Abstract
Purpose
To explore the potential effect of ageing on the corneal curvature and corrected visual acuity in patients with long-term keratoconus because of the paucity of these patients older than 50 years.
Methods
Records of keratoconic patients, who had initially presented to a specialized contact lens clinic and followed for more than 20 years after disease onset, were reviewed. Collected information included age, gender, date of first and last examination, date of onset of the disease, central corneal curvature, refraction, best corrected visual acuity (BCVA), therapeutic modality and clinical signs.
Results
Age of patients at last examination was 53.8 years ± 7.2 (range 44–67 years). Disease onset was self-reported to be at age 18.4 years ± 3.8. First examination was at age 25.1 years ± 9.4 and the mean number of years between first and last examination was 28.7 years. Mean central corneal curvature was 6.87 mm (48.77 D) ± 0.65 and 6.56 mm (51.09 D) ± 0.74, at first and last examination, respectively, a difference which was significant (p < 0.001). However, the last measurement of corneal curvature was found to remain approximately constant over the years from about 20 to 50 years after onset. Mean BCVA was not significantly different between first and last examination and was found to be approximately constant over the years.
Conclusion
Corneal curvature became steeper possibly within the first 20 years after disease onset but remained approximately unchanged afterwards. Likewise, BCVA remained practically constant over the years indicating relative stability of the disease after 20 years.
Keywords: Keratoconus, Age, Corneal curvature, BCVA
Resumen
Objetivo
Explorar el efecto potencial del envejecimiento sobre la curvatura de la córnea y la agudeza visual corregida en pacientes con queratocono a largo plazo, ya que hay una insuficiencia de estos pacientes con edades superiores a 50 años.
Métodos
Revisión de las historias de los pacientes con queratocono, que habían acudido inicialmente a una clínica especializada en lentes de contacto, y seguimiento durante más de 20 años desde el inicio de la enfermedad. La información recolectada incluyó edad, sexo, fecha de la primera y la última valoración, fecha de aparición de la enfermedad, curvatura central de la córnea, agudeza visual mejor corregida (BCVA), modalidad terapéutica y signos clínicos.
Resultados
La edad de los pacientes en el último examen fue de 53,8 años ± 7,2 (rango de 44 a 67 años). Los pacientes auto-reportaron el inicio de la enfermedad a los 18,4 años ± 3,8. El primer examen se realizó a los 25,1 años ± 9,4, siendo el número medio de años entre el primero y el último examen de 28,7 años. La media de la curvatura central de la córnea fue de 6,87 mm (48,77 D) ± 0,65 y 6,56 mm (51,09 D) ± 0,74, en el primero y el último examen, respectivamente, una diferencia que resultó significativa (p < 0,001). Sin embargo, se comprobó que la última medición de la curvatura de la córnea permanecía más o menos constante durante el transcurso de los años, durante 20 a 50 años desde el inicio. La BCVA media no resultó estadísticamente diferente entre el primero y el último examen, siendo más o menos constante con el paso de los años.
Conclusión
La curvatura de la córnea resultó más elevada dentro de los 20 primeros años desde la aparición de la enfermedad, pero no sufrió cambios posteriores. De igual modo, la BCVA permaneció prácticamente constante con el paso de los años, lo que indica una estabilidad relativa de la enfermedad con el transcurso de 20 años.
Palabras clave: Queratocono, Edad, Curvatura de la córnea
Introduction
It is a common observation that keratoconus (KC) is seen much less commonly in patients older than 50 years,1, 2, 3, 4, 5, 6, 7 who had been diagnosed in their youth with the disease, although in one study the number of these patients was found to be substantial.8 Nevertheless, it is surprising because KC is a chronic disease and one would expect to find a larger prevalence among patients older than 50 years than in a younger sample. Yet most studies of KC with this older age group have reported very low proportions: 7.4%,3 7.6%,5 10%,1 15%,9 except 40% in one study.8
It could be thought that the proportion of the younger age group would be increased because in the last couple of decades more people have been diagnosed with the disease than before, owing to the wide usage of corneal topography and pachymetry. Moreover, allergic conditions which have become more prevalent in the general population10 are known to be associated with many cases of KC,11 and may also contribute to increasing the number of young subjects. Another consideration suggested by several authors3, 4, 7 is that KC patients have a shorter life expectancy than the general population due to associated conditions, such as mitral valve prolapse,12, 13, 14 asthma and obesity15 or obstructive sleep apnea.15, 16, 17 However, a study conducted in England in which the mortality rate for KC patients was compared with the general population showed no significant difference between the two groups,2 although this study may have underestimated the true figure since the majority of patients examined was under 50 years of age. Still, some evidence of equal longevity of keratoconic and non-keratoconic patients was provided in a contact lens practice.4 Underestimation of KC prevalence in the old may also result from patients who have developed a stable disease, who are managed and satisfied with spectacles or contact lenses; or who are resigned to poor vision and do not bother to attend hospital or private clinics; or perhaps have relocated after retirement.
