Abstract
Objective
Circulating long-chain free fatty acids (FFAs) are important metabolic signals that acutely enhance fatty acid oxidation, thermogenesis, energy expenditure, and insulin secretion. However, if chronically elevated, they provoke inflammation, insulin resistance, and β-cell failure. Moreover, FFAs act via multiple signaling pathways as very potent regulators of gene expression. In human skeletal muscle cells differentiated in vitro (myotubes), we have shown in previous studies that the expression of CSF3, the gene encoding granulocyte colony-stimulating factor (G-CSF), is markedly induced upon FFA treatment and exercise.
Methods and results
We now report that CSF3 is induced in human myotubes by saturated, but not unsaturated, FFAs via Toll-like receptor 4-dependent and -independent pathways including activation of Rel-A, AP-1, C/EBPα, Src, and stress kinases. Furthermore, we show that human adipocytes and myotubes treated with G-CSF become insulin-resistant. In line with this, a functional polymorphism in the CSF3 gene affects adipose tissue- and whole-body insulin sensitivity and glucose tolerance in human subjects with elevated plasma FFA concentrations.
Conclusion
G-CSF emerges as a new player in FFA-induced insulin resistance and thus may be of interest as a target for prevention and treatment of type 2 diabetes.
Keywords: Granulocyte colony-stimulating factor (G-CSF), Saturated fatty acid-induced myokine, Fatty acid-induced insulin resistance
Highlights
-
•
CSF3, the gene encoding G-CSF, is induced in human myotubes by saturated, but not unsaturated, FFAs.
-
•
CSF3 expression is induced via Toll-like receptor 4-dependent and -independent pathways.
-
•
Human adipocytes and myotubes treated with G-CSF become insulin-resistant.
-
•
A CSF3 SNP affects insulin sensitivity and glucose tolerance in human subjects with elevated plasma FFA concentrations.
-
•
G-CSF emerges as a new player in FFA-induced insulin resistance.
Under physiological conditions, long-chain free fatty acids (FFAs) represent high-caloric fuels that are, in the anabolic state, predominantly stored in adipocytes in the form of triglycerides and, in the catabolic state, released for energy supply. Acute elevation of plasma FFAs resulting from dietary fat intake or fasting- or exercise-induced adipose tissue lipolysis is known to enhance fatty acid oxidation, thermogenesis, energy expenditure, and insulin secretion [1], [2]. However, if chronically elevated, e.g., as a consequence of permanently deregulated lipolysis due to obesity, circulating FFAs can be ectopically stored in many cell types and provoke endoplasmic reticulum stress, cell apoptosis, tissue inflammation, insulin resistance, and β-cell failure, lipotoxic effects that are currently discussed as contributing to the pathogenesis of type 2 diabetes [3], [4], [5].
Only recently, long-chain FFAs were recognized as humoral signals that, via multiple cellular signaling pathways including transmembrane and nuclear receptors, trigger alterations in gene expression (for recent review, see [6]). In an attempt to assess the impact of FFAs on the expression and secretion of metabolically relevant muscle-derived secretory factors, so-called myokines [7], we treated primary human skeletal muscle cells differentiated in vitro (myotubes) with long-chain FFAs and performed a pilot array-based gene expression analysis [8]. In this earlier study, we identified and characterized angiopoietin-like protein 4 (ANGPTL4) as a new FFA-induced myokine with lipolytic properties in humans in vivo [8]. In addition, we found granulocyte colony-stimulating factor (G-CSF) to be the second strongest up-regulated myokine behind ANGPTL4 [8]. Moreover, we recently reported that modeling exercise in vitro by electric pulse stimulation of human myotubes provokes induction and secretion of multiple myokines including G-CSF [9].
G-CSF was first purified in 1985 [10] and is known for its profound effects on immune cells. G-CSF potently stimulates the proliferation and release of peripheral blood progenitor cells into the bloodstream and is therefore used to treat neutropenia after chemotherapy [11], [12]. Furthermore, G-CSF levels are elevated upon intensive exercise leading to increased neutrophil counts [13], which are predominantly due to delayed neutrophil apoptosis [14]. G-CSF displays strong anti-apoptotic activity in mature neurons, induces neuronal differentiation, and improves recovery after spinal cord injury in rats [15], [16]. Potentially, of metabolic interest may be the finding that the expression of the G-CSF gene CSF3 is increased in adipose tissue of obese subjects [17].
The metabolic regulation and function of skeletal muscle cell-derived G-CSF is hitherto not well understood. In the present study, therefore, we investigated in more detail FFA-induced CSF3 expression, the underlying molecular mechanism(s), and G-CSF's functions in human myotubes and adipocytes. In addition, we aimed to translate the metabolic G-CSF effects shown in vitro to humans in vivo by testing the impact of CSF3 tagging single nucleotide polymorphisms (SNPs) on human metabolic traits.
1. Research design and methods
1.1. Cell culture
Primary myoblasts were obtained from healthy volunteers by needle biopsy of the vastus lateralis muscle. Culture and differentiation to myotubes were described earlier [18]. Following differentiation, myotubes were treated with FFAs, insulin, and different chemical compounds either alone or in combination for the indicated time spans. BSA conjugation of long-chain FFAs was described earlier [19]. Conditioned culture media were collected for G-CSF quantitation. Primary subcutaneous preadipocytes were obtained from healthy volunteers by periumbilical needle biopsy. Culture and differentiation to adipocytes was described recently [20]. Following differentiation, the cells were treated with insulin and G-CSF either alone or in combination for the indicated time spans.
1.2. Quantitative real-time reverse transcription PCR (RT-PCR)
Cells were washed with PBS, lysed with RLT buffer, and homogenized using QIAshredder (Qiagen, Hilden, Germany). Total RNA isolation (RNeasy Mini Kit, Qiagen), transcription into cDNA (Transcriptor First Strand cDNA Synthesis Kit, Roche Diagnostics, Indianapolis, IN, USA), and RT-PCR were performed as described before [21]. PCR primers were purchased from TIB Molbiol (Berlin, Germany). Primer sequences and PCR conditions can be provided upon request. All gene expression data were normalized to the housekeeping gene RPS13 using the ΔΔCt method.
1.3. RNA interference
Small interfering RNA (siRNA) oligonucleotides targeting TLR2, TLR4, NFKB1, NFKB2, REL, RELA, RELB, JUN, and CEBPA were purchased as siGENOME-SMART-pools (Thermo Scientific, Rockford, IL, USA). As control, we used an unrelated siRNA targeting firefly luciferase as reported earlier [8]. Transfection of human myotubes was performed using the transfection reagent VIROMER BLUE (Lipocalyx, Halle, Germany) according to the manufacturer's instructions. Twenty-four hours after transfection, cells were treated for additional 24 h with palmitate (0.5 mmol/L) or BSA for control.
1.4. Western blotting
Cells were washed with PBS and whole-cell lysates were generated using M-PER mammalian protein extraction reagent (Thermo Scientific, Rockford, IL, USA). Lysates were centrifuged at 13,000 g for 10 min, and the protein concentration was measured in the supernatant using the Bradford protein assay (Bio-Rad, Richmond, CA, USA). Equal amounts of protein were loaded onto an SDS polyacrylamide gel. After electrophoresis, proteins were transferred to nitrocellulose membranes (Amersham Life Sciences Inc., Arlington Hights, IL, USA) and incubated with primary anti-extracellular signal-regulated kinase (ERK), anti-phospho-ERK (p-ERK), anti-Akt, or anti-phospho-Akt (p-Akt Ser473) antibodies (1:1000 all, Cell Signaling Technology, Danvers, MA, USA). After three washes, membranes were incubated with appropriate peroxidase-conjugated secondary antibodies (Sigma–Aldrich, Munich, Germany). Specific signals were detected using enhanced chemiluminescence (Amersham Life Sciences Inc.). For quantification, EasyWin32 software (Herolab, Wiesloch, Germany) was used. Phosphorylation signals were normalized for the respective protein signals.
