Abstract
Aims
In transthyretin amyloid (ATTR) amyloidosis various principal phenotypes have been described: cardiac, neuropathic, or a mixed cardiac and neuropathic. In addition, two different types of amyloid fibrils have been identified (type A and type B). Type B fibrils have thus far only been found in predominantly early-onset V30M and in patients carrying the Y114C mutation, whereas type A is noted in all other mutations currently examined as well as in wild-type ATTR amyloidosis. The fibril type is a determinant of the ATTR V30M disease phenotype. 99mTc-DPD scintigraphy is a highly sensitive method for diagnosing heart involvement in ATTR amyloidosis. The objective of this study was to determine the relationship between ATTR fibril composition and 99mTc-DPD scintigraphy outcome in patients with biopsy-proven ATTR amyloidosis.
Methods
Altogether 55 patients with biopsy-proven diagnosis of ATTR amyloidosis and amyloid fibril composition determined were examined by 99mTc-DPD scintigraphy. The patients were grouped and compared according to their type of amyloid fibrils. Cardiovascular evaluation included ECG, echocardiography, and cardiac biomarkers. The medical records were scrutinized to identify subjects with hypertension or other diseases that have an impact on cardiac dimensions.
Results
A total of 97% with type A and none of the patients with type B fibrils displayed 99mTc-DPD uptake at scintigraphy (p < 0.001). Findings from analyses of cardiac biomarkers, ECG, and echocardiography, though significantly different, could not differentiate between type A and B fibrils in individual patients.
Conclusion
In ATTR amyloidosis, the outcome of 99mTc-DPD scintigraphy is strongly related to the patients’ transthyretin amyloid fibril composition.
Keywords: Amyloidosis hereditary, amyloid cardiomyopathy, echocardiography, scintigraphy, transthyretin
Introduction
Transthyretin (TTR) amyloid (ATTR) amyloidosis is a systemic disease in which mutant and/or wild-type (normal) TTR assemble into amyloid. Although ATTR amyloidosis is a systemic disease where amyloid can be found in most tissue types, the clinical manifestations vary depending on the disease-causing mutation but also on other, mainly unknown, factors (1). More than 100 different amyloidogenic mutations in the TTR gene have been described (2). A classification of mutations and their phenotypes has been suggested to separate them into three main types: neuropathic, cardiac, and mixed neuropathic and cardiac (3). However, this is an arbitrary classification, and substantial phenotypic variations are noted not only between, but also within the various TTR mutations (4).
The prevailing theory for ATTR formation is based on decreased stability of the TTR tetramer caused by ageing and/or amyloidogenic TTR mutations, which facilitates TTR’s separation into monomers that have a tendency to misfold and reassemble into extracellular amyloid deposits (5). However, an alternative pathway for ATTR fibril formation has been found for the Ser52Pro variant (6), where amyloid formation occurred after proteolysis of TTR at position 50, producing a highly amyloidogenic C-terminal fragment. Interestingly, two different types of ATTR fibrils have been identified within the ATTR V30M population (7,8). One type consists of a mixture of full-length TTR and C-terminal fragments (i.e. those starting at position 46 and later) (type A) and the other of full-length TTR only (type B) (9). Thus two pathways for amyloid formation may operate in ATTR amyloidosis patients, and two precursor proteins are present in their amyloid deposits.
The patients with type B fibrils suffer from early onset of disease and develop to a lesser degree symptomatic restrictive amyloid cardiomyopathy than do those with type A fibrils. Type A, however, is associated with later disease onset and both polyneuropathy and progressive cardiomyopathy (8). These two disease phenotypes have earlier been described as early- and late-onset disease (10,11).
Analyses of fibril composition performed in other TTR mutations and patients with senile systemic amyloidosis (SSA) have so far only shown type B fibrils in ATTR except for the rare ATTR Y114C mutation (12). Serial tissue sampling and analyses have been performed, and the fibril type remains the same over time and has been similar in all organs examined in the individual patient (13). Post-mortem examination of ATTR V30M patients disclosed presence of cardiac amyloid infiltration in both early- and late-onset cases and in both young and old patients (9).
The available medical treatments have not been evaluated with regard to amyloid fibril composition, and a recent publication by Marcoux et al. raises questions concerning the efficiency of treatment with tetramer stabilizers, e.g. Tafamidis, in patients with fragmented TTR (14). Additionally, in patients with cardiac or mixed phenotypes, the amyloid cardiomyopathy appears to progress despite liver transplantation, and life expectancy is significantly shortened (15,16).