There is of course the possibility that the corneas of KC patients stiffen with age, as is the case with normal corneas due to changes in corneal collagen fibrils,18, 19, 20, 21 resulting in a stable cornea or perhaps even a marked decrease in severity in some cases.22 In any case, progression of the disease is generally considered to have stopped before the age of 40 after approximately 20 years since onset, if any progression had occurred.5, 23, 24 It would seem reasonable to assume that patients and practitioners would be greatly interested to know what to expect in the long term, beyond 20 years of disease. The aim of this longitudinal retrospective study was to compare the central curvature and best corrected visual acuity (BCVA) of keratoconic patients examined at least 20 years earlier, with their recent findings, in order to determine whether their corneas continued to progress, stabilize or regress with time.
Methods
The clinical records from a long established, specialized private contact lens practice in Tel Aviv, Israel were used to provide data for this study. Between 70% and 80% of the patients attending this clinic have KC.11 Patients had been diagnosed previously with KC in at least one eye by an ophthalmologist and referred to this clinic for contact lens management rather than surgery. Records of all consecutive keratoconic patients who had a minimum of 20 years since onset, and who had presented between October 2012 and October 2014 were retrospectively reviewed. Exclusion criteria included any patient with a history of ophthalmic surgery bilaterally (e.g. keratoplasty). Half of these cases had been operated before the age of 40 years and a younger age is known to be a predictive factor for keratoplasty, along with deep scarring, very steep cornea, poor visual acuity or discomfort with contact lens wear.25 Also excluded was any ocular disease other than KC and aged less than 40 years. The study adhered to the tenets of the Declaration of Helsinki.
From the records of all eligible patients we analyzed the data regarding corrected Snellen visual acuity, refraction, Javal-Schiotz keratometry, slit-lamp examination and topography. Topography was only available in the last set of data. Computerized topography used with all patients was carried out with the Sirius topographer (CSO, Firenze, Italy). The model of the instrument used analyses only the Placido image by obtaining 25 sections of the cornea. Prior to this examination most patients removed their contact lenses on the day of appointment, but only two hours before in a few cases. Although most patients paid multiple visits to the clinic, data used in the analysis were those of the initial and last visit. All initial examinations had been performed by the same optometrist (SB). The last examinations were mostly performed by the same optometrist (SB) and in some cases by another (IO). Demographic data included the age of onset of the disease or the age of initial visual symptoms, the date of the first visit to the clinic and therapeutic modality (spectacles, contact lenses or corneal transplant). The main outcome was to compare the central curvature within the 3 mm zone (mean of steep K and flat K values) measured initially with keratometry, and with topography (Sim K) at the last examination more than 20 years later. Topography was not available when the patients were first seen many years ago and a comparison could only be made with the initial keratometry findings. However, very good agreement has been shown to occur between the Javal-Schiotz keratometer and a topography system.26 Best corrected visual acuity (BCVA) was also determined as an indication of the patient's visual performance with their correction, using high contrast Snellen letters at distance.
All data were entered into an Excel spreadsheet for subsequent analysis. Normality of data was assessed using the Kolmogorov–Smirnov test. If normally distributed, data were analyzed as mean change in central corneal curvature and BCVA between the initial and last visit using a t-test for related samples. Pearson correlation coefficients as well as regression lines were also determined. If not normally distributed, non-parametric tests were used. Values of p ≤ 0.05 were considered significant.