1.5. Cell proliferation assays
Two different assays were used to assess cell proliferation according to the manufacturer's instructions: a water-soluble tetrazolium 1 (WST-1) assay and a bromodeoxyuridine (BrdU) incorporation assay (both from Roche Molecular Biochemicals, Mannheim, Germany). Myoblasts were seeded at 5000 cells per well in a 96-well plate. Cells were either treated for five consecutive days with G-CSF or for 24/48 h with G-CSF and palmitate alone or in combination prior to the assay.
1.6. Determination of glycogen synthesis
Glycogen synthesis was measured as reported earlier [18] with the exception that the cells were pre-incubated with insulin with or without additional G-CSF.
1.7. Human clinical data
Data from 1859 pre-diabetic participants of the Tübingen Family (TÜF) study for type 2 diabetes [22] were analyzed (66% women, 34% men; age 39.6 ± 13.2 years, BMI 30.2 ± 9.3 kg/m2, means ± SD). All procedures followed were in accordance with the Helsinki Declaration and with the ethical standards of the responsible committee on human experimentation (Ethics Committee of the Eberhard Karls University Tübingen). The study protocol was approved by this Ethics Committee, and informed written consent was obtained from all participants of the study.
1.8. Selection of tagging SNPs and genotyping
Using genetic information provided by the International HapMap Project (release #28, August 2010, CEU population, http://www.hapmap.org/index.html.en), we analyzed the CSF3 gene on human chromosome 17q11.2 (2.4 kb, five exons) including 5 kb of its 5′-flanking and 1 kb of its 3′-flanking sequence and identified 12 common SNPs (minor allele frequency >5%). Among these, four non-linked SNPs tagged all the other common variants in this locus with an r2 ≥ 0.8: rs8078723 (T/C) in the 5′-flanking region, rs2071369 (C/T) in intron 2, rs25645 (G/A) in exon 4 (Leu185Leu), and rs2827 (C/T) in the 3′-untranslated region. For genotyping, DNA was isolated from whole blood using a commercial kit (NucleoSpin, Macherey & Nagel, Düren, Germany). The tagging SNPs were genotyped by mass spectrometry according to the manufacturer's instructions (Sequenom, Hamburg, Germany) with call rates ≥98%.
1.9. Laboratory measurements
Plasma glucose was measured with a bedside glucose analyzer (glucose oxidase method, Yellow Springs Instruments, Yellow Springs, OH, USA), serum insulin with a commercial chemiluminescence assay for ADVIA Centaur (Siemens Medical Solutions, Fernwald, Germany). Plasma FFA concentrations were determined with an enzymatic method (WAKO Chemicals, Neuss, Germany). Insulin sensitivity during an oral glucose tolerance test (OGTT) was calculated by the insulin sensitivity index proposed by Matsuda and DeFronzo [23]. Insulin resistance of adipose tissue was calculated as the product of fasting serum insulin and fasting plasma FFAs. The G-CSF concentration in serum and conditioned cell culture media was measured using a Quantikine Immunoassay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Media were measured either undiluted or (1:3) diluted.
1.10. Statistical analyses
Data are usually presented as means ± SEM or as individual data. Two-group comparisons in the in vitro setting were performed by matched-pairs and in the in vivo setting by unpaired Student's t-tests. For linear regression analysis, data with skewed distribution were loge-transformed to approximate normal distribution. Differences in metabolic data between the CSF3 genotypes were tested in the additive and dominant inheritance models using multiple linear regression analysis (least squares method) with gender, age, and BMI as covariates. For all in vitro analyses, p-values < 0.05 were considered statistically significant. To minimize the risk of statistical type I errors in the SNP analyses due to multiple testing, a Bonferroni-corrected significance threshold of p < 0.0127 was chosen accounting for the four tagging SNPs tested in parallel. In these analyses, a p-value range of 0.0127 ≤ p < 0.05 was considered as nominally significant. For all statistical analysis, the software package JMP 8.0.2 (SAS Institute, Cary, NC, USA) was used.
2. Results
2.1. Regulation of CSF3 expression by saturated and unsaturated long-chain FFAs (SFAs and UFAs)
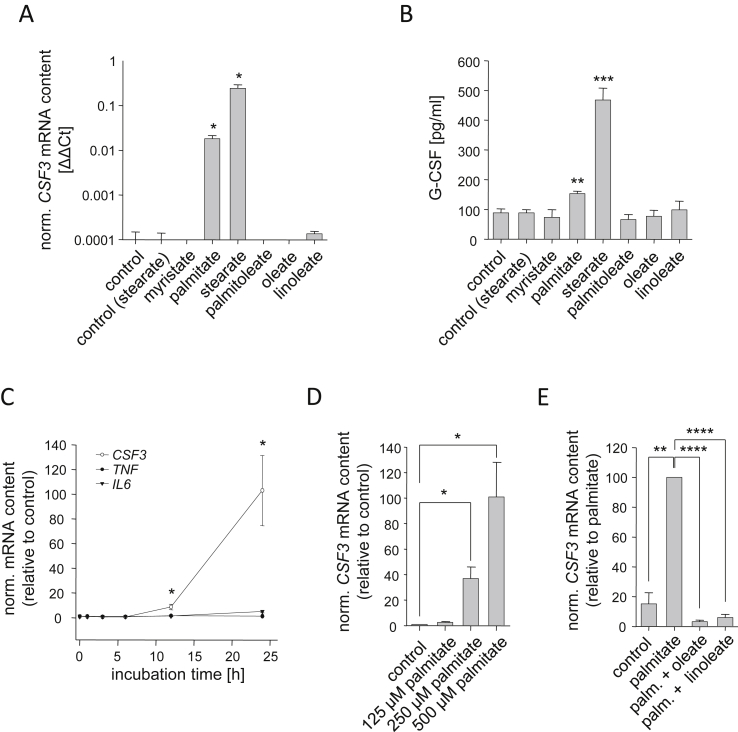
To better understand FFA-induced CSF3 expression, we treated primary human myotubes with a panel of long-chain SFAs and UFAs. In untreated cells, CSF3 mRNA contents ranged at the detection limit of the RT-PCR method. Only the SFAs palmitate (C16:0) and stearate (C18:0) were able to significantly induce CSF3 expression whereas the SFA myristate (C14:0) and the UFAs palmitoleate (C16:1ω7), oleate (C18:1ω9), and linoleate (C18:2ω6) did not change its expression (Figure 1A). Elevated CSF3 expression resulted in increased G-CSF concentrations in culture media conditioned by cells treated with SFAs (Figure 1B). In this regard, stearate was more potent than palmitate (Figure 1A,B). As stearate was described as a potent lipotoxic agent [19], [24], we preferred the SFA palmitate in most of our treatment experiments. Palmitate treatment did not provoke any signs of lipotoxicity in human myotubes at the concentrations used. The palmitate-induced CSF3 expression was time- (Figure 1C) as well as dose-dependent (Figure 1D). CSF3 expression was significantly induced as early as 12 h after start of treatment (Figure 1C) and was much stronger induced compared to other myokine genes, like TNF and IL6 (Figure 1C). It was known that UFAs can counteract SFA effects [24], [25]. Therefore, we co-incubated human myotubes with palmitate on the one hand and linoleate or oleate, respectively, on the other hand. Both UFAs completely blunted the CSF3-inducing effect of palmitate (Figure 1E).
Figure 1.