The diagnosis of heart involvement in ATTR amyloidosis has been based mainly on endomyocardial biopsy (EMB) and the echocardiographic finding of ventricular wall thickness greater than 12 mm (17–19). Electrocardiography (ECG) is commonly used both in diagnosis and follow-up, and a number of more or less specific findings related to ATTR cardiomyopathy have been identified (3,20,21). Since the report by Puille et al. on 99mtechnetium-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) scintigraphy for visualization of amyloid deposition in ATTR amyloidosis, several additional studies have reported 99mTc-DPD to have a high affinity and sensitivity for ATTR myocardial infiltration (22–24), but lack of uptake or only low-grade uptake (grade 1) has been observed in patients with immunoglobulin light chain amyloid protein (AL) cardiac amyloidosis which signifies that the precursor protein has an impact on the outcome of the investigation. Similarly, Longhi et al. recently described positive 99mTc-DPD scans in apolipoprotein AI-related disease, and negative in disease related to apolipoprotein AII (25). In a study by Rapezzi et al. (26), positive 99mTc-DPD scintigraphy was found in patients without echocardiographic findings consistent with heart hypertrophy, and endomyocardial biopsies confirmed the presence of ATTR amyloid. These findings substantiate the sensitivity of the 99mTc-DPD scintigraphy and its potential use to identify ATTR cardiomyopathy before the patient develops symptoms or signs of disease. To our knowledge, endomyocardial biopsies verifying absence of amyloid infiltration were not performed in patients with negative scans.
The mechanism behind the high sensitivity and specificity of 99mTc-DPD scintigraphy has not been settled, but it has been suggested that the high calcium content in ATTR amyloid facilitates binding to phosphate in the radiotracer, similarly to that of serum amyloid P-component that binds to amyloid fibrils in a highly calcium-dependent manner (27). However, an explanation for the low or absent accumulation of the tracer in immunoglobulin light chain (AL-) amyloid cardiomyopathy has not been presented. In addition, the possible correlations between histopathological findings in ATTR amyloidosis and 99mTc-DPD uptake have not been studied.
Since ATTR amyloid fibrils contain two different components—full-length TTR (type B) or a mixture of C-terminal fragments and full-length TTR (type A)—we hypothesized that the affinity of 99mTc-DPD to ATTR may be related to amyloid fibril composition and therefore also to the findings on 99mTc-DPD scintigraphy. The present study aims to clarify this question.
Material and methods
Patient selection and data collection
Since March 2012 all patients under evaluation for ATTR amyloidosis at the Swedish centre for ATTR amyloidosis, Umeå University Hospital, have been investigated by 99mTc-DPD scintigraphy. Up to May 2014, 55 patients with biopsy-proven ATTR amyloidosis and a TTR mutation have undergone 99mTc-DPD scintigraphy and could be studied. In three patients, reliable typing of the ATTR fibrils was not possible due to insufficient amount of amyloid in the biopsy tissue (Table I); these patients were excluded from the analysis.
Table I.
Patient characteristics.
| V30M | Non-V30M | |
|---|---|---|
| Sex male/female, n | 34/14 | 2/5 |
| Type of amyloid fibril | ||
| Type A fibrils, n | 25 | 6 |
| Type B fibrils, n | 21 | 0 |
| Not typed, n | 2 | 1 |
| Age at onset median (range), years | 63 (26–82) | 60 (38–69) |
| Age at examination median (range), years | 68 (30–83) | 65 (41–72) |
| Previous liver transplant, n | 12 | 1 |
| PND score, na | ||
| 0 | 10 | 3 |
| I | 20 | 0 |
| II | 10 | 0 |
| IIIA | 2 | 0 |
| IIIB | 6 | 3 |
| IV | 0 | 0 |
| Not applicableb | 0 | 1 |
| Primary symptoms, n | ||
| Cardiac | 4 | 4 |
| Neuropathy | 40 | 1 |
| Other | 4 | 2 |
| Total | 48 | 7 |
Type A amyloid fibrils are composed of full-length and truncated transthyretin, and type B of full-length transthyretin only.
Polyneuropathy disability (PND) score: 0 = no sensory disturbances; I = sensory disturbances in the feet, but no impaired walking capability; II = walking impairment, but walking without aid; IIIA = walking with one stick or crutch; IIIB = walking with two sticks or crutches; IV = in wheelchair or immobilized.
Patient with post-polio syndrome that causes neurological impairment.
All patients had positive genetic testing for TTR mutation. The V30M mutation (n = 48) dominated, but seven other non-V30M mutations were found, each represented by one individual: Glu54Leu, Ala45Ser, His88Arg, Ala97Ser, Tyr60Ala, Ala45Gly, and Val122Ile.
Patient characteristics
Clinical records were reviewed for information on medications, disease onset (defined as onset of symptoms related to amyloid disease reported by the patients), and primary symptoms (defined as the symptoms leading to health care contact) (Table I). The most common symptom, polyneuropathy, was graded according to severity using the polyneuropathy disability (PND) score, where 0 signifies no symptoms of polyneuropathy, and IV signifies a patient bedridden or confined to a wheelchair (28). Information concerning other causes of left ventricular hypertrophy, such as hypertension, valvular disease, and chronic kidney disease, was also retrieved from the records. Hypertension was defined by previous or current antihypertensive treatment or two separate measurements of systolic blood pressure exceeding 140 mmHg. Since many of the patients also had polyneuropathy, reliable assessment of New York Heart Association (NYHA) class was not possible.
