Abstract
Protease-activated receptor-1 (PAR-1) has a significant role in the pathogenesis of various malignancies and its expression mainly affects the survivals of cancer patients. The aim of the present study was to determine the clinical significance of the serum concentrations of PAR-1 in patients with gastric carcinoma. A total of 63 pathologically confirmed gastric cancer patients were enrolled in this study, with a median age of 62 years. Serum PAR-1 concentrations were determined by the enzyme-linked immunosorbent assay method and no significant difference in the baseline serum PAR-1 concentrations was found between patients and normal controls (P=0.5). The investigated clinical variables, including patient age, gender, localization of lesion, histology, grade of pathology, disease stage and serum tumor markers (lactate dehydrogenase, carcinoembryonic antigen and carbohydrate antigen 19-9) were not correlated with serum PAR-1 levels (P>0.05). Furthermore, no association was identified between the serum PAR-1 level and chemotherapy responsiveness (P=0.43). Serum PAR-1 level also had no prognostic role for survival (P=0.27). In conclusion, the serum PAR-1 concentration has no diagnostic, predictive and prognostic values in gastric cancer patients.
Keywords: serum, protease-activated receptor-1, gastric cancer
Introduction
Protease-activated receptor-1 (PAR-1), the prototypic member of the PAR family, is activated by thrombin following cleavage of its extracellular amino terminus domain (1–7). PAR-1 and its activating factors, which are expressed on tumor cells and their stroma, induce coagulation and have a significant role in promoting tumor progression in several carcinomas such as breast, pancreas, laryngeal and gastric cancer (1,7).
Gastric cancer constitutes multifactorial etiology and its genetic and immunological background remains to be elucidated. Cultured gastric cancer cell lines produce extreme concentrations of cytokines and growth factors with pleiotropic biological activities in in vitro trials. Among them, PAR-1 functions as an autocrine and paracrine factor that induces a number of cellular functions, including tumor growth, angiogenesis, invasion and metastasis (1–4).
Expression and secretion of the PAR-1 isoform in gastric cancer cells has been determined by previous studies (1–4). Increased PAR-1 expression levels were associated with increased gastric cancer cell proliferation and metastatic potential (1–4). Currently however, the molecular function of PAR-1 and the possible clinical significance of PAR-1 in gastric cancer patients remain to be elucidated.
Although all the available data have been provided from preclinical trials, thus far there is no clinical study to investigate the clinical value of the PAR-1 isoform in serum or plasma in patients with gastric cancer. Therefore, the significance of the circulating PAR-1 levels in gastric cancer patients remains to be elucidated. Thus, the serum levels of PAR-1 in gastric cancer patients was investigated, and its association with the prognosis, various clinical variables and response to chemotherapy was assessed, so as to confirm whether this biomarker may be useful for the diagnosis and in the assessment of the prognosis, and for use in the treatment of gastric cancer.
Materials and methods
Patients and therapy
The present study included 63 consecutive patients with histologically confirmed gastric cancer who were admitted to the Institute of Oncology (Istanbul University, Istanbul, Turkey). All the patients had not received any type of therapy, chemotherapy or chemoradiation, during the last 6 months. The staging was according to the American Joint Committee on Cancer and Union for International Cancer Control staging systems (8). Detailed patient history, physical examination and blood tests including complete blood count and biochemistry analyses were performed for each patient. Patients with Eastern Cooperative Oncology Group performance status ≤2, and suitable blood test results received chemotherapy included different combinations of various chemotherapeutic agents, such as fluorouracil, folinic acid, epirubicine, cisplatin, capecitabine and docetaxel. Chemotherapy responsiveness was determined by the revised Response Evaluation Criteria in Solid Tumors criteria version 1.1 (9).
A total of 30 healthy control subjects were included in the analysis. Informed consent was provided from all the patients. The ethics committee of the Institute of Oncology reviewed and approved the study.
Measurement of serum PAR-1 levels
Blood serum samples of patients were provided on first admission by venipuncture prior to chemotherapy or follow-up, and were clotted at room temperature. The sera were collected following centrifugation and were frozen at −20°C until analysis.
The human PAR-1 enzyme-linked immunosorbent assay (ELISA) (Wuhan EIAab Science Co., Ltd., Wuhan, China) used a double-antibody sandwich ELISA to determine the level of human PAR-1 in the samples.
Measurement of lactate dehydrogenase (LDH), carcinoembryonic antigen (CAE) and carbohydrate antigen 19-9 (CA 19-9)
The serum CEA and CA 19-9 levels were determined by the microparticle enzyme immunoassay (Abbott Diagnostics, Chicago, IL, USA). Serum LDH activity was determined immediately following collection by the kinetic method on a Targa-3000 autoanalyzer (Pointe Scientific Inc., Lincoln Park, MI, USA).