Fatty acid-regulated CSF3 expression in human myotubes. (A) Human myotubes differentiated in vitro were treated for 24 h with the indicated fatty acids (0.5 mmol/L), and CSF3 mRNA expression was measured by RT-PCR. (B) G-CSF concentration was measured in the conditioned culture media. (C) CSF3, TNF, and IL6 mRNA contents were measured at different time-points in human myotubes treated with palmitate (0.5 mmol/L). (D) Human myotubes were treated for 24 h with the indicated palmitate concentrations, and CSF3 mRNA expression was measured. (E) CSF3 mRNA content was assessed in human myotubes treated with palmitate alone or in combination with oleate or linoleate, respectively (0.5 mmol/L each). Data are given as means ± SEM. Two-group comparisons were performed using matched-pairs Student's t-test (N ≥ 3; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
2.2. The role of Toll-like receptors (TLRs) in SFA-induced CSF3 expression
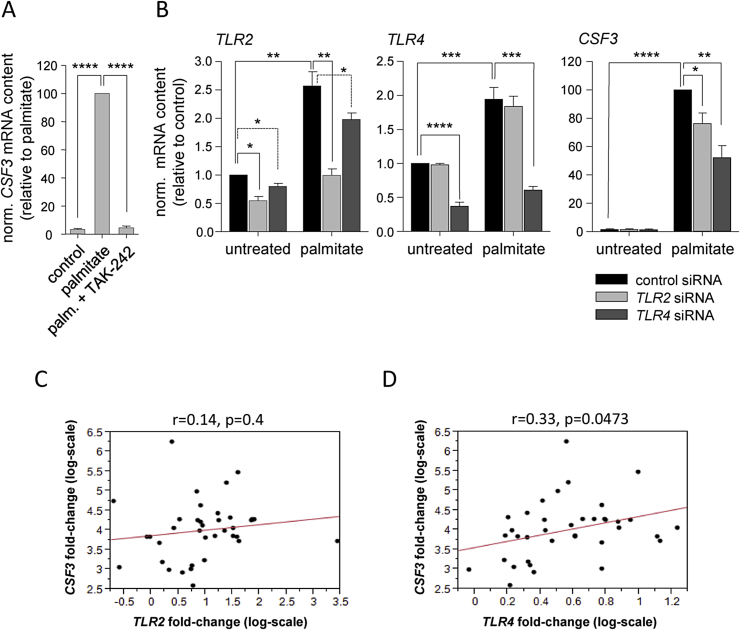
The finding that UFAs counteracted SFA-induced CSF3 expression pointed to an involvement of TLR2 or TLR4 as FFA receptor since it was known that SFAs activate these receptors whereas UFAs block them [26], [27], [28]. Therefore, we co-incubated human myotubes with palmitate and the TLR4 inhibitor TAK-242 [29] and observed a complete inhibition of the palmitate effect (Figure 2A). Furthermore, we applied TLR2 and TLR4 knockdown by RNA interference (Figure 2B). We observed an up-regulation of TLR2 and TLR4 mRNA in palmitate-treated human myotubes, which was reported before [30]. Treatment of cells with TLR4 siRNA did not only significantly reduce the basal and palmitate-induced expression of TLR4, but also slightly impaired TLR2 expression. TLR2 siRNA, on the other hand, was more specific and reduced basal and palmitate-induced TLR2 expression without having any effect on TLR4 expression (Figure 2B). In TLR4 siRNA-treated cells, palmitate-induced CSF3 expression was markedly reduced by 40% (Figure 2B). TLR2 siRNA treatment also reduced palmitate-induced CSF3 expression, however to a lesser extent (22%, Figure 2B). As shown above (Figure 1A), CSF3 expression was induced most strongly by stearate. Even though it is supposed to be more cytotoxic than palmitate we used this SFA to reach maximum amplitudes of CSF3 induction and a sufficient spread for correlational analyses in myotubes from 33 human donors. We measured CSF3, TLR2, and TLR4 expression and detected a positive correlation between stearate-stimulated CSF3 induction and TLR4 induction (Figure 2D), but not between CSF3 induction and TLR2 induction (Figure 2C). These experiments supported a relevant role of TLR4 in SFA-induced CSF3 expression.
Figure 2.
Saturated fatty acid-induced CSF3 expression via Toll-like receptors. (A) CSF3 mRNA expression was measured in human myotubes pretreated for 6 h with TAK-242 (1 μg/mL) prior to 24-hour treatment with palmitate (0.5 mmol/L). (B) Human myotubes were pretreated for 24 h with siRNA for TLR2 or TLR4, respectively, prior to 24-hour treatment with palmitate (0.5 mmol/L), and TLR2, TLR4, and CSF3 mRNA contents were quantified. Data are given as means ± SEM. Two-group comparisons were performed using matched-pairs Student's t-test (N ≥ 3; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). (C and D) Myotube cultures derived from 33 human donors were treated for 24 h with stearate (0.5 mmol/L), stearate-stimulated induction rates (fold-changes) of CSF3, TLR2, and TLR4 mRNA expression were determined, and their inter-relationships were analyzed by simple linear regression analysis.
2.3. Transcription factors and kinases involved in SFA-induced CSF3 expression
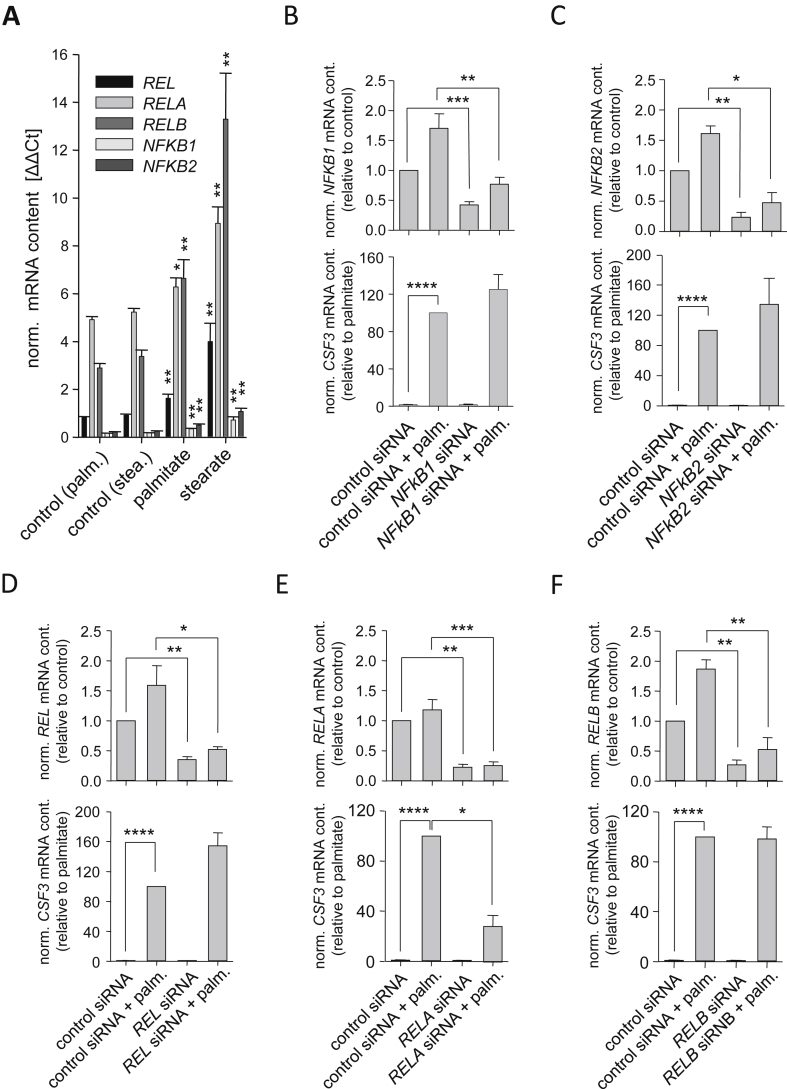
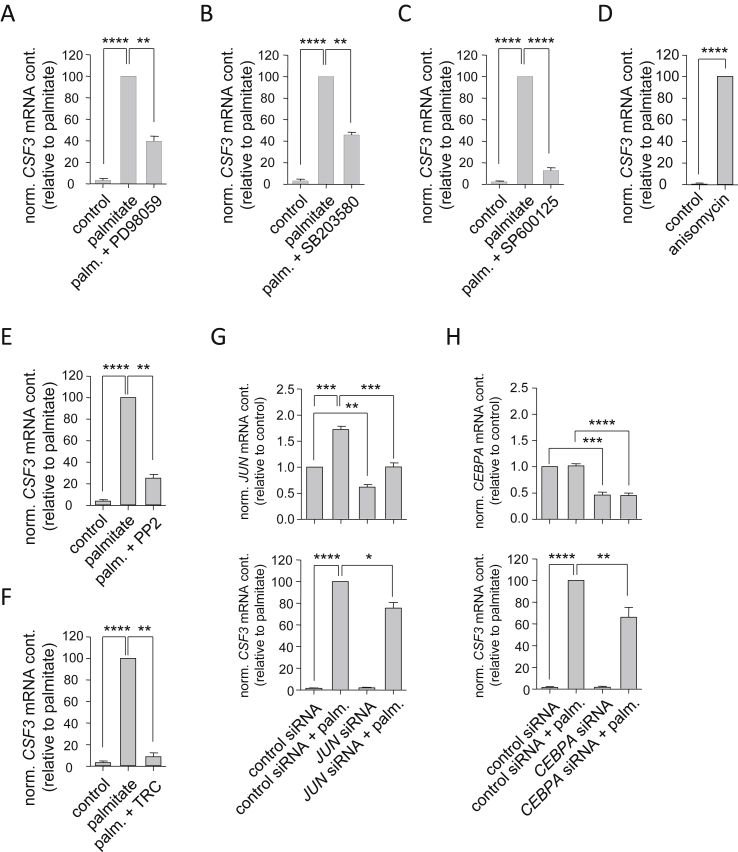
Analyzing the promoter region of CSF3 using a search engine based on SABiosciences' text mining application and the UCSC genome browser (http://www.sabiosciences.com/chipqpcrsearch.php), we identified putative binding sites for several transcription factors known to be downstream of TLR2 and TLR4, namely NFκB1, Rel-A, and AP-1 [31]. Human myotubes expressed all five NFκB family members with RELA and RELB showing the strongest gene expression (Figure 3A). Myotubes treated with the SFAs palmitate and stearate revealed significant induction of all five NFKB genes (Figure 3A). We down-regulated all five genes by RNA interference and observed an impairment of palmitate-induced CSF3 expression exclusively in cells treated with RELA siRNA (Figure 3E), knockdown of REL, RELB, NFKB1, and NFKB2 had no impact on palmitate-induced CSF3 expression (Figure 3B,C,D and F). Apart