Results
67 patients who presented to the clinic during the study period met the eligibility criteria. Seven patients were excluded because they exhibited severe corneal distortions or extensive scarring binocularly, which made it impossible to record any viable data at their last examination. The data of another seven patients were too incomplete to compare the initial with the last measurements. Hence, we collected data on 53 patients at baseline and last examination. 23 (43%) patients were females and 30 (57%) males. Of this cohort 12 patients (23%) had unilateral penetrating keratoplasty and another three patients (6%) had unilateral KC; other patients had missing data from one eye, almost always due to a distorted image of the cornea, which had made it impossible to take a measurement. Thus, the analysis was carried out on 72 eyes of 53 patients.27 At the last examination, most patients had been fitted with rigid gas permeable lenses, but six eyes had been fitted with scleral and three with piggyback lenses. At the first examination patients were corrected with hard lenses; five of them with PMMA, one with spectacles and one eye with a soft lens, and the others with rigid gas permeable lenses. The mean number of hours of wear per day was 13.8 h ± 2.3. The reasons for their last visit were varied; the most common was annual check up (33%), followed by a demand for new lenses (19%), a lost lens (16%) and blurred vision (9%) due to lens scratching or distortion. 15% of patients did not give any reason for their visit. Discomfort was only mentioned by 7% of patients, in one instance with a scleral lens. Several patients presented mild scarring.
The mean age of self-reported disease onset was 18.4 years (±3.8; range 23–50) and the mean age of the patients at first presentation was 25.1 (±9.4). The mean age at the last examination was 53.8 years ± 7.2; (44–67 years) and the mean total number of years since onset was 34.9 (±7.3). The average number of years between the first and last examination was 28.7 years (range 20–49 years). The mean corneal peak as measured by topography (sagittal) at the last examination was equal to 53.8 D, ±7.2; (45 D to 78 D), which represents mostly moderate KC with some severe types, based on the classification given in Romero-Jimenez.28
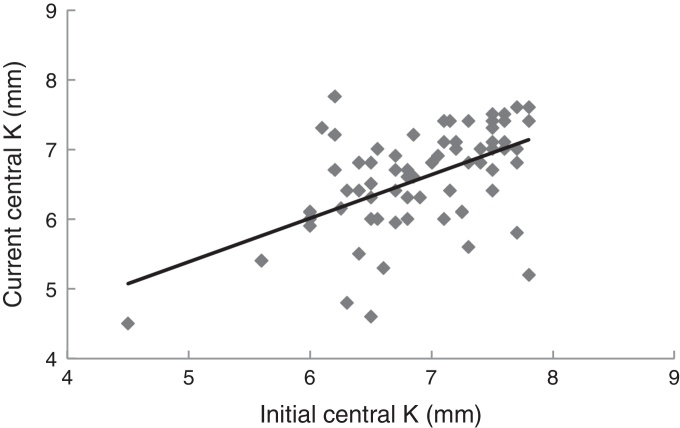
The mean central corneal curvatures at first and last examination are presented in Table 1. The difference was statistically different (paired t = 3.9, p < 0.001). Fig. 1 shows the relationship between the initial and last mean central corneal curvature. The slope of the regression line is 0.63 indicating a steeper cornea in the older group. The Pearson correlation coefficient between the two variables was r = 0.54, which is significant (p < 0.001). Omitting the two outliers, the correlation coefficient became r = 0.62, indicating a strong relationship between the two variables. However, most patients had unequal change in curvature over the years. The majority of eyes became steeper (50 out 72 eyes; 69%), i.e. progressed; 10% remained the same and 21% slightly flatter, i.e. regressed.
Table 1.
Mean central corneal curvature (K) and best corrected visual acuity (BCVA) at first and last examinations.
| First examination | Last examination | Years in between | P value | |
|---|---|---|---|---|
| Mean K (SD) in mm | 6.87 (±0.65) | 6.56 (±0.74) | 28.7 (±6.9) | <0.001 |
| In dioptres | 48.77 (±5.1) | 51.09 (±4.49) | ||
| Mean BCVA in decimal | 0.65 (±0.24) | 0.61 (±0.18) | 28.7 (±6.9) | =0.25 |
| In metres | 6/9.2 | 6/9.8 |
Figure 1.
