Abstract
The aim of the present study was to investigate the clinical application of molecular pathological diagnosis for the prognosis of Kazakh patients with esophageal squamous cell carcinoma (ESCC) using centromere enumeration probes (CEPs) for chromosomes 3 and 17. A total of 40 patients with ESCC that had received radical surgical treatment and 10 healthy control participants were enrolled in the present study. Touch preparations of fresh cancerous and normal tissues were completed and fluorescence in situ hybridization (FISH) was performed to count the copy numbers of CEP 3 and 17, and abnormalities were analyzed, in comparison with routine pathological diagnoses. FISH analysis demonstrated that abnormal copy numbers of CEP 3 and 17 (aneuploidy) were detected in all 40 patients with ESCC. CEP 3 and 17 polyploidy rates differed significantly between poorly differentiated, moderately differentiated and well-differentiated ESCC groups (P<0.05): Well-differentiated, 35.38 and 30.92%; moderately differentiated, 55.81 and 44.43%; and poorly differentiated, 76.26 and 71.90%, respectively. Furthermore, polyploidy rates were significantly increased in the group with lymph node metastasis, as compared with the group without (CEP 3, P=0.0001; CEP 17, P=0.012). Variations in the copy numbers of CEP 3 and 17 were demonstrated to be correlated with the level of differentiation and lymph node metastasis in patients with ESCC. Therefore, the present results indicate that DNA probes may be used to predict prognostic factors in patients with ESCC. Furthermore, FISH technology is an objective and qualitative method that is capable of detecting variations in CEP 3 and 17; therefore, FISH may be used in the genetic diagnosis of ESCC in Kazakh patients.
Keywords: DNA probe, fluorescence in situ hybridization, aneuploid, esophageal cancer, Kazakh patients
Introduction
Esophageal cancer (EC) is among the most common types of cancer associated with the digestive system. Due to a lack of efficient early diagnostic measurements, the majority of patients with EC are in an advanced stage when treatment is initiated, which is associated with a poor prognosis. Mortality rates of patients with EC are high (>300,000 patients per year), and the incidence of EC among Kazakh people in the Xinjiang Uyghur Autonomous Region of northwest China is 155.9/100,000, which is higher than any other ethnic group in China (1–3).
Conventional methods of pathological diagnosis provide crucial information regarding tumor differentiation and the morphological characteristics (4,5) in patients with EC. However, clinical diagnosis and decisions on treatment are difficult due to various limitations, including tissue disfigurement following extrusion, inadequate biopsy depth and discrepancies between pathological diagnosis and actual diagnosis. Therefore, a more objective and quantitative method is required to improve the accuracy of EC diagnosis (6).
Previous studies have suggested that the incidence and evolution of EC is associated with various chromosomal anomalies (7,8). During carcinogenesis, cells undergo molecular cytogenetic changes prior to any alterations in morphology. Nuclear chromosome abnormality, which is observed in cancer cells, is an early event during the process of tumorigenesis, and it has become the objective index for determining cancerous cells (9,10). Nuclear aneuploidy is a prevalent feature of various types of cancer, including EC (9,10); therefore, the detection of aneuploidy, which is usually found in aneusomic nuclei, may be used to detect cancerous cells. Fluorescence in situ hybridization (FISH) technology is a rapid and sensitive method used for the detection of aneusomy on a specific chromosome (11). FISH has been utilized for the diagnosis of various types of cancer with high sensitivity and specificity, including hematological malignancies and lung, breast and kidney cancer (12,13). The advantage of FISH is that it is an objective and quantitative method for identifying cancerous cells. Previous studies have demonstrated that the priority of FISH in cancer detection is higher than conventional cytology and, therefore, may be advantageous to the early diagnosis of various types of cancer (14,15).
In the present study, chromosomal loci centromere of chromosome (CEP) 3 and 17 were selected to fabricate the DNA probe as they have an increased aberration rate in esophageal and lung cancer (16,17). In order to analyze the clinical application of FISH and any correlations between the prognosis of patients in the diagnosis of ESCC, 40 Kazakh patients with esophageal squamous cell carcinoma (ESCC) underwent FISH examination and conventional pathological diagnosis using surgical samples.