from NFκB, stress kinases were reported to be activated upon TLR4 activation [32]. Therefore, we pharmacologically inhibited mitogen-activated protein kinase (MAPK)/ERK kinases (MEK) 1 and 2 (Figure 4A), p38 MAPK (Figure 4B), and c-Jun N-terminal kinase (JNK) (Figure 4C), respectively. All these treatments resulted in impairment of palmitate-induced CSF3 expression, with JNK inhibition showing the strongest effect compared to MEK1/2 and p38 MAPK inhibition. On the other hand, palmitate-independent activation of p38 MAPK and JNK by anisomycin resulted in strong CSF3 induction (Figure 4D). These findings further highlighted the importance of TLR4 signaling via stress kinases and Rel-A in SFA-induced CSF3 induction.
Figure 3.
Role of NFκB in palmitate-induced CSF3 expression. (A) The mRNA expression of the indicated genes was measured in human myotubes treated for 24 h with palmitate (0.5 mmol/L) or stearate (0.5 mmol/L), respectively. (B–F) Human myotubes were pretreated for 24 h with the indicated siRNA prior to 24-hour treatment with palmitate (0.5 mmol/L), and knockdown of the target gene and its influence on CSF3 mRNA expression was assessed by RT-PCR. Data are given as means ± SEM. Two-group comparisons were performed using matched-pairs Student's t-test (N ≥ 3; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Figure 4.
Additional signaling components of palmitate-induced CSF3 expression. (A, B, and C) Human myotubes were pre-treated for 30 min with the MEK1/2 inhibitor PD98059 (20 μmol/L; A), the p38 MAPK inhibitor SB203580 (5 μmol/L; B), or the JNK inhibitor SP600125 (50 μmol/L; C), respectively, prior to 24-hour treatment with palmitate (0.5 mmol/L). (D) Human myotubes were incubated for 20 h with anisomycin (25 μg/mL). (E and F) Human myotubes were pretreated for 150 min with the Src inhibitor PP2 (15 μmol/L; E) or for 30 min with the long-chain fatty acyl-CoA synthetase inhibitor triacsin C (TRC, 5 μmol/L; F), respectively, prior to 24-hour treatment with palmitate (0.5 mmol/L). (G and H) Human myotubes were pretreated for 24 h with JUN (G) or CEBPA (H) siRNA, respectively, prior to 24-hour treatment with palmitate (0.5 mmol/L). JUN, CEBPA, and CSF3 mRNA expression was quantified by RT-PCR. Data are given as means ± SEM. Two-group comparisons were performed using matched-pairs Student's t-test (N ≥ 3; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Notably, pharmacological inhibition of TLR4-independent steps of palmitate signaling, such as Src activation (Figure 4E) and metabolic activation of intracellular palmitate by long-chain acyl-CoA synthetase (Figure 4F), also resulted in markedly reduced palmitate-induced CSF3 expression providing evidence that palmitate induced CSF3 expression via multiple parallel signaling pathways including pathways independent (signaling via the membrane receptor TLR4) as well as dependent on cellular FFA uptake and subsequent metabolic activation.
Finally, knockdown of the transcription factors c-Jun, a component of the heterodimeric transcription factor complex AP-1 (Figure 4G), and C/EBPα (Figure 4H) significantly reduced palmitate-induced CSF3 expression as well. The list of putative transcription factors potentially binding and trans-activating the CSF3 promoter was comprehensive (http://www.sabiosciences.com/chipqpcrsearch.php). These findings pointed to a complex combinatorial regulation of CSF3 gene transcription.
2.4. Mitogenic effect of G-CSF on human myoblasts
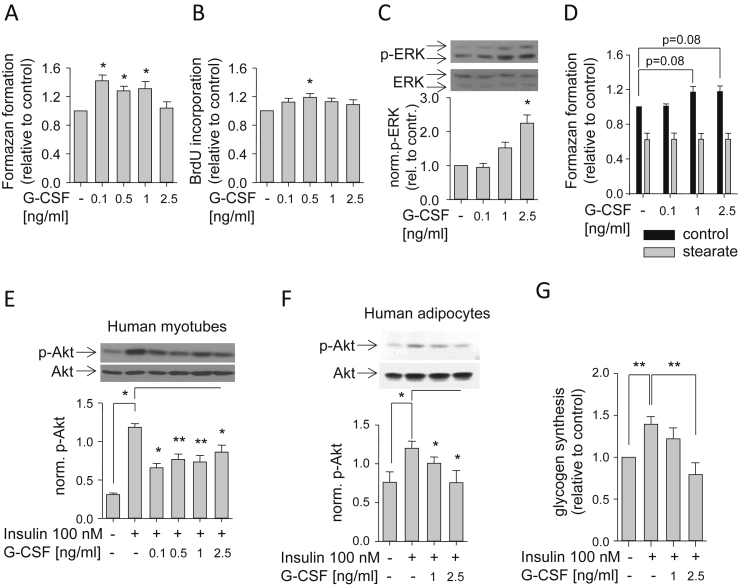
We then addressed the issue whether G-CSF can exert effects on muscle cells thereby opening the possibility of local auto-/paracrine actions of muscle-derived G-CSF. A mitogenic effect of G-CSF on murine myoblasts was described earlier [33]. Therefore, we treated undifferentiated human myoblasts with different concentrations of G-CSF for five days and detected a moderate and concentration-dependent stimulatory effect on cell proliferation by WST-1 (Figure 5A) and BrdU incorporation (Figure 5B) assays. In accordance, we found concentration-dependent increments in ERK phosphorylation after 10 min of incubation with G-CSF (Figure 5C). Anti-apoptotic effects of G-CSF were known from other cell types [14]. Therefore, we examined whether G-CSF can prevent stearate-induced reduction of culture growth/viability using the WST-1 assay. Co-treatment of human myoblasts with stearate and G-CSF in different concentrations for 48 h did not prevent stearate-induced reduction of culture growth/viability (Figure 5D). These findings suggested mitogenic rather than an anti-apoptotic effects of G-CSF on human myoblasts.