Histopathological diagnosis and typing of the amyloid fibrils
In all patients except one, diagnostic biopsies were obtained from subcutaneous abdominal fat, and in the remaining patient the amount of amyloid in the cardiac biopsy was insufficient for ATTR fibril typing. Three patients had proven amyloid deposits both in abdominal fat and in endomyocardial biopsies. No patient had a positive abdominal fat biopsy and a negative heart biopsy.
For the histopathological diagnosis and typing of the fibrils, abdominal adipose tissue biopsies were used, and they were prepared and examined as previously described (29). To detect full-length TTR as well as C-terminal TTR fragments, Western blot utilizing a polyclonal antiserum produced in rabbit against TTR50-127 was used (9,30,31).
99mTc-DPD scintigraphy
All patients were scanned using a hybrid single-photon emission computed tomography (SPECT)-CT gamma camera (General Electric Medical Systems, Milwaukee, WI, USA, Infinia Hawkeye) with a low-energy high-resolution (LEHR) collimator after intravenous injection of 740 MBq of 99mTc-DPD. Whole-body planar images were acquired 5 min and 3 h post-injection in a 256 × 1024 matrix followed by a cardiac SPECT-CT with a low-dose, non-contrast CT scan. SPECT acquisition was performed with a 128 × 128 matrix size in 30 projections followed by an iterative (OSEM, 3 iterations, 10 subsets) reconstruction with CT-based attenuation correction. SPECT-CT reconstruction and image fusion were performed on the Celeries (GE Healthcare, Waukesha, WI, USA) workstation. The CT volume data were reconstructed into 5 mm slice thickness.
Visual scoring of cardiac retention was carried out according to a suggested method (i.e. score 0, absent cardiac uptake and normal bone uptake; score 1, mild cardiac uptake, inferior to bone uptake; score 2, moderate cardiac uptake accompanied by attenuated bone uptake; score 3, strong cardiac uptake with mild/absent bone uptake) (23,32). A score of 1, 2, or 3 was considered as 99mTc-DPD-positive. Two experienced specialists in nuclear medicine independently performed the image analyses. There were no disagreements.
Echocardiography
Patients were investigated with two-dimensional, M-mode, and tissue Doppler echocardiography (Vivid 9, GE Medical Systems, Horten, Norway) with a phased-array transducer (1.5–4.0 MHz). The examinations were performed by one experienced examiner (P.L.), and measurements were made in accordance with the standards of the European Society of Cardiovascular Imaging (33). One experienced operator (B.P.) performed all measurements. When measuring end-diastolic interventricular septum thickness (IVSD), left ventricular (LV) end-diastolic diameter, and LV posterior wall thickness, attention was paid to exclude the trabecular and chordal structures from both the left and right side cavity by using the leading edge methodology. LV mass was calculated according to Devereux et al. (34). Left atrial end-systolic diameter was measured from parasternal long axis projection. E/é was used to estimate LV filling pressures and calculated using ratios of early diastolic blood flow velocity (E) to early myocardial diastolic (é) velocities, the latter measured in the basal lateral left ventricular free wall (35). Left ventricular ejection fraction (LVEF) was calculated using the biplane method of discs (modified Simpson’s rule) (33).
Cardiac biomarkers
The cardiac biomarkers troponin T and NT-proBNP were analysed at the clinical chemistry laboratory of Umeå University Hospital according to routine practice using a Cobas 8000 machine and troponin T hs STAT and proBNP II STAT reagents (Roche Scandinavia, Bromma, Sweden).
Electrocardiogram
ECGs were interpreted according to the Minnesota code and then categorized as normal or pathological in accordance with criteria known to be associated with amyloid cardiomyopathy (3).
Atrial abnormalities found and categorized as pathological were atrial fibrillation, atrial flutter, and atrial pacing. AV-block 1 or higher, left bundle branch block, right bundle branch block, left anterior hemiblock, extreme left axis deviation, and ventricular pacing were considered to be pathological conduction disorders. STT abnormalities, abnormal R- progression, or Q-waves were other findings categorized as pathological.
Cardiovascular magnetic resonance
In addition, 13 patients underwent cardiovascular magnetic resonance (CMR) imaging at the same time as 99mTc-DPD scintigraphy. Two of them were not included in the analysis, since their fibril type could not be determined. The investigations were performed on a Philips 1.5 T Intera or Achieva scanner (Philips, Best, The Netherlands). Cine (steady-state free-precession; SSFP) images included a stack of short-axis images covering the heart from apex to base. Ventricular volumes and mass were calculated according to standard methods, and data were compared with previously published reference values (36). For late gadolinium enhancement (LGE), phase-sensitive inversion recovery sequences were applied 10–20 min after injection of gadolinium (gadopentetate dimeglumine) covering the ventricles in three orthogonal planes.