Patients and methods
Patients
Between June 2011 and September 2012, 40 Kazakh patients with ESCC were admitted to the Department of Thoracic Surgery at The First Affiliated Hospital of Xinjiang Medical University (Urumqi, China) and underwent surgical resection. Among the 40 patients enrolled in the present study, 34 were male and 6 were female, with an average age of 57.4 years (Table I). None of the patients had previously received any preoperative radiotherapy, chemotherapy or other treatment. Pathology was graded according to the 7th American Joint Committee on Cancer staging manual (18). Final post-operative pathological diagnosis confirmed ESCC in all 40 cases, including well differentiated (n=13), moderately differentiated (n=16) and poorly differentiated tumors (n=11). Lymph node metastasis was detected in 55% (22/40) of cases; therefore, 45% (18/40) of the cases enrolled in the present study were non-metastatic. A total of 10 esophageal tissue samples (>5 cm distant from the tumor) were harvested as normal controls from healthy individuals. Informed consent was obtained from all patients.
Table I.
Clinical characteristics of the patients with esophageal squamous cell carcinoma.
| Case | Age (years) | Gender | Pathology |
|---|---|---|---|
| 1 | 71 | M | T1bN0M0, IA |
| 2 | 75 | M | T2N0M0, IB |
| 3 | 71 | M | T3N0M0, IIA |
| 4 | 55 | M | T2N0M0, IB |
| 5 | 58 | M | T3N1M0, IIIA |
| 6 | 54 | F | T3N0M0, IIB |
| 7 | 69 | M | T2N0M0, IB |
| 8 | 55 | M | T3N2M0, IIIB |
| 9 | 62 | M | T2N0M0, IB |
| 10 | 45 | F | T3N1M0, IIIA |
| 11 | 54 | M | T2N0M0, IB |
| 12 | 60 | M | T2N0M0, IIA |
| 13 | 57 | M | T1N0M0, IA |
| 14 | 70 | M | T3N2M0, IIIB |
| 15 | 58 | F | T2N0M0, IIA |
| 16 | 56 | M | T3N1M0, IIIA |
| 17 | 73 | M | T2N1M0, IIB |
| 18 | 68 | M | T3N1M0, IIIA |
| 19 | 40 | M | T1bN0M0, IA |
| 20 | 43 | F | T2N1M0, IIB |
| 21 | 54 | M | T3N2M0, IIIB |
| 22 | 47 | M | T3N1M0, IIIA |
| 23 | 44 | M | T3N1M0, IIIA |
| 24 | 56 | M | T2N1M0, IIB |
| 25 | 42 | M | T3N1M0, IIIA |
| 26 | 45 | M | T3N0M0, IIB |
| 27 | 50 | M | T3N1M0, IIIA |
| 28 | 56 | F | T2N0M0, IB |
| 29 | 72 | M | T3N1M0, IIIA |
| 30 | 65 | M | T2N1M0, IIB |
| 31 | 67 | F | T3N1M0, IIIA |
| 32 | 52 | M | T1aN0M0, IA |
| 33 | 50 | M | T3N1M0, IIIA |
| 34 | 48 | M | T3N1M0, IIIA |
| 35 | 62 | M | T2N1M0, IIB |
| 36 | 50 | M | T2N0M0, IB |
| 37 | 63 | M | T3N0M0, IIB |
| 38 | 55 | M | T1bN0M0, IA |
| 39 | 72 | M | T3N1M0, IIIA |
| 40 | 50 | M | T2N1M0, IIB |
Pathology graded according to the 7th American Joint Committee on Cancer staging manual (18). M, male; F, female.
FISH method
Touch preparations of cells were performed on glass slides from fresh specimens and air-dried for 24 h at room temperature and were subsequently stored at −80°C in preparation for FISH. The same specimens were stained with hematoxylin and eosin for pathological evaluation (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China).