Figure 5.
G-CSF effects on human myoblasts, myotubes and adipocytes. (A and B) Proliferating human myoblasts were treated for five days with the indicated G-CSF concentrations, and culture growth was assessed via formazan formation (A) or BrdU incorporation (B), respectively. (C) After 10-minute treatment with the indicated G-CSF concentrations, proliferating myoblasts were lysed, and phospho-ERK (p-ERK; i.e., phospho-p42 and phospho-p44) and ERK (i.e., p42 and p44) signals were visualized by Western blotting; phosphorylated p42 was normalized for total p42 protein. (D) Human myoblasts were incubated for 48 h with the indicated G-CSF concentrations in the absence (control) or presence of stearate (0.5 mmol/L), respectively, and viability was assessed via formazan formation. (E and F) Human myotubes (E) and adipocytes (F) differentiated in vitro were pretreated for 30 min with the indicated G-CSF concentrations prior to 5-minute treatment with insulin (100 nmol/L), and Akt and phospho-Akt (p-Akt) were visualized by Western blotting; phosphorylated Akt was normalized for total Akt protein. (G) Human myotubes were pretreated for 60 min with insulin (100 nmol/L) and the indicated G-CSF concentrations prior to addition of glucose (5.5 mmol/L) for an additional 60 min, and glycogen synthesis was determined as described in Materials and Methods. Data are given as means ± SEM. Two-group comparisons were performed using matched-pairs Student's t-test (N ≥ 3; *p < 0.05, **p < 0.01).
2.5. G-CSF effects on insulin action in human myotubes and adipocytes
CSF3 was found overexpressed in visceral and subcutaneous adipose tissue of morbidly obese subjects [17], [34]. The majority of these subjects were reported to be insulin-resistant. Therefore, we studied G-CSF's effect on insulin action in human myotubes and adipocytes. Both cell types were pretreated for 30 min with different concentrations of G-CSF prior to incubation with insulin. In both cell types, we observed reduced insulin-induced Akt phosphorylation by G-CSF treatment (Figure 5E,F). Additionally, we quantified insulin-stimulated glycogen synthesis in myotubes treated with G-CSF. Co-incubation of myotubes with insulin and G-CSF prevented insulin-induced glycogen synthesis (Figure 5G). In conclusion, G-CSF had insulin-desensitizing properties and, as a SFA-induced myokine, could contribute to SFA-induced insulin resistance of muscle and adipose tissue.
2.6. Impact of CSF3 tagging SNPs on insulin sensitivity and myotube CSF3 expression
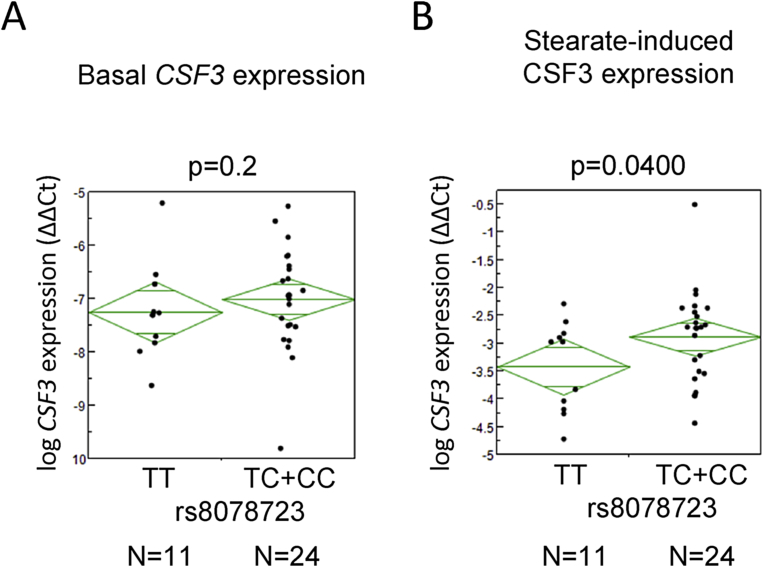
Encouraged by the inhibitory effect of G-CSF on insulin action in human myotubes and adipocytes, we analyzed the influence of four CSF3 tagging SNPs (rs8078723, rs2071369, rs25645, and rs2827) on insulin sensitivity and glucose tolerance in 1,859 metabolically characterized subjects of the ongoing TÜF study. One SNP (rs8078723) revealed significant associations (p < 0.0127, dominant model) with the area under the curve (AUC) of plasma glucose and adipose tissue insulin resistance and a nominal association (0.0127 < p < 0.05, dominant model) with whole-body insulin sensitivity with carriers of the minor C-allele showing elevated plasma glucose, increased adipose tissue insulin resistance, and reduced whole-body insulin sensitivity compared to homozygous carriers of the major T-allele (Table 1). In accordance with G-CSF's well-known proliferative effects on leukocytes, we also detected a significant SNP effect on blood leukocyte number (p < 0.0127, both additive and dominant model) with minor C-allele carriers showing increased leukocyte numbers (Table 1). Since CSF3 was shown to be a FFA-regulated gene, we stratified the human cohort into subjects with low plasma FFA (lowest two quintiles of data distribution) and high plasma FFA (highest two quintiles) concentrations, respectively (the middle quintile was excluded from analysis as grey zone). CSF3 SNP rs8078723 did not show any effect in subjects with low FFA levels who, according to our in vitro data, probably did not express appreciable amounts of CSF3 mRNA in muscle (Table 1). However, in subjects with high FFA levels, the effects seen in the overall cohort were conserved (Table 1). Since G-CSF inhibited insulin action in primary human myotubes and SNP rs8078723 affected insulin sensitivity in the human cohort, we hypothesized that the SNP should be associated with increased CSF3 expression in the myotubes. Even though basal CSF3 expression was borderline and not different between the genotypes (Figure 6A), myotubes from minor allele carriers upon stearate treatment showed the expected higher levels of CSF3 expression compared to homozygous major allele carriers (Figure 6B). These results indicated that minor allele carriers of CSF3 SNP rs8078723 were more responsive to SFAs with respect to CSF3 gene induction and, via elevated G-CSF expression and auto-/paracrine G-CSF signaling, could thus experience impairments in insulin sensitivity of muscle and adipose tissue.
Table 1.