Analysis
The patients were divided into groups according to fibril type and IVSD. If any amount of truncated transthyretin was detected on the Western blot, the fibril type was categorized as type A. If only full-length fibrils were present it was categorized as type B. The groups were compared with regard to results of the 99mTc-DPD scintigraphy, CMR, biochemical, electrocardiographic, and echocardiographic findings. The impact of sex and previous liver transplantation was also analysed. Additionally, according to IVSD, the patients were divided into three groups, one with no cardiomyopathy (IVSD <13 mm), one with suspected cardiomyopathy (IVSD 13–14 mm), and one with cardiomyopathy (IVSD >14 mm), and results from 99mTc-DPD scintigraphy were compared between these groups.
Statistics
Statistical analysis was performed using IBM® SPSS® Statistics version 22. Values were expressed as median ranges (min–max). Non-parametric tests were utilized: Mann–Whitney U for analysis of differences in numerical data between groups and Fisher’s exact test for analysis of distribution of categorical data. For correlations Spearman’s rank correlation coefficient was used. Values of p < 0.05 were considered statistically significant.
Ethics
The study was conducted according to the Helsinki declaration, and all patients had given written consent to participate in the study. The central ethical review board at Umeå University has approved all study procedures.
Results
Patient characteristics
A majority of the ATTR V30M patients were males, whereas for non-ATTR V30M the majority were females (Table I). Neuropathy was the most common symptom that brought the patients to the attention of the health care system, but, interestingly, four of the ATTR V30M patients (8%) sought medical attention for heart-related symptoms.
There were no statistically significant differences between numbers of males and females with type A amyloid fibrils (54% versus 68% respectively [p = 0.30]) or regarding IVSD thickness between the genders (14 [8–25] versus 13 [9–23] mm respectively; p = 0.5). When analysing differences between transplanted and non-transplanted patients, transplanted patients were younger (59 [39–72] versus 69 [40–83] years; p = 0.03) and had a longer disease duration (7 [3–22] versus 2 [0–11] years; p < 0.01), but the IVSD thickness was not different (12 [8–22] versus 14 [8–25] mm; p = 0.4).
Amyloid fibril composition and uptake of 99mTc-DPD
None of the patients with type B fibrils displayed uptake of 99mTc-DPD at scintigraphy (Table II and Figure 1). All patients with type A fibrils except one showed cardiac 99mTc-DPD uptake (p < 0.0001). Patients with type A fibrils were older, had significantly thicker heart muscle, lower LVEF, and higher LV mass (echo and CMR), E/é, troponin T, and NT-proBNP than those with type B fibrils; and fewer of them had normal ECG. Pacemaker treatment was equally common in type A and B fibril groups, as was the prevalence of hypertension. It is worth noting that all patients with hypertension were on adequate treatment. In the group of patients with type A fibrils, nearly 20% sought medical attention because of heart symptoms compared with 5% of those with type B.
Table II.
Clinical findings and outcome of DPD scintigraphy.
| Type A (n = 31) | Type B (n = 21) | p value | |
|---|---|---|---|
| Age at examination median (range), years | 71 (41–83) | 57 (30–75) | <0.01 |
| Sex male/female, n | 18/13 | 15/6 | 0.15 |
| V30M, n | 26 (83.9%) | 21 (100%) | |
| 99mTc-DPD-positive, n | 30 (96.8%) | 0 (0%) | <0.01 |
| Grade 0, n | 1 | 21 | |
| Grade 1, n | 0 | 0 | |
| Grade 2, n | 11 | 0 | |
| Grade 3, n | 19 | 0 | |
| Positive cardiac biopsy, n (total performed) | 2 (2) | 1 (1) | |
| Previous liver transplant, n | 5 (16.1%) | 8 (38.1%) | <0.01 |
| NT-proBNP median (range), ng/La | 1240 (37–9829) | 67 (10–711) | <0.01 |
| Hs-troponin T median (range), ng/La | 33 (10–95) | 10 (7–31) | <0.01 |
| CMR increased LV mass, n/total | 6/6 | 0/5 | <0.01 |
| CMR LGE, n/total | 3/6 | 1/5 | 0.35 |
| IVSD median (range), mm | 17 (12–25) | 11 (8–15) | <0.01 |
| IVSD >12 mm, n (%) | 29 (82.9%) | 6 (17.1%) | <0.01 |
| Left atrial diameter (range), mm | 42 (24–51) | 36 (27–52) | 0.02 |
| LV mass (range), g | 307 (136–552) | 175 (117–444) | <0.01 |
| LVEF (range), % | 50 (30–70) | 58 (51–68) | 0.01 |
| E/é median (range) | 14.0 (5.8–24.7) | 7.0 (5.1–16.3) | <0.01 |
| Normal ECG, n (%) | 5 (16.1%) | 13 (61.9%) | <0.01 |
| Pacemaker, n (%)b | 5 (16.1%) | 3 (14.3%) | 1.00 |
| Age of onset median (range), years | 65 (38-82) | 49 (26-75) | <0.01 |
| Primary symptomsc | 0.19 | ||
| Neuropathy, n (%) | 22 (71.0%) | 17 (81%) | |
| Cardiomyopathy, n (%) | 6 (19.4%) | 1 (4.8%) | |
| Other, n (%) | 3 (9.7%) | 3 (14.3%) | |
| Hypertension, n (%) | 16 (65.2%) | 6 (21.6%) | 0.15 |
Type A amyloid fibrils are composed of full-length and truncated transthyretin, type B of full-length transthyretin only.