Relationship between the central corneal curvature (mean K) measured at first and last visit. N = 72 eyes. Mean time difference between the current and initial measurements: 28.7 (±6.9) years. The regression line is y = 0.63x + 2.26.
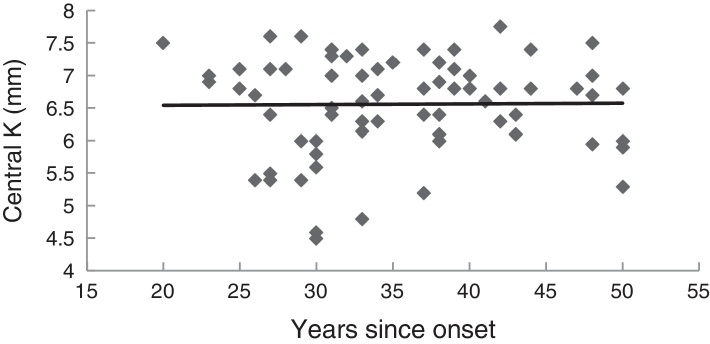
Fig. 2 illustrates the relationship between the number of years since disease onset and the mean K measured at the last examination for all eyes. The slope of the regression line was equal to 0.001, and was not significantly different from zero (p = 0.99), thus indicating that mean corneal curvature in KC remained approximately the same over the years, at least beyond 20 years after onset.
Figure 2.
Relationship between the last central curvature (mean K) and the number of years since the onset of the disease. N = 72 eyes. The regression line is y = 0.001x + 6.5.
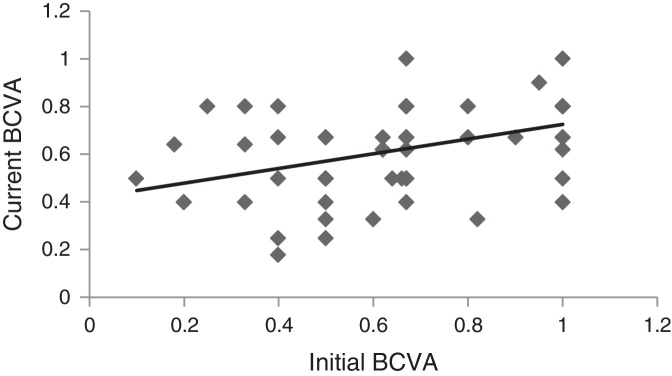
The mean BCVA at first and last examination are presented in Table 1. The difference was not statistically significant (z = 1.14, p = 0.25), using Wilcoxon signed ranks test. Fig. 3 shows the relationship between the initial and last BCVA. The slope of the regression line is 0.31 indicating a tendency towards a lower BCVA at the last examination. The correlation coefficient between the two variables was r = 0.44, which is significant (p < 0.001), using Spearman's rank correlation test. More eyes had worse BCVA (35 eyes; 49%); 20% had remained the same and 32% had better BCVA at the last examination.
Figure 3.
Relationship between the best corrected visual acuity (BCVA) measured at first visit and recently. N = 72 eyes. Mean time difference between the present and the initial measurements: 28.7 years. The regression line is: y = 0.31x + 0.42.
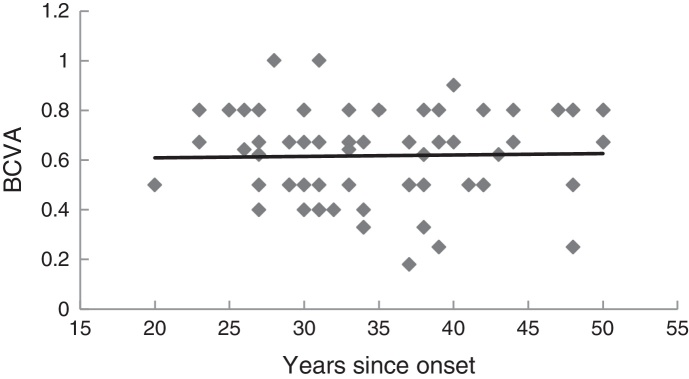
Fig. 4 illustrates the relationship between the number of years since disease onset and the last measurements of BCVA. The slope of the regression line was 0.0006, which was not significantly different from zero (p = 0.99), thus indicating that BCVA remains approximately constant over the years, at least beyond 20 years after onset.