Cells were denatured with 70% formaldehyde at 74°C in a water bath and subsequently washed twice with standard saline citrate (SSC; Abbott Molecular, Inc., Des Plaines, IL, USA) at room temperature. Slides were dehydrated through a graded ethanol series (70, 85 and 100%) prior to the application of 10 µl hybridization solution, containing 1 µl orange fluorescently-labeled CEP 3 probe, 1 µl green fluorescently-labeled CEP 17 probe, 7 µl hybridization buffer and 1 µl double-distilled water (all Abbott Molecular, Inc.). Slides were covered with a cover slip and sealed with rubber cement (Fixogum; Marabu GmbH & Co. KG, Tamm, Germany). Following incubation for 16 h at 42°C in a humidity-controlled chamber, the slides were washed with SSC at 74°C and at room temperature for 2 min. Subsequently, 5 µl diamidinophenylindole (DAPI II; Abbott Molecular, Inc.) was applied to each spot and covered with a cover slip.
Two fluorescently-labeled probes were used, Cy3 and FITC (Thermo Fisher Scientific, Inc., Waltham, MA, USA), and each of the protocols included at least one normal esophageal tissue as a control. FISH signal analysis was performed according to the kit instructions (Vysis CEP 3 (D3Z1) SpectrumOrange Probe Kit and CEP 17 Probe Kit; Abbott Molecular, Inc.) A fluorescence microscope and an image acquisition and analysis system (DM6000; Leica Microsystems GmbH, Wetzlar, Germany) was used to determine the signal count. Results were analyzed by two individual statisticians. All cells were evaluated, with the exception of damaged cells or those with overlapping nuclei. A total of 100 nuclei were counted from each patient, and the total number of centromeric signals was recorded. Cells were deemed positive for aneuploidy when the percentage of hyperdisomic nuclei with >3 copies of at least one nucleus was >10%.
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used to perform statistical analyses in the present study. The Rank sum test and t-test were used to analyze the data from each group. Throughout the study, P<0.05 was considered to indicate a statistically significant difference.
Results
Polyploidy rates of the centromeres of chromosomes 3 and 17 were detected using CEPs and FISH analysis
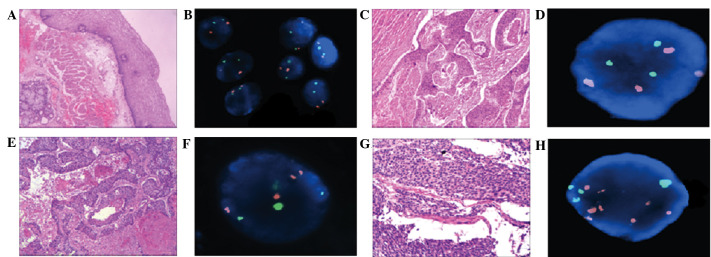
The percentage of diploid CEP3 and 17 was >93% in the control group (Table II). Abnormal polyploidy copy numbers of CEP3 and 17 were detected in all 40 ESCC specimens (Fig. 1).
Table II.
Results of fluorescence in situ hybridization in normal esophageal tissue.
| Aneuploidy (CEP 3/CEP 17) | ||||
|---|---|---|---|---|
| Case | 2 copies | 3 copies | 4 copies | Multiple copies |
| 1 | 96/96 | 1/2 | 3/2 | 4/4 |
| 2 | 97/95 | 2/3 | 1/2 | 3/5 |
| 3 | 97/97 | 2/2 | 1/1 | 3/3 |
| 4 | 98/98 | 2/2 | 0 | 2/2 |
| 5 | 96/97 | 3/2 | 1/1 | 4/3 |
| 6 | 96/95 | 4/2 | 0/3 | 4/5 |
| 7 | 95/95 | 2/2 | 3/3 | 5/5 |
| 8 | 95/96 | 3/2 | 2/2 | 5/4 |
| 9 | 93/94 | 3/3 | 4/3 | 7/6 |
| 10 | 97/95 | 2/3 | 1/2 | 3/5 |
CEP, centromere of chromosome.
Figure 1.