Statistical analysis
Parameters were classified as median values and as cut-off points. Comparisons between clinical or laboratory parameters and serum PAR-1 assay levels were performed using Mann-Whitney U test. Survival estimations of patients were determined by Kaplan-Meier method and differences of survivals were performed by the log-rank statistics. P≤0.05 was considered to indicate a statistically significant difference. The SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses.
Results
Patient characteristics
In total, 63 patients with gastric cancer were enrolled in the study. The demographic and histopathological features of the patients are shown in Table I. The median age of patients was 62 years (range, 28–82 years).
Table I.
Characteristics of patients and disease status.
| Parameter | Patients, n (%) |
|---|---|
| Total patients | 63 (100) |
| Age, years | |
| ≥60 | 35 (56) |
| <60 | 28 (44) |
| Gender | |
| Male | 25 (40) |
| Female | 38 (60) |
| Localization of tumor | |
| Cardia | 21 (33) |
| Antrum | 27 (43) |
| Undetermined | 15 (24) |
| Histology | |
| Adenocarcinoma | 42 (67) |
| Signet-ring cell | 21 (33) |
| Grade | |
| I–II | 10 (16) |
| III | 44 (70) |
| Undetermined | 9 (14) |
| Tumor (T) stage | |
| 1–3 | 14 (22) |
| 4 | 22 (35) |
| Unknown | 27 (43) |
| No. of involved lymph node | |
| 0–2 | 12 (19) |
| ≥3 | 13 (21) |
| Unknown | 38 (60) |
| Disease stage | |
| Non-metastatic | 32 (51) |
| Metastatic | 31 (49) |
| Liver metastasisa | |
| Yes | 14 (45) |
| No | 17 (55) |
| Curative surgeryb | |
| Yes | 17 (53) |
| No | 9 (28) |
| Unknown | 6 (19) |
| Hemoglobin level, g/dl | |
| Low, <12 | 35 (56) |
| Normal, ≥12 | 28 (44) |
| WBC count | |
| Normal, <10,000 | 52 (83) |
| High, ≥10,000 | 11 (17) |
| Platelet count | |
| Normal, <350,000 | 54 (86) |
| High, >350,000 | 9 (14) |
| LDH level, U/l | |
| Normal, <450 | 43 (68) |
| High, ≥450 | 10 (16) |
| Unknown | 10 (16) |
| ESR, /h | |
| High, ≥50 | 16 (25) |
| Normal, <50 | 10 (16) |
| Unknown | 37 (59) |
| CEA level, ng/ml | |
| Normal, <10 | 44 (70) |
| High, ≥10 | 13 (21) |
| Unknown | 6 (9) |
| CA 19–9 level, IU/ml | |
| Normal, <40 | 32 (51) |
| High, ≥40 | 25 (40) |
| Unknown | 6 (9) |
| Chemotherapy responsiveness | |
| Responsive | 13 (43) |
| Non-responsive | 17 (57) |
| Last status | |
| Alive | 28 (44) |
| Succumbed | 35 (56) |
In metastatic patients
in nonmetastatic patients. WBC, white blood cell; LDH, lactate dehydrogenase; ESR, erythrocyte sedimentation rate; CEA, carcinoembryonic antigen; CA 19–9, carbohydrate antigen 19–9.
Serum PAR-1 levels
No significant difference was observed in the serum PAR-1 levels between the gastric cancer patients and healthy subjects (P=0.5) (Table II). The demographic, pathological, clinical and biochemistry variables, including patient age, gender, localization of lesion, histology, pathological grade, stage of disease, and serum tumor markers including LDH, CAE and CA 19-9 were not correlated with serum PAR-1 levels (P>0.05) (Table III). Similarly, no association was identified between the serum PAR-1 level and chemotherapy responsiveness (P=0.43) (Table III).
Table II.
Serum PAR-1 levels in the gastric cancer patients and controls.
| Median PAR-1 (range), ng/ml | |||
|---|---|---|---|
| Assay | Patients, n=63 | Controls, n=30 | P-value |
| PAR-1 | 0.06 (0.01–4.05) | 0.08 (0.03–8.67) | 0.5 |
PAR-1, protease-activated receptor-1.
Table III.
