major cAMP-dependent protein kinase (A-kinase) substrate in adipocytes is perilipin, a protein found exclusively at the surface of the lipid storage droplets. Using anti-perilipin serum, we have isolated two related classes of full-length coding cDNAs, designated perilipin A and B, from a rat adipocyte cDNA expression library. The two cDNAs derive from two mRNA species that arise by differential splicing. The mRNAs are predicted to encode perilipins A and B, proteins of 517 aa (56,870 Da) and 422 aa (46,420 Da), respectively, which share a common 406-aa N-terminal sequence. The predicted perilipin A contains peptides present in proteolytic digests of the purified 62-kDa form of perilipin from rat adipocytes, as well as the requisite consensus A-kinase phosphorylation sites. Like perilipin A, the B form is expressed in adipocytes and is associated with lipid storage droplets. Modeling of predicted secondary structures fails to reveal an underlying basis for the tenacious association of perilipins with lipid droplets. These proteins exhibit a significant sequence relationship (approximately 65% similarity through 105 aa) with only one other known protein, the adipocyte differentiation-related protein (ADRP). Like the perilipins, ADRP appears to be adipocyte-specific, which suggests that they interact in a related intracellular pathway. The molecular probes for perilipins A and B described here will permit detailed analyses of their functional role(s) in lipid metabolism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992 May 11;20 (Suppl):2013–2018. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Egan J. J., Greenberg A. S., Chang M. K., Londos C. Control of endogenous phosphorylation of the major cAMP-dependent protein kinase substrate in adipocytes by insulin and beta-adrenergic stimulation. J Biol Chem. 1990 Nov 5;265(31):18769–18775. [PubMed] [Google Scholar]
- Egan J. J., Greenberg A. S., Chang M. K., Wek S. A., Moos M. C., Jr, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8537–8541. doi: 10.1073/pnas.89.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Hergt M., Grund C. Rearrangement of the vimentin cytoskeleton during adipose conversion: formation of an intermediate filament cage around lipid globules. Cell. 1987 Apr 10;49(1):131–141. doi: 10.1016/0092-8674(87)90763-x. [DOI] [PubMed] [Google Scholar]
- Garnier J. Protein structure prediction. Biochimie. 1990 Aug;72(8):513–524. doi: 10.1016/0300-9084(90)90115-w. [DOI] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990 Apr;3(5):433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I. Protein N-myristoylation: simple questions, unexpected answers. Clin Res. 1990 Oct;38(3):517–528. [PubMed] [Google Scholar]
- Greenberg A. S., Egan J. J., Wek S. A., Garty N. B., Blanchette-Mackie E. J., Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991 Jun 15;266(17):11341–11346. [PubMed] [Google Scholar]
- Honnor R. C., Dhillon G. S., Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J Biol Chem. 1985 Dec 5;260(28):15122–15129. [PubMed] [Google Scholar]
- Jiang H. P., Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7856–7860. doi: 10.1073/pnas.89.17.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Berger S. L. Preparation of cDNA and the generation of cDNA libraries: overview. Methods Enzymol. 1987;152:307–316. doi: 10.1016/0076-6879(87)52035-3. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R. Different molecular mechanisms for cAMP regulation of gene expression during Dictyostelium development. Dev Biol. 1987 Jul;122(1):163–171. doi: 10.1016/0012-1606(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Moos M., Jr, Nguyen N. Y., Liu T. Y. Reproducible high yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988 May 5;263(13):6005–6008. [PubMed] [Google Scholar]
- Murphy D. J., Keen J. N., O'Sullivan J. N., Au D. M., Edwards E. W., Jackson P. J., Cummins I., Gibbons T., Shaw C. H., Ryan A. J. A class of amphipathic proteins associated with lipid storage bodies in plants. Possible similarities with animal serum apolipoproteins. Biochim Biophys Acta. 1991 Jan 17;1088(1):86–94. doi: 10.1016/0167-4781(91)90156-g. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussak C. E., Almazan M. T., Tseng B. Y. Peptide production from proteins separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis. Anal Biochem. 1989 May 1;178(2):233–238. doi: 10.1016/0003-2697(89)90630-1. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Jones M. K., De Loof H., Brouillette C. G., Venkatachalapathi Y. V., Anantharamaiah G. M. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992 Feb;33(2):141–166. [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Stone K. L., Williams K. R. High-performance liquid chromatographic peptide mapping and amino acid analysis in the sub-nanomole range. J Chromatogr. 1986 May 30;359:203–212. doi: 10.1016/0021-9673(86)80074-7. [DOI] [PubMed] [Google Scholar]
- Tempst P., Riviere L. Examination of automated polypeptide sequencing using standard phenyl isothiocyanate reagent and subpicomole high-performance liquid chromatographic analysis. Anal Biochem. 1989 Dec;183(2):290–300. doi: 10.1016/0003-2697(89)90482-x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Meinkoth J. L., Kimmel A. R. Northern and Southern blots. Methods Enzymol. 1987;152:572–581. doi: 10.1016/0076-6879(87)52064-x. [DOI] [PubMed] [Google Scholar]