Comparison of hematoxylin and eosin (H&E) staining and fluorescence in situ hybridization (FISH) in normal and abnormal esophageal tissues. (A) Normal esophageal membranes following H&E staining (magnification, 40). (B) Normal representative nuclei carrying 2 copies of the centromeres of chromosomes (CEP) 3 (orange) and 17 (green). (C) Well-differentiated esophageal squamous cell carcinoma (ESCC) following H&E staining (magnification, 40). (D) FISH representative nuclei carrying 3 copies of CEP 3 and 3 copies of CEP 17. (E) Moderately differentiated ESCC following H&E staining (magnification, 40). (F) FISH representative nuclei carrying 4 copies of CEP 3 and 3 copies of CEP 17. (G) Poorly differentiated ESCC H&E staining (magnification, 100). (H) FISH representative nuclei carrying 7 copies of CEP 3 and 4 copies of CEP 17.
CEP3 and CEP17 aberrations correlated with lymph node metastasis
Average mutation rates of CEP 3 and 17 were 61.2% and 50.95%, respectively (Table III). The sensitivity and specificity of the FISH results were 100% with no false positives or negatives, calculated according to the final pathological diagnosis. Lymph node metastasis was detected in 55% of cases, therefore, 45% of the cases enrolled in the present study were non-metastatic. Furthermore, polyploidy rates were significantly increased in the metastatic lymph node group, as compared with the non-metastatic group (CEP 3, P=0.0001; CEP 17, P=0.012) (Table IV).
Table III.
Results of fluorescence in situ hybridization in esophageal cancer tissue.
| Aneuploidy (CEP 3/CEP 17) | ||||||
|---|---|---|---|---|---|---|
| Case | Pathology | 2 copies | 3 copies | 4 copies | ≥5 copies | Multiple copies |
| 1 | WDSCC | 45/39 | 25/31 | 20/22 | 10/8 | 55/61 |
| 2 | WDSCC | 71/73 | 19/15 | 10/12 | 0/0 | 29/27 |
| 3 | WDSCC | 73/77 | 17/12 | 9/11 | 1/0 | 27/23 |
| 4 | WDSCC | 65/63 | 21/16 | 11/12 | 3/9 | 35/37 |
| 5 | WDSCC | 60/72 | 28/12 | 5/8 | 7/8 | 40/28 |
| 6 | WDSCC | 82/80 | 17/15 | 0/5 | 1/0 | 18/20 |
| 7 | WDSCC | 76/74 | 13/11 | 6/7 | 5/8 | 24/26 |
| 8 | WDSCC | 72/68 | 13/15 | 7/9 | 8/8 | 28/32 |
| 9 | WDSCC | 66/72 | 20/17 | 10/9 | 4/2 | 34/28 |
| 10 | WDSCC | 59/68 | 26/21 | 6/7 | 9/4 | 41/32 |
| 11 | WDSCC | 60/65 | 21/19 | 11/8 | 8/8 | 40/35 |
| 12 | WDSCC | 50/57 | 20/17 | 25/20 | 5/6 | 50/43 |
| 13 | WDSCC | 71/74 | 11/11 | 10/10 | 8/5 | 29/26 |
| 14 | MDSCC | 41/59 | 11/12 | 36/20 | 12/9 | 59/41 |
| 15 | MDSCC | 38/56 | 25/17 | 28/20 | 9/7 | 62/44 |
| 16 | MDSCC | 43/59 | 28/18 | 13/12 | 16/11 | 57/41 |
| 17 | MDSCC | 64/65 | 18/13 | 12/15 | 6/7 | 36/35 |
| 18 | MDSCC | 38/57 | 26/29 | 28/11 | 8/3 | 62/43 |
| 19 | MDSCC | 31/56 | 36/20 | 19/12 | 16/12 | 69/44 |
| 20 | MDSCC | 52/78 | 32/17 | 13/4 | 3/1 | 48/22 |