Reference values: Left atrial diameter, 27–40 mm; Hs-troponin T, <14.51 ng/L; NT-proBNP, <150 ng/L if age <75 years, <450 ng/L if age >75 years.
Two patients were excluded from analysis due to advanced kidney disease.
Pacemaker: presence of cardiac pacemaker at the time of 99mTc-DPD scintigraphy.
Primary symptoms: patient reported symptoms leading up to diagnosis. Other symptoms included gastrointestinal tract disturbances, renal failure, and cranial nerve affection.
CMR = cardiovascular magnetic resonance; E/é = mitral E-wave velocity/lateral wall é-wave velocity; IVSD = end-diastolic interventricular septum thickness (normal reference 6–10 mm); LGE = late gadolinium enhancement; LV mass = left ventricular mass; LVEF = left ventricular ejection fraction.
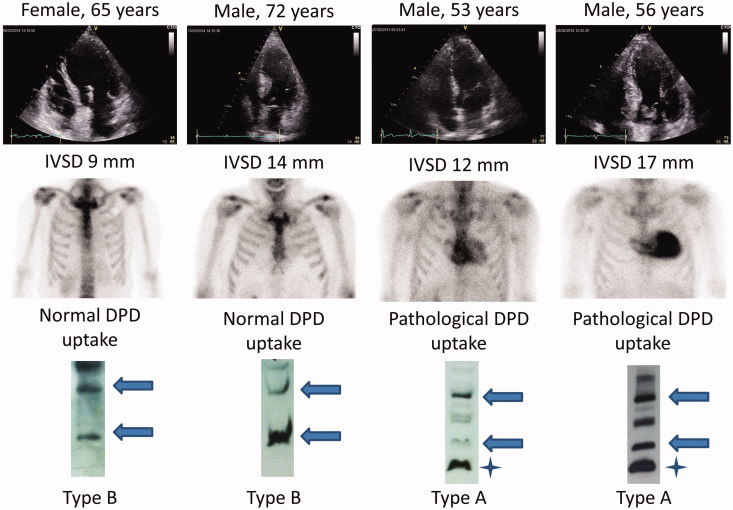
Figure 1.
Images from echocardiography (top), DPD scintigraphy (middle), and the Western blot analyses (bottom) from four patients. The arrows point at the monomeric (lower arrow) and dimeric (upper arrow) full-length TTR, while the stars mark fragmented TTR. The band between the arrows in type A amyloid has not been characterized but may be a TTR fragment dimer.
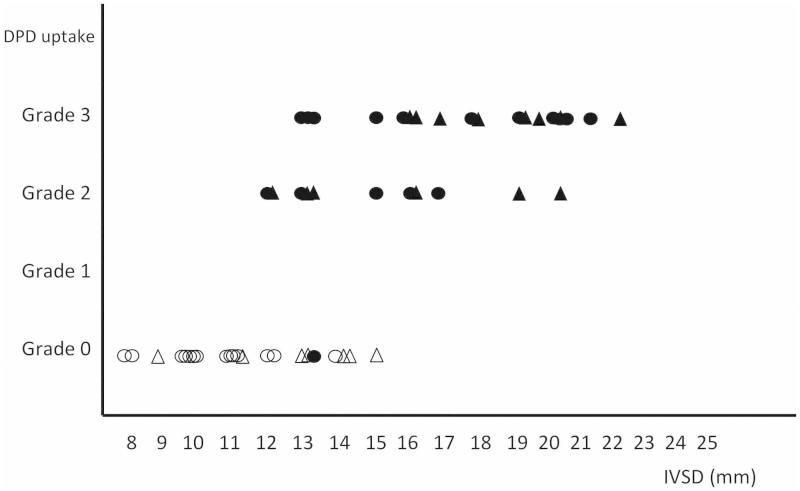
When grouping the patients into three groups according to their IVSD, there was a significant difference between type A and B fibrils and the outcome of the 99mTc-DPD scintigraphy for all groups (Table III). In addition, there was a correlation between grade of cardiac hypertrophy and 99mTc-DPD uptake (p < 0.01) (Figure 2).
Table III.