Figure 4.
Relationship between the last best corrected visual acuity (BCVA) and the number of years since the onset of the disease. N = 72 eyes. The regression line is: y = 0.0006x + 0.59.
Discussion
The results of this study showed that the mean central corneal curvature of keratoconic eyes became significantly steeper with time, from 48.8 D to 51.1 D. It is likely that most of the corneal changes occurred in the first 20 years after disease onset and up to the age of 40–45 years as has been reported in several studies.5, 29, 30, 31 However, not all patients progress in that period; Ertan and Muftuoglu5 reported mean K values of 50.9 D in a group of KC patients younger than 20 years, 49.1 D in another group between the age 21–40 years, and 50.8 D in a third group of patients older than 41 years. There are also several longitudinal studies reporting a high percentage of patients who did not progress. In one such a study, 61 eyes out of 85 eyes (72%) did not progress.32 In another study 75% of patients with mild KC followed for 14 years did not progress24 and in a third study 85% of patients with a follow-up of 10 years did not progress.33 In our study of long-standing KC, 31% of eyes did not progress, 10% remained the same and 21% exhibited a slight regression. Nevertheless, it would appear that the evolution of KC is bimodal with one group of patients progressing and the other non-progressing.
However, the changes in corneal curvature of long-standing KC corneas (up to 50 years) did not become significantly steeper than those of patients who have had the disease for 20 or 25 years (Fig. 2). This relative constancy over the years is similar to normal eyes in which the mean radius of corneal curvature is age-independent.34, 35 It would suggest that KC corneas progress or change up to a maximum, which remains approximately the same with ageing. Resistance to further corneal changes could be due to natural collagen cross-linking, not unlike the growth of collagen fibrils20 and stiffening of corneal tissue,19, 21, 22 which occurs in the stroma of normal eyes with age.
Keratoconus usually appears in the teenage years or early adulthood. Self-reported onset in our study was 18.4 years. Not all patients develop the disease in their youth, such as the reported onset in a patient aged 51 years.36 During the years of progression and corneal changes the condition which may have begun in one eye only becomes bilateral in most, but not in all cases. In one study it was reported that 50% of the non-affected fellow eyes developed the disease within 16 years.37 Generally only a few percent of cases remain unilateral. It was 6% in our study and slightly less in some other reports.8, 37, 38, 39 It would seem that bilateral KC occurs in the first 20 years of disease.
Best corrected visual acuity (BCVA) was not found to be statistically different between first and last examination (average time difference 28.7 years) nor was there any deterioration over the years among all subjects. If any deterioration of corrected visual acuity had occurred it may have happened in the first few years after onset when BCVA was no longer efficiently correctable with spectacles and the patients were referred to the clinic for contact lens therapy. Our data of BCVA did not show any significant reduction over the years, thus substantiating the benefit of the contact lens modality in these patients. Alternatively, the use of high contrast letters in our study may have failed to detect a reduction of BCVA. In the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study40 a decrease of 10 or more letters was noted at 7-year follow-up in 30.8% of patients with low contrast letters whereas it was only 19% with high contrast letters. In another study BCVA changed significantly only in eyes that progressed and not in those that did not.41 A true deterioration of BCVA may have been concealed in our study since 31% of eyes did not progress and we used high contrast letters.