Distribution and survival associations of serum PAR-1 levels on the clinical parameters in gastric cancer patients.
| Serum PAR-1 level, P-value | ||
|---|---|---|
| Parameters | Distribution | Survival |
| Age, years | 0.06 | 0.61 |
| <60/≥60 | ||
| Gender | 0.59 | 0.56 |
| Male/female | ||
| Localization of tumor | 0.10 | 0.04 |
| Cardia/antrum | ||
| Histology | 0.27 | 0.22 |
| Adenocarcinoma/signet ring | ||
| Grade | 0.41 | 0.10 |
| I–II/III | ||
| Tumor (T) stage | 0.12 | 0.06 |
| 1–3/4 | ||
| No. of involved lymph node | 0.57 | 0.21 |
| 0–2/≥3 | ||
| Curative surgery | 0.46 | 0.36 |
| Yes/no | ||
| Metastasis | 0.99 | 0.03 |
| Yes/no | ||
| Liver metastasis | 0.38 | 0.11 |
| Yes/no | ||
| Hemoglobin level | 0.23 | 0.34 |
| Low/normal | ||
| WBC count | 0.06 | 0.30 |
| High/normal | ||
| Platelet count | 0.68 | 0.51 |
| High/normal | ||
| ESR | 0.97 | 0.02 |
| High/normal | ||
| LDH level | 0.70 | 0.11 |
| High/normal | ||
| CEA level | 0.66 | 0.01 |
| High/normal | ||
| CA 19–9 level | 0.92 | 0.04 |
| High/normal | ||
| Chemotherapy responsiveness | 0.43 | 0.05 |
| Yes/no | ||
| Serum PAR-1 level | – | 0.27 |
| Median, < or ≥ | ||
PAR-1, protease-activated receptor-1; WBC, white blood cell; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; CA 19–9, carbohydrate antigen 19–9.
The median survival time for patients was 42.0 weeks. The 1-year survival rate was 42.2%. The presence of distant metastasis (P=0.03), antrum site (P=0.04), high erythrocyte sedimentation rate (P=0.02), elevated serum CEA levels (P=0.01), high serum CA 19-9 levels (P=0.04) and chemotherapy responsiveness (P=0.05) were significantly poor outcome parameters (Table III). However, serum PAR-1 levels were not associated with survival (P=0.27) (Table III and Fig. 1).
Figure 1.
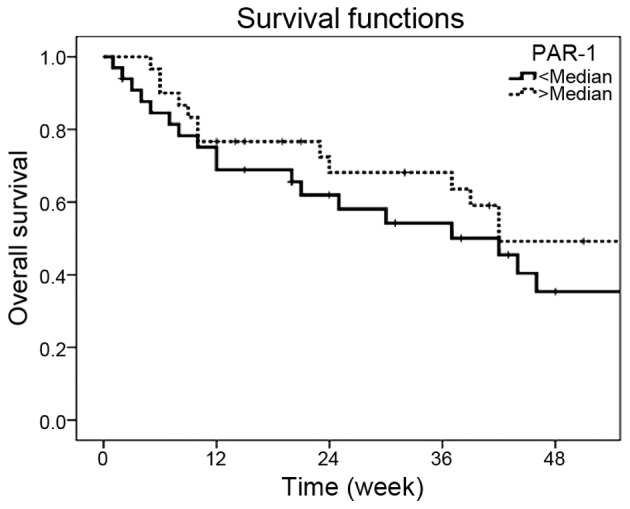
Survival curves in the gastric cancer patients according to the serum PAR-1 concentrations (P=0.27). PAR-1, protease-activated receptor-1.
Discussion
Although overexpression of PAR-1 has been determined in gastric cancer, its clinical significance has remained ambiguous in patients with gastric cancer. The possible cause of this situation is limited data; only a few trials have been performed thus far (1–4).
Although PAR-1 expression has been correlated with tumor invasion and metastasis in several types of cancer, the pioneering study presented the associations between immunohistochemical (IHC) status of PAR-1 and clinicopathological factors and outcome in gastric cancer in 2008 (1). An IHC study was performed in 129 samples of gastric cancer by the anti-PAR monoclonal antibody. Of the 129 gastric cancer specimens, tumor tissues of 58 (45%) observed positive immunoreactivity for PAR-1. The PAR-1 expression was highly intensive on the cell membranes. Significant associations between PAR-1 staining and wall invasion depth (P=0.0028) and peritoneal dissemination (P=0.041) were found. However, no correlation was identified between PAR-1 staining and histopathological stage, histological differentiation, macroscopic type, lymph node metastasis, liver metastasis or surgical curability. The patient survival analysis for gastric cancer overexpressing PAR-1 showed a higher risk of fatality compared with no overexpression (P<0.0001). Therefore, univariate and multivariate analysis identified that PAR-1 expression was an independent prognostic factor. The investigators concluded that the gastric cancer tissue produces matrix metalloproteinase-1 (MMP-1), cleaving PAR-1 to generate a new receptor N-terminus in the autocrine and paracrine manner, and activated PAR-1 causes cell invasion and metastasis. Another study also showed that the PAR-1 protein level was only significantly correlated with tumor size (2). In this study, ncRuPAR, a novel long non-coding RNA molecule that can upregulate PAR-1, inhibited the development of gastric cancer, and its possible underlying mechanism involves the PAR-1 inhibition.