99mTc-DPD uptake and amyloid fibril types according to diastolic interventricular septum thickness (IVSD).
| Positive DPD-scintigraphy | Negative scintigraphy | Fisher exact probability test: TP | |
|---|---|---|---|
| IVSD <13 mm | <0.01 | ||
| Type A fibrils | 2 | 0 | |
| Type B fibrils | 0 | 15 | |
| IVSD ≥13 and <15 mm | 0.02 | ||
| Type A fibrils | 6 | 1 | |
| Type B fibrils | 0 | 5 | |
| IVSD ≥15 mm | 0.04 | ||
| Type A fibrils | 22 | 0 | |
| Type B fibrils | 0 | 1 |
Type A amyloid fibrils are composed of full-length and truncated transthyretin, type B of full-length transthyretin only.
Figure 2.
Relationship between interventricular thickness (IVSD), 99mTc-DPD uptake, hypertension, and fibril type. Filled figures indicate patients with type A fibrils, and empty figures patients with type B fibrils. Triangles indicate patients with hypertension, and circles patients without. Type A amyloid fibrils are composed of full-length and truncated transthyretin, type B of full-length transthyretin only. The uptake of 99mTc-DPD is strongly correlated to IVSD (Spearman RS = 0.76; p < 0.01). DPD uptake grade = uptake of 99mTc-DPD graded according to Puille et al (22).
The patient with type A fibrils and negative scintigraphy had a minimal amount of truncated fibrils in the biopsy material and minor ventricular hypertrophy (IVSD 13 mm); however, she had also suffered from a long-standing but well-treated hypertension. Two patients with fibril type A and no signs of amyloid cardiomyopathy on echocardiography and ECG examinations (IVSD <12 mm and normal ECG) displayed uptake on 99mTc-DPD scintigraphy. Both patients were without cardiac symptoms and had disease duration of only 2 years.
In the group with type B fibrils, six patients (29%) had increased IVSD (>12 mm), but none had cardiac 99mTc-DPD uptake. However, three of these patients had other diseases known potentially to increase left ventricular thickness: one had renal failure and a history of hypertension, and two had well-treated hypertension only. The patient with kidney disease had, as expected, very high NT-proBNP value. In the remaining three patients with abnormal septal thickness (IVSD 13 mm in two and 14 mm in one) the NT-proBNP values were normal, but two of them had elevated serum troponin T concentrations (17 and 24 ng/L), and one had focal gadolinium uptake on CMR. No other causes than amyloid cardiomyopathy could be identified in these three patients. In addition, one patient had increased thickness of the apical septum, elevated filling pressures on heart catheterization, and a positive endomyocardial biopsy, but negative 99mTc-DPD scintigraphy.
All patients with non-V30M TTR mutations had type A amyloid fibrils and displayed a positive 99mTc-DPD uptake.
Discussion
The main finding of this investigation was the strong association between 99mTc-DPD uptake and type A amyloid fibrils, i.e. fibrils with C-terminally truncated ATTR. The finding of two patients with positive scintigraphy in the group of patients with normal heart dimensions, both of whom had type A fibrils, and, conversely, the absence of uptake in all patients with type B fibrils, strongly suggests a direct relationship between fibril type and 99mTc-DPD uptake.
In the present study only one EMB was performed on a patient with type B fibrils, and even though it was positive, it may be argued that other patients’ hearts did not contain amyloid. However, in a study of six deceased Swedish ATTR V30M patients’ hearts, all contained amyloid, even that from a patient with an early onset of disease (at the age of 32) and without an enlarged heart (37). Furthermore, in another post-mortem examination of hearts from Swedish wild-type and ATTR V30M patients, amyloid consisting of either type A or type B fibrils was found in all hearts (9). However, the distribution of the two types within the heart differed. Type A fibrils had a homogeneous but patchy distribution within the cardiac tissue and were composed of tightly packed, short, randomly orientated fibrils. In type B, the amyloid appeared as thin streaks throughout the cardiac tissue; often surrounding individual muscle cells as long fibrils arranged in parallel bundles, often penetrating into the myocytes (9).
Even though only a small part of our study cohort underwent CMR at the time of evaluation, the findings are similar to those of a larger study, where late gadolinium enhancement (LGE) was seen mainly on late-onset patients, but were absent in the group with early-onset, neuropathic, V30M ATTR amyloidosis, i.e. patients that probably had amyloid type B fibrils (38).
These previous studies clearly show that the heart is a target organ for ATTR deposits regardless of fibril type. However, the implication for the patient depends on fibril type, with more pronounced development of heart failure symptoms and cardiomyopathy in type A ATTR patients (8,39). 99mTc-DPD scintigraphy remains an excellent tool to identify patients with ATTR cardiomyopathy as well as those carrying a high risk of developing cardiomyopathy, an important finding in patients who are considered candidates for liver transplantation (38). With the emergence and testing of novel pharmacological treatments, classifying patients with regard to amyloid formation pathways might be essential.