| 21 | MDSCC | 60/70 | 9/11 | 12/12 | 9/7 | 40/30 |
| 22 | MDSCC | 45/55 | 26/21 | 12/9 | 17/15 | 55/45 |
| 23 | MDSCC | 39/46 | 45/39 | 9/13 | 7/2 | 61/54 |
| 24 | MDSCC | 48/57 | 25/20 | 19/16 | 8/7 | 52/43 |
| 25 | MDSCC | 44/54 | 31/26 | 23/15 | 3/5 | 56/46 |
| 26 | MDSCC | 45/52 | 25/25 | 12/8 | 18/15 | 55/48 |
| 27 | MDSCC | 24/24 | 55/36 | 9/30 | 12/10 | 76/76 |
| 28 | MDSCC | 15/26 | 35/44 | 20/20 | 30/10 | 85/74 |
| 29 | MDSCC | 80/75 | 19/15 | 1/10 | 0/0 | 20/25 |
| 30 | PDSCC | 30/38 | 27/21 | 24/26 | 19/15 | 70/62 |
| 31 | PDSCC | 25/28 | 40/40 | 25/23 | 10/9 | 75/72 |
| 32 | PDSCC | 21/27 | 30/23 | 39/40 | 10/10 | 79/73 |
| 33 | PDSCC | 13/9 | 55/46 | 17/30 | 15/15 | 87/91 |
| 34 | PDSCC | 14/30 | 41/29 | 23/30 | 22/11 | 86/70 |
| 35 | PDSCC | 14/6 | 40/41 | 20/24 | 26/31 | 86/94 |
| 36 | PDSCC | 28/40 | 28/25 | 27/23 | 17/12 | 72/60 |
| 37 | PDSCC | 32/40 | 39/26 | 25/24 | 4/10 | 68/60 |
| 38 | PDSCC | 12/25 | 58/52 | 12/12 | 18/11 | 88/75 |
| 39 | PDSCC | 32/24 | 47/55 | 10/12 | 11/9 | 68/76 |
| 40 | PDSCC | 40/42 | 20/17 | 27/26 | 13/15 | 60/58 |
WDSCC, well-differentiated squamous cell carcinoma; MDSCC, moderately differentiated squamous cell carcinoma; PDSCC, poorly differentiated squamous cell carcinoma; CEP, centromere of chromosome.
Table IV.
CEP3 and CEP17 aberrations correlated with lymph node metastasis.
| Lymph node metastasis | CEP3 | CEP17 |
|---|---|---|
| Negative | 44.38±3.820 | 40.57±3.486 |
| Positive | 66.32±3.946 | 56.21±4.898 |
| T-value | −3.990 | −2.639 |
| P-value | 0.000 | 0.012 |
CEP, centromere of chromosome
Results of differentiation degree of CEP3 and CEP17 in three groups
The polyploidy rates for CEP3 and 17 were 35.38 and 30.92% in well-differentiated ESCC, 55.81 and 44.43% in moderately differentiated ESCC, and 76.27 and 71.90% in poorly differentiated ESCC, respectively. Significant differences were detected between the poorly differentiated, moderately differentiated and well-differentiated ESCC specimens (CEP 3, P<0.05; CEP 17, P<0.05) (Table V).
Table V.
Differentiation degree of CEP3 and CEP17 in the three groups.
| Differentiation | CEP3 | CEP17 |
|---|---|---|
| WD | 35.3846±12.2647 | 30.9231±10.9199 |
| MD | 55.8125±15.3849 | 44.4375±14.5692 |
| PD | 76.2727±9.5403 | 71.9091±12.0785 |
| F-value | 8.616 | 7.975 |
| P-value | <0.05 | <0.05 |
CEP, centromere of chromosome; WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated.
Discussion
The incidence of EC is high in the Xinjiang Uyghur Autonomous Region of northwestern China, and the Kazakh ethnic group in particular suffers from an increased incidence of EC, as compared with other ethnic groups (2). Therefore, improved diagnosis and treatment of EC is required to enhance the survival rate and quality of life of patients with EC.