Associations of CSF3 SNP rs8078723 in the human cohort.
| Overall (N = 1,859) | |||||
| TT | TC | CC | padd | pdom | |
| N (women/men) | 758 (484/274) | 870 (586/284) | 231 (161/70) | – | – |
| AUC glucose (mmol/L) | 14.4 ± 0.1 | 14.9 ± 0.1 | 14.7 ± 0.2 | 0.0139 | 0.0007 |
| ISI-OGTT (1019 L2/mol2) | 13.8 ± 0.2 | 13.2 ± 0.2 | 13.6 ± 0.5 | 0.1 | 0.0295 |
| IRI-AT (10−18 mol2/L2) | 34,085 ± 817 | 37,464 ± 902 | 37,460 ± 1575 | 0.0231 | 0.0126 |
| Leukocytes (μL−1) | 6,478 ± 115 | 6,640 ± 61 | 6,714 ± 118 | 0.0066 | 0.0029 |
| Low FFAs (N = 743) | |||||
| TT | TC | CC | padd | pdom | |
| N (women/men) | 321 (163/158) | 335 (181/154) | 87 (54/33) | – | – |
| AUC glucose (mmol/L) | 13.9 ± 0.1 | 14.2 ± 0.1 | 13.8 ± 0.3 | 0.5 | 0.2 |
| ISI-OGTT (1019 L2/mol2) | 14.4 ± 0.4 | 14.0 ± 0.4 | 13.6 ± 0.7 | 0.1 | 0.1 |
| IRI-AT (10−18 mol2/L2) | 23,064 ± 723 | 24,123 ± 708 | 25,988 ± 1715 | 0.1 | 0.2 |
| Leukocytes (μL−1) | 6,627 ± 248 | 6,535 ± 99 | 6,875 ± 225 | 0.2 | 0.4 |
| High FFAs (N = 742) | |||||
| TT | TC | CC | padd | pdom | |
| N (women/men) | 269 (206/63) | 372 (291/81) | 101 (78/23) | – | – |
| AUC glucose (mmol/L) | 15.1 ± 0.2 | 15.7 ± 0.1 | 15.1 ± 0.3 | 0.2 | 0.0113 |
| ISI-OGTT (1019 L2/mol2) | 14.0 ± 0.5 | 12.2 ± 0.3 | 13.8 ± 0.8 | 0.2 | 0.0117 |
| IRI-AT (10−18 mol2/L2) | 45,755 ± 1664 | 51,280 ± 1660 | 48,834 ± 2633 | 0.06 | 0.0215 |
| Leukocytes (μL−1) | 6,313 ± 104 | 6,799 ± 95 | 6,630 ± 159 | 0.0038 | 0.0002 |
The human cohort was left unstratified (overall) or was stratified into subjects with low plasma FFA (lowest two quintiles of data distribution combined) and high plasma FFA (highest two quintiles combined) concentrations, respectively (the mid quintile was excluded from analysis as grey zone). Metabolic data were adjusted for gender, age, and BMI. The SNP was analyzed in the additive and dominant inheritance model (padd, pdom). Significant associations (p < 0.0127 after Bonferroni correction for four SNPs) are marked by using bold fonts. AUC – area under the curve; FFA – free fatty acid; IRI-AT – insulin resistance index adipose tissue; ISI-OGTT – insulin sensitivity index oral glucose tolerance test; SNP – single nucleotide polymorphism.
Figure 6.
Effect of CSF3 SNP rs8078723 on CSF3 mRNA expression in human myotubes. (A and B) Effects of CSF3 SNP rs8078723 in the dominant inheritance model on basal (A) and stearate-induced (B) CSF3 mRNA expression in human myotubes differentiated in vitro. Two-group comparisons were performed using hypothesis-driven one-sided unpaired Student's t-tests.
In order to replicate our findings with the CSF3 SNP rs8078723, we looked into publicly available data of the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC, http://www.magicinvestigators.org). With respect to the associations described in our TÜF study, i.e., associations with glucose tolerance and insulin sensitivity, we interrogated age-, gender-, and BMI-adjusted data for 2-hour glucose from OGTTs (N = 30,620) and for fasting insulin as a rough estimate of insulin resistance (N = 51,750). AUC glucose data were not available. Even though not significant, minor C-allele carriers revealed trends towards higher 2-hour glucose and fasting insulin levels (p = 0.16 and p = 0.13, respectively).
Finally, we measured fasting serum G-CSF concentrations in 238 subjects randomly selected from the TÜF study. The serum G-CSF concentrations averaged 22.0 ± 0.6 pg/mL. Using multiple linear regression analysis, fasting serum G-CSF was positively and independently associated with female gender (p = 0.0011) and BMI (p = 2.8 × 10−8), but neither with age (p = 0.7) nor with the CSF3 SNP rs8078723 (p = 0.6, both additive and dominant model). Moreover, we did not observe associations of fasting serum G-CSF with AUC glucose, adipose tissue insulin resistance, or whole-body insulin resistance (p ≥ 0.6, all). Based on these clearly negative data, the compared to the serum levels up to 25-fold higher concentrations reached in media conditioned by SFA-treated human myotubes (Figure 1B), and the observation that the fasting serum levels are at least 5-fold lower than those found to be effective in cultured cells (Figure 5), we concluded that myocyte-derived G-CSF is of auto-/paracrine rather than endocrine importance.
3. Discussion
In this study, we analyzed the mechanism underlying FFA-induced CSF3 expression in primary human myotubes. Since we found its expression to be regulated by TLR4, a cell membrane receptor known to be involved in inflammatory signaling and insulin resistance [35], we also assessed the effect of this gene's product, G-CSF, on insulin action in human myotubes and adipocytes. In an attempt to translate our findings to the human situation, in vivo effects of CSF3 SNPs on insulin sensitivity and glucose tolerance were detected.
Muscle CSF3 expression induced by palmitate and electric pulse stimulation (an in vitro exercise model) was reported before [8], [9]. Here, we show that CSF3 expression is induced by long-chain SFAs (palmitate and stearate) but not UFAs (oleate and linoleate). In addition, long-chain UFAs are able to very potently blunt palmitate-induced CSF3 expression reflecting UFAs' beneficial effects observed earlier: for instance, co-incubation of SFAs with UFAs was shown to prevent SFA-induced apoptosis of human pancreatic β-cells [36] and human coronary artery endothelial cells [24] as well as SFA-induced inflammation and impaired insulin signaling in skeletal muscle cells [25].
Based on the aforementioned observation, we suggested that the effects of SFAs on CSF3 expression are mediated by TLRs [26], [27], [28]. Indeed, pharmacological TLR4 inhibition and down-regulation of TLR4 expression resulted in reduced CSF3 expression. Furthermore, CSF3 expression correlated with TLR4 expression in myotube cultures from 33 donors, further underlining this receptor's importance for CSF3 expression. TLR4 expression is critical for LPS effects in mice [37] and for insulin homeostasis in human and murine β-cells [38]. The best known pathway downstream of TLR4 includes NFkB, however, stress kinases are activated as well [31]. We identified putative binding sites for NFκB1 and Rel-A in the human CSF3 promoter region in silico, and our data indicate that palmitate-induced CSF3 expression is Rel-A- rather than NFκB1-dependent. Moreover, human myocyte CSF3 expression is strongly induced by stress kinases, such as JNK, p38 MAPK, and MEK1/2. Some studies report preferred stress kinase activation upon TLR activation by SFAs: during endurance training, elevated extracellular FFA levels activate p38 MAPK and JNK via TLR2 and TLR4 [39]. Additionally, JNK activation via TLR4 rather than NFkB activation is crucial for palmitate-induced INS-1 beta cell death [40]. However, we also have to stress that, apart from TLR4-dependent pathways, TLR4-independent pathways may also contribute to palmitate-induced CSF3 gene expression. We analyzed two TLR4-independent axes, i.e., Src activation and metabolic activation of palmitate by long-chain fatty acyl-CoA synthetase. Upon palmitate treatment, Src is relocated to lipid rafts where it gets activated and subsequently stimulates JNK [41]. Long-chain fatty acyl-CoA synthetase catalyzes the synthesis of fatty acyl-CoA leading to ER-stress and thereby to JNK activation [42]. Pharmacological inhibition of Src and long-chain fatty acyl-CoA synthetase reduced palmitate-induced CSF3 expression. Since both pathways result in JNK activation [41], [42], these findings may highlight the importance of the JNK pathway for SFA-induced CSF3 expression.