The obvious limitation of this study is the lack of cardiac biopsies in patients with negative 99mTc-DPD scintigraphies. The study was performed on patients undergoing our routine programme for patients with hereditary ATTR amyloidosis, and heart biopsies are not part of the evaluation if it does not have an impact on the clinical management and treatment of the patients. Furthermore, the amount of amyloid material received from EMB is in our experience often insufficient for reliable Western blot analysis.
Another limitation is the small number of CMR performed. However, MR examination is not included in our clinical programme, basically because of limited access to a laboratory. High prevalence of pacemaker carriers and the use of 99mTc-DPD scintigraphy as a preferred method for identifying amyloid cardiomyopathy are other reasons.
The outcome of Western blot analysis is dependent on the amount of amyloid in the tissue. Low amyloid content makes the examination difficult to interpret, since the bands corresponding to full-length and truncated TTR will be weak. In the present investigation, three biopsy samples without clear bands (close to 5% of all biopsies) could not be analysed regarding fibril type and were therefore not included in the statistical analysis. We cannot exclude the possibility that some samples with very small amounts of truncated ATTR fibrils were mistakenly assigned to the type B group. However, the patient denoted as carrying type A ATTR fibrils and whose 99mTc-DPD scintigraphy was negative displayed a weak band corresponding to truncated TTR, which emphasizes that it is the truncated TTR in the heart that determines the outcome of 99mTc-DPD scintigraphy.
In summary, the excellent diagnostic accuracy of 99mTc-DPD scintigraphy appears to be related to the precursor protein in ATTR amyloidosis. The presence of C-terminal fragments found predominantly in ATTR V30M amyloidosis patients with late onset and in non-V30M patients is significantly related to uptake of the tracer and outcome of the examination.
Acknowledgments
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by FAMY, FAMY Norrbotten and Amyl Foundation, Swedish Heart and Lung Foundation (P.L., O.B.S.), Västerbotten County Council, and Selander’s Foundation.
References
- 1.Benson MD, Kincaid JC.. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve 2007;36:411–23. [DOI] [PubMed] [Google Scholar]
- 2.Rowczenio DM, Noor I, Gillmore JD, Lachmann HJ, Whelan C, Hawkins PN, et al. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat 2014;35:E2403–12. [DOI] [PubMed] [Google Scholar]
- 3.Rapezzi C, Quarta CC, Obici L, Perfetto F, Longhi S, Salvi F, et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J 2013;34:520–8. [DOI] [PubMed] [Google Scholar]
- 4.Coelho T, Maurer MS, Suhr OB.. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin 2013;29:63–76. [DOI] [PubMed] [Google Scholar]
- 5.Merlini G, Westermark P.. The systemic amyloidoses: clearer understanding of the molecular mechanisms offers hope for more effective therapies. J Intern Med 2004;255:159–78. [DOI] [PubMed] [Google Scholar]
- 6.Mangione PP, Porcari R, Gillmore JD, Pucci P, Monti M, Porcari M, et al. Proteolytic cleavage of Ser52Pro variant transthyretin triggers its amyloid fibrillogenesis. Proc Natl Acad Sci U S A 2014;111:1539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergstrom J, Murphy CL, Weiss DT, Solomon A, Sletten K, Hellman U, et al. Two different types of amyloid deposits–apolipoprotein A-IV and transthyretin–in a patient with systemic amyloidosis. Lab Invest 2004;84:981–8. [DOI] [PubMed] [Google Scholar]
- 8.Ihse E, Ybo A, Suhr O, Lindqvist P, Backman C, Westermark P.. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol 2008;216:253–61. [DOI] [PubMed] [Google Scholar]
- 9.Bergstrom J, Gustavsson A, Hellman U, Sletten K, Murphy CL, Weiss DT, et al. Amyloid deposits in transthyretin-derived amyloidosis: cleaved transthyretin is associated with distinct amyloid morphology. J Pathol 2005;206:224–32. [DOI] [PubMed] [Google Scholar]
- 10.Suhr OB, Lindqvist P, Olofsson BO, Waldenstrom A, Backman C.. Myocardial hypertrophy and function are related to age at onset in familial amyloidotic polyneuropathy. Amyloid 2006;13:154–9. [DOI] [PubMed] [Google Scholar]
- 11.Sobue G, Koike H, Misu K, Hattori N, Yamamoto M, Ikeda S, et al. Clinicopathologic and genetic features of early- and late-onset FAP type I (FAP ATTR Val30Met) in Japan. Amyloid 2003;10(Suppl 1):32–8. [PubMed] [Google Scholar]
- 12.Ihse E, Rapezzi C, Merlini G, Benson MD, Ando Y, Suhr OB, et al. Amyloid fibrils containing fragmented ATTR may be the standard fibril composition in ATTR amyloidosis. Amyloid. 2013;20:142–50. [DOI] [PubMed] [Google Scholar]