The clinical impression that there are very few old keratoconic patients does not necessarily mean that they are subject to a higher mortality rate than the general population, based on the limited evidence provided by Moodaley and Woodward.2 Further evidence was provided in a comparison study between keratoconic and nonkeratoconic populations in a specialized contact lens practice; the keratoconic group actually formed a slightly older cohort than the nonkeratoconic group, at least in the population sample which did not exceed 71 years of age. The reason for this difference is that patients who wear contact lenses for aesthetic reasons are more likely to discontinue them in old age, whereas contact lenses are still needed for vision in keratoconic patients.4 Moreover, patients with keratoconus require more care than non-keratoconic patients because of diminished acuity and also abrasions.42 The patient population of our specialized contact lens clinic also consists of a vast majority of patients younger than 70 years and based on our clinical records we see a fair amount of KC patients older than 50 years but as they demand much less care than young KC patients they do not impress on our minds. Nevertheless, the absence of KC patients older than 70 years compared to non-KC seems to be substantiated, based on a cross-section analysis of several specialist contact lens practices.7 The results of the present study do not explain why there are fewer old KC patients only to indicate that their absence is not due to a natural disappearance of the disease, a question that was rightly posed by Krachmer,22 but to a stability of the disease, at least up to about 70 years of age. Further research is needed to establish what happens to older KC patients. There is also the question of what happens to KC patients who do not come back to the clinic for either a replacement lens because of a dirty lens covered with proteins, or who have lost a lens, a question also posed by many authors.1, 2, 3, 4, 5, 6, 7 Hence, we took hold of records from the year 1984 and telephoned the first 11 keratoconic patients whom we had not seen for many years. We invited them to come to the clinic for a free check-up. None of them agreed and they told us that they were satisfied with their contact lenses and their vision and refused to oblige. We found out that some patients had had cataract surgery and had ceased contact lens wear because the implant imparted adequate vision.
There are certain limitations to this study. We used only conventional high contrast Snellen letters which, as mentioned above may have affected BCVA results. However, we used the same letter chart for both the initial and last examination. The age of onset is based on patients’ verbal history, which is always subject to recall, especially when it comes to specifying the date. However, the self-reported ages are similar to other published data.30, 42 The uncertainty about whether patients presented with contact lenses at the first examination performed several decades earlier may have led to an underestimation of the difference in curvature between the two measurements. We also acknowledge that removal of contact lenses on the day of appointment may have resulted in warpage that flattened the cornea thus underestimating the true steepness, but this prevailed with all patients. As these patients cannot function without their lenses they would only remove them on that day. Most importantly this study is limited only to patients who presented to the clinic and we cannot surmise what happens to other old KC patients who do not present to the clinic.
In conclusion, this is the first longitudinal, retrospective study of changes occurring in keratoconic corneas over a long span of years since onset. It showed that central corneal curvature became significantly steeper in most cases, although this progression most likely occurred in the first 20 years after disease onset. It was also found that after some 20 years following disease onset corneal curvature remained approximately constant. BCVA also remained approximately constant over the years. This study may enable clinicians to give more informed counselling regarding the expected progression of this disease.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgement
Mr Ilya Ortenberg and Ms Karen Lahav-Yacouel are practitioners at the Microlens practice.
References
- 1.Lass J.H., Lembach R.G., Park S.B. Clinical management of keratoconus. Ophthalmology. 1990;97:433–445. doi: 10.1016/s0161-6420(90)32569-1. [DOI] [PubMed] [Google Scholar]
- 2.Moodaley L.C.M., Woodward E.G., Liu C.S.C., Buckley R.J. Life expectancy in keratoconus. Br J Ophthalmol. 1992;76:590–591. doi: 10.1136/bjo.76.10.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pobelle-Frasson C., Velou S., Huslin V., Massicault B., Colin J. Keratocone: que deviennent les patients ages. J Fr Ophtalmol. 2004;27:779–782. doi: 10.1016/s0181-5512(04)96213-4. [DOI] [PubMed] [Google Scholar]
- 4.Yeung K.K., Tai J.H., Weissman B.A. Where have all the keratoconic patients gone? Int Cont Lens Clin. 1998;25:109–113. [Google Scholar]
- 5.Ertan A., Muftuoglu O. Keratoconus clinical findings according to different age and gender groups. Cornea. 2008;27:1109–1113. doi: 10.1097/ICO.0b013e31817f815a. [DOI] [PubMed] [Google Scholar]
- 6.Reeves S.W., Ellwein L.B., Kim T., Constantine R., Lee P.P. Keratoconus in the medicare population. Cornea. 2009;28:40–42. doi: 10.1097/ICO.0b013e3181839b06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMonnies C.W. Quo vadis older keratoconus patients? Do they die at younger ages? Cornea. 2013;32:496–502. doi: 10.1097/ICO.0b013e31825aba0e. [DOI] [PubMed] [Google Scholar]
- 8.Yldiz E.H., Diehl G.F., Cohen E.J., Hammersmith K.M., Laibson P.R., Rapuano C.J. Demographics of patients older than 50 years with keratoconus. Eye Contact Lens. 2009;35:309–311. doi: 10.1097/ICL.0b013e3181be5784. [DOI] [PubMed] [Google Scholar]
- 9.Zadnik K., Barr J.T., Edrington T.B. Baseline findings in the Collaborative Longitudinal valuation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998;39:2537–2546. [PubMed] [Google Scholar]
- 10.Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both. Immunology. 2004;112:352–363. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shneor E., Millodot M., Blumberg S., Ortenberg I., Behrman S., Gordon-Shaag A. Characteristics of 244 patients with keratoconus seen in an optometric contact lens practice. Clin Exp Optom. 2013;96:219–224. doi: 10.1111/cxo.12005. [DOI] [PubMed] [Google Scholar]
- 12.Beardsley T.C., Foulks G.N. An association of keratoconus and mitral valve prolapsed. Ophthalmology. 1982;89:35–37. doi: 10.1016/s0161-6420(82)34857-5. [DOI] [PubMed] [Google Scholar]
- 13.Sharif K.W., Casey T.A., Coltart J. Prevalence of mitral valve prolapsed in keratoconus patients. J R Soc Med. 1992;85:446–448. [PMC free article] [PubMed] [Google Scholar]
- 14.Rabbanikkah Z., Javadi M.A., Rostami P. Association between acute corneal hydrops in patients with keratoconus and mitral valve prolapsed. Cornea. 2011;30:154–157. doi: 10.1097/ICO.0b013e3181e846a2. [DOI] [PubMed] [Google Scholar]
- 15.Kristinsson J.K., Carlson A.N., Kim T. Keratoconus and obesity – a connection. Invest Ophthalmol Vis Sci. 2003;44 E-Abstract 912. [Google Scholar]
- 16.Gupta P.K., Stinett S.S., Carlson A.N. Prevalence of sleep apnea in patients with keratoconus. Cornea. 2012;31:595–599. doi: 10.1097/ICO.0b013e31823f8acd. [DOI] [PubMed] [Google Scholar]
- 17.Pihlblad M.S., Schaefer D.P. Eyelid laxity, obesity and obstructive sleep apnea in keratoconus. Cornea. 2013;32:1232–1236. doi: 10.1097/ICO.0b013e318281e755. [DOI] [PubMed] [Google Scholar]
- 18.Andreassen T.T., Simonsen A.H., Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980;31:435–441. doi: 10.1016/s0014-4835(80)80027-3. [DOI] [PubMed] [Google Scholar]
- 19.Cartwright N.E.K., Tyrer J.R., Marshall J. Age-related differences in the elasticity of the human cornea. Invest Ophthalmol Vis Sci. 2011;52:4324–4329. doi: 10.1167/iovs.09-4798. [DOI] [PubMed] [Google Scholar]
- 20.Daxer A., Misof K., Grabner B., Etti A., Fratzl P. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci. 1998;39:644–648. [PubMed] [Google Scholar]
- 21.Elsheikh A., Geraghty B., Rama P., Campanelli M., Meek K.M. Characterization of age-related variation in corneal biochemical properties. J R Soc Interface. 2010;7:1475–1485. doi: 10.1098/rsif.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krachmer J.H. Potential research projects. Cornea. 2007;26:243–245. doi: 10.1097/ICO.0b013e318030e396. [DOI] [PubMed] [Google Scholar]