To investigate how PAR-1 has a significant role in gastric cancer cells, a few studies were performed in addition to the studies aforementioned (3,4). In gastric cancer cells, activation of PAR-1 can trigger an array of responses, thus, it would support tumor cell growth and tumor invasion (3). Overexpression of nuclear factor-κB, epidermal growth factor receptor (EGFR) and tenascin-C are among the effects of PAR-1 activation, and tenascin-C promotes EGFR activation by the autocrine route. Furthermore, in another study, galectin-3, MMP-3 and PAR-1 were highly expressed and co-localized in cancerous tissues from patients with gastric cancer. Galectin-3 increased cell migration and invasion via PAR-1 upregulation (4). The investigators of these two studies concluded that PAR-1 was a potentially significant target for the gastric cancer therapy.
All these findings regarding PAR-1 were provided by preclinical trials. Thus far, PAR-1 was not studied in the sera of gastric cancer patients. The aim of the present study was to investigate the clinical significance of the serum PAR-1 levels in gastric cancer patients. The serum levels of PAR-1 were quantitatively analyzed by ELISA. The results showed that serum PAR-1 was not able to discriminate between the gastric carcinoma patients and controls, indicating that PAR-1 was not a good serological diagnostic marker in gastric cancer. Furthermore, no significant associations were identified between the levels of serum PAR-1 and the tumor features such as stage, histology, grade and serum tumor markers. Similarly, the serum level of PAR-1 was not associated with outcome. Thus, serum PAR-1 levels could not be used as a prognostic indicator to predict tumor prognosis. In addition, no link between serum PAR-1 concentrations and sensitivity to chemotherapy has raised the possibility of using PAR-1 as predictors of chemotherapy responsiveness in patients scheduled to undergo chemotherapy regimens. The serum PAR-1 concentrations may not be a potential predictor of clinical responsiveness to chemotherapy. Thus, it means that these findings are inconsistent with the aforementioned data provided from preclinical trials.
In conclusion, the serum PAR-1 levels have no diagnostic, predictive and prognostic values in gastric cancer patients. Although the small sample size and the short follow-up time are the limitations and may have influenced the results, the present study contributes significant information to the literature in that it was carried out with the serum instead of tissue, and it contained all stages of the disease. Larger scale studies in larger patient populations are required to determine the exact role of serum PAR-1 in gastric cancer patients.
References
- 1.Fujimoto D, Hirono Y, Goi T, Katayama K, Yamaguchi A. Prognostic value of protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) in gastric cancer. Anticancer Res. 2008;28(2A):847–854. [PubMed] [Google Scholar]
- 2.Liu L, Yan B, Yang Z, Zhang X, Gu Q, Yue X. ncRuPAR inhibits gastric cancer progression by down-regulating protease-activated receptor-1. Tumour Biol. 2014;35:7821–7829. doi: 10.1007/s13277-014-2042-6. [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto D, Hirono Y, Goi T, Katayama K, Matsukawa S, Yamaguchi A. The activation of proteinase-activated receptor-1 (PAR1) mediates gastric cancer cell proliferation and invasion. BMC Cancer. 2010;10:443. doi: 10.1186/1471-2407-10-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SJ, Shin JY, Lee KD, Bae YK, Choi IJ, Park SH, Chun KH. Galectin-3 facilitates cell motility in gastric cancer by up-regulating protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) PLoS One. 2011;6:e25103. doi: 10.1371/journal.pone.0025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henrikson KP, Salazar SL, Fenton JW, II, Pentecost BT. Role of thrombin receptor in breast cancer invasiveness. Br J Cancer. 1999;79:401–406. doi: 10.1038/sj.bjc.6690063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudroff C, Schafberg H, Nowak G, Weinel R, Scheele J, Kaufmann R. Characterization of functional thrombin receptors in human pancreatic tumor cells (MIA PACA-2) Pancreas. 1998;16:189–194. doi: 10.1097/00006676-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann R, Schafberg H, Rudroff C, Nowak G. Thrombin receptor activation results in calcium signaling and protein kinase C-dependent stimulation of DNA synthesis in HEp-2g laryngeal carcinoma cells. Cancer. 1997;80:2068–2074. doi: 10.1002/(SICI)1097-0142(19971201)80:11<2068::AID-CNCR5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592–5598. doi: 10.1002/cncr.25550. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]