- 13.Ihse E, Suhr OB, Hellman U, Westermark P.. Variation in amount of wild-type transthyretin in different fibril and tissue types in ATTR amyloidosis. J Mol Med 2011;89:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcoux J, Mangione PP, Porcari R, Degiacomi MT, Verona G, Taylor GW, et al. A novel mechano-enzymatic cleavage mechanism underlies transthyretin amyloidogenesis. EMBO Mol Med 2015;7:1337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stangou AJ, Hawkins PN, Heaton ND, Rela M, Monaghan M, Nihoyannopoulos P, et al. Progressive cardiac amyloidosis following liver transplantation for familial amyloid polyneuropathy: implications for amyloid fibrillogenesis. Transplantation 1998;66:229–33. [DOI] [PubMed] [Google Scholar]
- 16.Ericzon B-G, Wilczek HE, Larsson M, Wijayatunga P, Stangou A, Pena JR, et al. Liver transplantation for hereditary transthyretin amyloidosis: after 20 years still the best therapeutic alternative? Transplantation 2015;99:1847–54. [DOI] [PubMed] [Google Scholar]
- 17.Falk RH, Dubrey SW.. Amyloid heart disease. Progr Cardiovasc Dis 2010;52:347–61. [DOI] [PubMed] [Google Scholar]
- 18.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270–6. [DOI] [PubMed] [Google Scholar]
- 19.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol 2005;79:319–28. [DOI] [PubMed] [Google Scholar]
- 20.Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M.. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol 2005;95:535–7. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson P, Karp K, Bjerle P, Olofsson BO.. Disturbances of cardiac rhythm and conduction in familial amyloidosis with polyneuropathy. Brit Heart J 1984;51:658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puille M, Altland K, Linke RP, Steen-Muller MK, Kiett R, Steiner D, et al. 99mTc-DPD scintigraphy in transthyretin-related familial amyloidotic polyneuropathy. Eur J Nucl Med Mol Imag 2002;29:376–9. [DOI] [PubMed] [Google Scholar]
- 23.Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005;46:1076–84. [DOI] [PubMed] [Google Scholar]
- 24.Glaudemans AW, van Rheenen RW, van den Berg MP, Noordzij W, Koole M, Blokzijl H, et al. Bone scintigraphy with (99m)technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid 2014;21:35–44. [DOI] [PubMed] [Google Scholar]
- 25.Longhi S, Bonfiglioli R, Obici L, Gagliardi C, Milandri A, Lorenzini M, et al. Etiology of amyloidosis determines myocardial 99mTc-DPD uptake in amyloidotic cardiomyopathy. Clin Nucl Med 2015;40:446–7. [DOI] [PubMed] [Google Scholar]
- 26.Rapezzi C, Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imag 2011;38:470–8. [DOI] [PubMed] [Google Scholar]
- 27.Pepys MB, Dyck RF, de Beer FC, Skinner M, Cohen AS.. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol 1979;38:284–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Suhr O, Danielsson A, Holmgren G, Steen L.. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med 1994;235:479–85. [DOI] [PubMed] [Google Scholar]
- 29.Westermark P, Davey E, Lindbom K, Enqvist S.. Subcutaneous fat tissue for diagnosis and studies of systemic amyloidosis. Acta Histochem 2006;108:209–13. [DOI] [PubMed] [Google Scholar]
- 30.Westermark P, Sletten K, Olofsson BO.. Prealbumin variants in the amyloid fibrils of Swedish familial amyloidotic polyneuropathy. Clin Exp Immunol 1987;69:695–701. [PMC free article] [PubMed] [Google Scholar]
- 31.Westermark P, Westermark GT, Suhr OB, Berg S.. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci 2014;119:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imag 2011;4:659–70. [DOI] [PubMed] [Google Scholar]
- 33.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 34.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- 35.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93. [DOI] [PubMed] [Google Scholar]
- 36.Maceira AM, Prasad SK, Khan M, Pennell DJ.. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417–26. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson A, Eriksson P, Olofsson BO, Thornell LE.. The cardiac atrioventricular conduction system in familial amyloidosis with polyneuropathy. A clinico-pathologic study of six cases from Northern Sweden. Acta Pathol Microbiol Immunol Scand A 1983;91:343–9. [DOI] [PubMed] [Google Scholar]
- 38.Deux JF, Damy T, Rahmouni A, Mayer J, Plante-Bordeneuve V.. Noninvasive detection of cardiac involvement in patients with hereditary transthyretin associated amyloidosis using cardiac magnetic resonance imaging: a prospective study. Amyloid 2014;21:246–55. [DOI] [PubMed] [Google Scholar]
- 39.Gustafsson S, Ihse E, Henein MY, Westermark P, Lindqvist P, Suhr OB.. Amyloid fibril composition as a predictor of development of cardiomyopathy after liver transplantation for hereditary transthyretin amyloidosis. Transplantation 2012;93:1017–23. [DOI] [PubMed] [Google Scholar]