TLR4 activation plays an important role in SFA-induced insulin resistance [43], [44]. On the one hand, TLR4 activates downstream kinases, such as JNK and p38 MAPK, which directly inhibit insulin action by serine phosphorylation of insulin receptor substrates [44], [45]. On the other hand, TLR4 activation leads to enhanced expression and secretion of cytokines, like TNFα, which in turn inhibit insulin action [44], [45]. G-CSF not only belongs to the list of TLR4-induced cytokines, it also appears to be induced faster than IL6 and TNFα: CSF3 expression upon palmitate treatment showed an earlier and stronger onset compared to IL6 and TNF expression (Figure 1C) possibly indicating that it may be, upon TLR4 activation, among the earliest secreted factors inducing insulin resistance in an auto-/paracrine manner. Hence, its impact on insulin action was assessed in primary human myotubes and adipocytes. In both cell types, G-CSF inhibited insulin action confirming our assumption that G-CSF is a novel player in TLR4-mediated insulin resistance.
G-CSF plasma levels are known to increase upon intensive exercise [13], and there are reports describing reduced insulin signaling shortly after intensive exercise, such as marathon runs [46]. According to our data pointing to insulin-desensitizing G-CSF effects, elevated G-CSF plasma levels upon exercise could contribute to this temporary insulin resistance that is thought to favor fat combustion and to spare glucose for the brain. Palmitate and stearate are major plasma FFAs that, according to our data, may represent metabolic risk factors due to their CSF3-inducing properties. However, UFAs, like palmitoleate, that are usually simultaneously present in blood may efficiently counteract this permanent risk and may therefore be indicative of insulin sensitivity [47]. This fits well our data showing efficient inhibition of TLR4 activation and expression/release of the insulin-desensitizing cytokine G-CSF by UFAs.
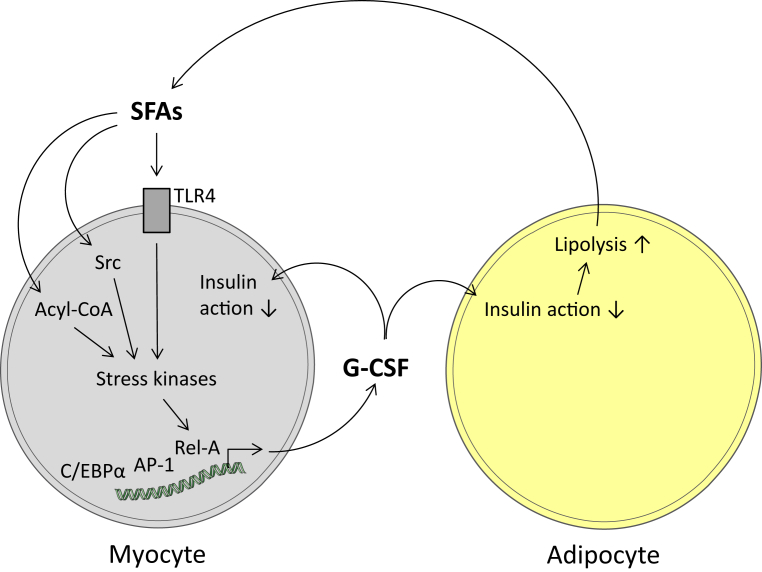
Chronically high plasma SFA concentrations due to, e.g., obesity-mediated deregulated lipolysis provoke chronic insulin resistance. Even though a systemic role of G-CSF in metabolic diseases is not established, CSF3 expression was found elevated in adipose tissue of morbidly obese subjects [17] possibly contributing to local adipose tissue insulin resistance, as our adipocyte data may suggest. Moreover, obesity-related high plasma SFA concentrations, according to our data, should also increase CSF3 expression in skeletal muscle cells, and G-CSF secreted from skeletal muscle cells may not only impair insulin action in myocytes per se but also in the adjacent extramyocellular fat depots (Figure 7). Thus, it is conceivable that high local G-CSF concentrations in the, by mass, most important insulin-responsive tissues muscle and fat may essentially contribute to reduced whole-body insulin sensitivity. In accordance with this concept, the minor allele of a common SNP in the CSF3 gene (rs8078723) that was associated with increased CSF3 expression in human myotubes affected adipose tissue and whole-body insulin sensitivity in subjects with elevated FFA levels, without influencing plasma G-CSF concentrations.
Figure 7.
Schematic presentation of the hypothetical local role of G-CSF in insulin resistance. In human skeletal muscle cells, saturated long-chain fatty acids (SFAs) activate, via Toll-like receptor 4 (TLR4)-dependent and -independent pathways, stress kinases and, further downstream, transcription factors (Rel-A, AP-1, C/EBPα) involved in the combinatorial induction of the G-CSF gene. Enhanced G-CSF production and release provokes, in an auto-/paracrine way, impairments of insulin actions in myocytes and the adjacent adipocytes of the so-called extramyocellular fat depot. Based on the mass of skeletal muscle and its surrounding fat depot, it is conceivable that this local insulin resistance contributes to whole-body insulin resistance.
In conclusion, there is accumulating evidence that SFAs play an important role in the pathogenesis of insulin resistance and type 2 diabetes. With respect to the identification of novel targets for prevention and treatment of type 2 diabetes, it is crucial to clarify the mechanisms underlying SFA-induced insulin resistance. In this study, we identified TLR4-dependent and -independent pathways mediating SFA-induced expression of the novel myokine G-CSF. G-CSF treatment impaired insulin action in both human myotubes and adipocytes. Moreover, data from SNP analyses revealed effects of the CSF3 gene on adipose tissue and whole-body insulin sensitivity as well as glucose tolerance in subjects with high plasma FFA levels supporting an involvement of G-CSF in FFA-induced insulin resistance. In summary, our data point to G-CSF as a novel insulin-desensitizing myokine of relevance for human insulin sensitivity and glucose tolerance.
Author contributions
A.-M.O. and N.G. performed the in vitro experiments and analyzed the data, A.-M.O. designed the in vitro experiments and wrote the manuscript. C.H. and I.T. provided technical support and analyzed the data. A.B. and F.M. collected and analyzed the human data. N.S., A.F. and H.-U.H. designed and carried out the human study. H.S. designed the in vitro experiments, analyzed the data, and edited the manuscript. All authors discussed the results and commented on the manuscript.
Acknowledgments
We thank all participants of the TÜF Study for their cooperation. We thank Alke Guirguis, Daniela Thien, and Roman Werner for excellent technical assistance. This study was supported in part by a grant (01GI0925) from the German Federal Ministry of Education and Research (BMBF) to the German Centre for Diabetes Research (DZD e.V.). N.S. is supported by a Heisenberg professorship from the Deutsche Forschungsgemeinschaft (STE 1096/3-1).
Conflict of interest
The authors have no conflicts of interest related to this study to declare.