- 23.Choi J.A., Kim M.S. Progression of keratoconus by longitudinal assessment with corneal topography. Invest Ophthalmol Vis Sci. 2012;53:927–935. doi: 10.1167/iovs.11-8118. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez J.L.O., Jurado J.C.G., Rodriguez F.J.B., Laborda D.S. Keratoconus: age of onset and natural history. Optom Vis Sci. 1997;74:147–151. doi: 10.1097/00006324-199703000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Gordon M.O., Steger-May K., Szczotka-Flynn L. Baseline factors predictive of incident penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2006;142:1044–1050. doi: 10.1016/j.ajo.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Mehravaran S., Asgari S., Bigdeli S., Shahnazi A., Hashemi H. Keratometry with different techniques: a study of device repeatability and inter-device agreement. Int Ophthalmol. 2014;34:869–875. doi: 10.1007/s10792-013-9895-3. [DOI] [PubMed] [Google Scholar]
- 27.Murdoch I.E., Morris S.S., Cousens S.N. People and eyes: statistical approaches in ophthalmology. Br J Ophthalmol. 1998;82:971–973. doi: 10.1136/bjo.82.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero-Jimenez M., Santodomingo-Rubido J., Wolffsohn J.S. Keratoconus: a review. Contact lens Anterior Eye. 2010;33:157–166. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Phillips A.J. Can true monocular keratoconus occur? Clin Exp Optom. 2003;86:399–402. doi: 10.1111/j.1444-0938.2003.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 30.Rabinowitz Y.S. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 31.Krachmer J.H., Feder R.S., Belin M.W. Keratoconus and related noninflammatory corneal thinning disorder. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 32.Oshika T., Tanabe T., Tomidokoro A., Amano S. Progression of keratoconus assessed by Fourier analysis of videokeratography data. Ophthalmology. 2002;109:339–342. doi: 10.1016/s0161-6420(01)00903-4. [DOI] [PubMed] [Google Scholar]
- 33.Shirayama-Suzuki M., Amano Honda N., Usui T., Yamagami S., Oshoka T. Longitudinal analysis of corneal topography in suspected keratoconus. Br J Ophthalmol. 2009;93:815–819. doi: 10.1136/bjo.2008.140012. [DOI] [PubMed] [Google Scholar]
- 34.Eysteinsson T., Jonasson F., Sasaki H. Central corneal thickness, radious of the corneal curvature and intraocular pressure in normal subjects using non-contact techniques. Acta Ophthalmol. 2002;80:11–15. doi: 10.1034/j.1600-0420.2002.800103.x. [DOI] [PubMed] [Google Scholar]
- 35.Iyamu E., Osuobeni E. Age, gender, corneal diameter, corneal curvature and central corneal thickness in Nigerians with normal intraocular pressure. J Optom. 2012;5:87–97. [Google Scholar]
- 36.Tenkman L.R., Price M.O., Price F.W. Keratoconus onset after age 50. J Refract Surg. 2012;28:436–438. doi: 10.3928/1081597X-20120522-02. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Rabinowitz Y.S., Rasheed K., Yang H. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology. 2004;111:440–446. doi: 10.1016/j.ophtha.2003.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Holland D.R., Maeda N., Hannush S.B. Unilateral keratoconus incidence and quantitative topographic analysis. Ophthalmology. 1997;104:1409–1413. doi: 10.1016/s0161-6420(97)30123-7. [DOI] [PubMed] [Google Scholar]
- 39.Rabinowitz Y.S., Nesburn A.B., McDonnell P.J. Videokeratography of the fellow eye in unilateral keratoconus. Ophthalmology. 1993;100:181–186. doi: 10.1016/s0161-6420(93)31673-8. [DOI] [PubMed] [Google Scholar]
- 40.Davis L.J., Schechtman K.B., Wilson B.S. Longitudinal changes in visual acuity in keratoconus. Invest Ophthalmol Vis Sci. 2006;47:489–500. doi: 10.1167/iovs.05-0381. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki M., Amano S., Honda N., Usui T., Yamagami S., Oshika T. Longitudinal changes in corneal irregular astigmatism and visual acuity in eyes with keratoconus. Jpn J Ophthalmol. 2007;51:265–269. doi: 10.1007/s10384-007-0453-2. [DOI] [PubMed] [Google Scholar]
- 42.Weissman B.A., Chun M.W., Barnhart L.A. Corneal abrasion associated with contact lens correction of keratoconus a retrospective study. Optom Vis Sci. 1994;71:677–681. doi: 10.1097/00006324-199411000-00001. [DOI] [PubMed] [Google Scholar]