References
- 1.Nakamura M.T., Yudell B.E., Loor J.J. Regulation of energy metabolism by long-chain fatty acids. Progress in Lipid Research. 2014;53:124–144. doi: 10.1016/j.plipres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Boden G. Free fatty acids and insulin secretion in humans. Current Diabetes Reports. 2005;5:167–170. doi: 10.1007/s11892-005-0004-5. [DOI] [PubMed] [Google Scholar]
- 3.Cnop M., Igoillo-Esteve M., Cunha D.A., Ladriere L., Eizirik D.L. An update on lipotoxic endoplasmic reticulum stress in pancreatic beta-cells. Biochemical Society Transactions. 2008;36:909–915. doi: 10.1042/BST0360909. [DOI] [PubMed] [Google Scholar]
- 4.Boden G. Obesity, insulin resistance and free fatty acids. Current Opinion in Endocrinology Diabetes and Obesity. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel V.T., Shulman G.I. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masi L.N., Rodrigues A.C., Curi R. Fatty acids regulation of inflammatory and metabolic genes. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16:418–424. doi: 10.1097/MCO.0b013e32836236df. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen B.K. Muscle as a secretory organ. Comprehensive Physiology. 2013;3:1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- 8.Staiger H., Haas C., Machann J., Werner R., Weisser M., Schick F. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009;58:579–589. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheler M., Irmler M., Lehr S., Hartwig S., Staiger H., Al Hasani H. Cytokine response of primary human myotubes in an in vitro exercise model. American Journal of Physiology – Cell Physiology. 2013;305:C877–C886. doi: 10.1152/ajpcell.00043.2013. [DOI] [PubMed] [Google Scholar]
- 10.Welte K., Platzer E., Lu L., Gabrilove J.L., Levi E., Mertelsmann R. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheridan W.P., Morstyn G., Wolf M., Dodds A., Lusk J., Maher D. Granulocyte colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet. 1989;2:891–895. doi: 10.1016/s0140-6736(89)91552-3. [DOI] [PubMed] [Google Scholar]
- 12.Sheridan W.P., Begley C.G., Juttner C.A., Szer J., To L.B., Maher D. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339:640–644. doi: 10.1016/0140-6736(92)90795-5. [DOI] [PubMed] [Google Scholar]
- 13.Yamada M., Suzuki K., Kudo S., Totsuka M., Nakaji S., Sugawara K. Raised plasma G-CSF and IL-6 after exercise may play a role in neutrophil mobilization into the circulation. Journal of Applied Physiology (1985) 2002;92:1789–1794. doi: 10.1152/japplphysiol.00629.2001. [DOI] [PubMed] [Google Scholar]
- 14.Mooren F.C., Volker K., Klocke R., Nikol S., Waltenberger J., Kruger K. Exercise delays neutrophil apoptosis by a G-CSF-dependent mechanism. Journal of Applied Physiology (1985) 2012;113:1082–1090. doi: 10.1152/japplphysiol.00797.2012. [DOI] [PubMed] [Google Scholar]
- 15.Dittgen T., Pitzer C., Plaas C., Kirsch F., Vogt G., Laage R. Granulocyte-colony stimulating factor (G-CSF) improves motor recovery in the rat impactor model for spinal cord injury. PLoS One. 2012;7:e29880. doi: 10.1371/journal.pone.0029880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider A., Kruger C., Steigleder T., Weber D., Pitzer C., Laage R. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. Journal of Clinical Investigation. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinahones F.J., Coin A.L., Murri M., Oliva O.W., Mayas T., Barbarroja N. Caspase induction and BCL2 inhibition in human adipose tissue: a potential relationship with insulin signaling alteration. Diabetes Care. 2013;36:513–521. doi: 10.2337/dc12-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krutzfeldt J., Kausch C., Volk A., Klein H.H., Rett K., Haring H.U. Insulin signaling and action in cultured skeletal muscle cells from lean healthy humans with high and low insulin sensitivity. Diabetes. 2000;49:992–998. doi: 10.2337/diabetes.49.6.992. [DOI] [PubMed] [Google Scholar]
- 19.Eitel K., Staiger H., Rieger J., Mischak H., Brandhorst H., Brendel M.D. Protein kinase C delta activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes. 2003;52:991–997. doi: 10.2337/diabetes.52.4.991. [DOI] [PubMed] [Google Scholar]
- 20.Bohm A., Halama A., Meile T., Zdichavsky M., Lehmann R., Weigert C. Metabolic signatures of cultured human adipocytes from metabolically healthy versus unhealthy obese individuals. PLoS One. 2014;9:e93148. doi: 10.1371/journal.pone.0093148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heni M., Hennige A.M., Peter A., Siegel-Axel D., Ordelheide A.M., Krebs N. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS One. 2011;6:e21594. doi: 10.1371/journal.pone.0021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefan N., Machicao F., Staiger H., Machann J., Schick F., Tschritter O. Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia. 2005;48:2282–2291. doi: 10.1007/s00125-005-1948-3. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M., Defronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 24.Staiger K., Staiger H., Weigert C., Haas C., Haring H.U., Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;55:3121–3126. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 25.Coll T., Eyre E., Rodriguez-Calvo R., Palomer X., Sanchez R.M., Merlos M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. Journal of Biological Chemistry. 2008;283:11107–11116. doi: 10.1074/jbc.M708700200. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.Y., Sohn K.H., Rhee S.H., Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. Journal of Biological Chemistry. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.Y., Zhao L., Youn H.S., Weatherill A.R., Tapping R., Feng L. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. Journal of Biological Chemistry. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.Y., Ye J., Gao Z., Youn H.S., Lee W.H., Zhao L. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. Journal of Biological Chemistry. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 29.Matsunaga N., Tsuchimori N., Matsumoto T., Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Molecular Pharmacology. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 30.Reyna S.M., Ghosh S., Tantiwong P., Meka C.S., Eagan P., Jenkinson C.P. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akira S., Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 32.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Current Opinion in Immunology. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 33.Hara M., Yuasa S., Shimoji K., Onizuka T., Hayashiji N., Ohno Y. G-CSF influences mouse skeletal muscle development and regeneration by stimulating myoblast proliferation. Journal of Experimental Medicine. 2011;208:715–727. doi: 10.1084/jem.20101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbarroja N., Lopez-Pedrera C., Garrido-Sanchez L., Mayas M.D., Oliva-Olivera W., Bernal-Lopez M.R. Progression from high insulin resistance to type 2 diabetes does not entail additional visceral adipose tissue inflammation. PLoS One. 2012;7:e48155. doi: 10.1371/journal.pone.0048155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.Y., Hwang D.H. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Molecolar Cells. 2006;21:174–185. [PubMed] [Google Scholar]
- 36.Eitel K., Staiger H., Brendel M.D., Brandhorst D., Bretzel R.G., Haring H.U. Different role of saturated and unsaturated fatty acids in beta-cell apoptosis. Biochemical and Biophysical Research Communications. 2002;299:853–856. doi: 10.1016/s0006-291x(02)02752-3. [DOI] [PubMed] [Google Scholar]
- 37.Kalis C., Kanzler B., Lembo A., Poltorak A., Galanos C., Freudenberg M.A. Toll-like receptor 4 expression levels determine the degree of LPS-susceptibility in mice. European Journal of Immunology. 2003;33:798–805. doi: 10.1002/eji.200323431. [DOI] [PubMed] [Google Scholar]
- 38.Garay-Malpartida H.M., Mourao R.F., Mantovani M., Santos I.A., Sogayar M.C., Goldberg A.C. Toll-like receptor 4 (TLR4) expression in human and murine pancreatic beta-cells affects cell viability and insulin homeostasis. BMC Immunology. 2011;12:18. doi: 10.1186/1471-2172-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zbinden-Foncea H., Raymackers J.M., Deldicque L., Renard P., Francaux M. TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Medicine & Science in Sports & Exercise. 2012;44:1463–1472. doi: 10.1249/MSS.0b013e31824e0d5d. [DOI] [PubMed] [Google Scholar]
- 40.Lee S.M., Choi S.E., Lee J.H., Lee J.J., Jung I.R., Lee S.J. Involvement of the TLR4 (Toll-like receptor4) signaling pathway in palmitate-induced INS-1 beta cell death. Molecular and Cellular Biochemistrty. 2011;354:207–217. doi: 10.1007/s11010-011-0820-7. [DOI] [PubMed] [Google Scholar]
- 41.Holzer R.G., Park E.J., Li N., Tran H., Chen M., Choi C. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wymann M.P., Schneiter R. Lipid signalling in disease. Nature Reviews Molecular Cell Biology. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 43.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. Journal Clinical Investigation. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J.J., Sears D.D. TLR4 and insulin resistance. Gastroenterology Research and Practice. 2010;2010 doi: 10.1155/2010/212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 46.Tuominen J.A., Ebeling P., Bourey R., Koranyi L., Lamminen A., Rapola J. Postmarathon paradox: insulin resistance in the face of glycogen depletion. American Journal of Physiology. 1996;270:E336–E343. doi: 10.1152/ajpendo.1996.270.2.E336. [DOI] [PubMed] [Google Scholar]
- 47.Stefan N., Kantartzis K., Celebi N., Staiger H., Machann J., Schick F. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care. 2010;33:405–407. doi: 10.2337/dc09-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]