Previous studies have demonstrated that various genetic mutation occur during carcinogenesis (10,15). FISH is a sensitive and specific diagnostic technique that is capable of detecting various types of cytogenetic alterations, including aneusomy, amplification and deletion. This technique has become a widely used diagnostic method in cytogenetic studies (11,19); however, to the best of our knowledge there are no previous studies investigating the application of FISH in the diagnosis of Kazakh esophageal cancer.
Human chromosome 3 carries hyperdense genes, which are associated with numerous types of cancer, including raf-1 and erb-A; whereas chromosome 17 carries certain oncogenes and tumor suppressor genes, including p53, c-erbB2, BRCA1 and nm23 (9,14,20). Previous studies have demonstrated elevated aberration rates in CEP 3 and 17 in patients with EC, which indicates that EC may be correlated with constitutional cytogenetic alterations (12,20).
In the present study, CEP 3 and 17 DNA probes were selected, and the cut-off value for the percentage of hyperdisomic cells was set at 10%. Normal cells often have <6% hyperdisomic cells and this discrepancy is likely to be due to sister chromatids being counted as copies (6,8).
In a previous study conducted by Fiegl et al (21) FISH was applied in the diagnosis of lung, breast, liver and stomach cancer, and the confirmed diagnostic rate was increased. Furthermore, Fritcher et al (22) analyzed esophageal adenocarcinoma using the FISH method with c-Myc, P16, HER2 and 20q13 centromeric region probes, and demonstrated that the sensitivity of cytology was only 45% for the detection of esophageal adenocarcinoma; however, a detection rate of 100% was achieved using FISH.
In the present study, FISH was applied to Kazakh patients with ESCC to elucidate the diagnostic value of FISH in the detection of cancer cells and as a prognostic indicator. Polyploidy of CEP 3 and 17 was detected in all 40 ESCC specimens and significant differences in the rates of polyploidy were detected between the poorly differentiated, moderately differentiated and well-differentiated ESCC specimens for both CEPs (CEP 3, P<0.05; CEP 17, P<0.05). Furthermore, polyploidy was significantly increased in the metastatic lymph node group, as compared with the non-metastatic group (CEP 3, P=0.0001; CEP 17, P=0.012). The average mutation rates of CEP 3 and 17 were 61.2 and 50.95%, respectively.
The results of the present study demonstrated that the aberration rates of CEP 3 and 17 were correlated with the level of ESCC differentiation. This may due to the eating habits of the Kazakh population, in particular the over consumption of smoked meat, fermented food, heavy smoking or drinking, and the reduced consumption of fresh fruits and vegetables (2,23,24).
In conclusion, the present study successfully used CEP 3 and 17 probes to detect cancerous cells in Kazakh patients with ESCC. In particular, aneuploidy was significantly higher in poorly differentiated squamous cells and the metastatic lymph node group. Therefore, DNA probes may be used as predictive biological markers for the prognosis of patients with ESCC. Furthermore, as an objective and qualitative method, FISH technology is capable of detecting CEP 3 and 17 variations in the diagnosis of Kazakh patients with ESCC, which may be used to genetically diagnose EC in the future. Further studies are required.
Acknowledgements
This study was supported by the Returned Overseas Students to Science and Technology Activities Fund (no. 2012-111) and National Natural Science Foundation of China (no. 81160279). The authors thank the Department of Hematology at the First Affiliated Hospital of Xinjiang Medical University for technical support.
Glossary
Abbreviations
- FISH
fluorescence in situ hybridization
- EC
esophageal cancer
- ESCC
esophageal squamous cell carcinoma
- CEP
centromere of chromosome
- SSC
standard saline citrate
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Lu XM, Zhang YM, Lin RY, Arzi G, Wang X, Zhang YL, Zhang Y, Wang Y, Wen H. Relationship between genetic polymorphisms of metabolizing enzymes CYP2E1, GSTM1 and Kazakh's Esophageal squamous cell cancer (ESCC) in Xinjiang, China. World J Gastroenterol. 2005;11:3651–3654. doi: 10.3748/wjg.v11.i24.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krasna MJ. Multimodality therapy for esophageal cancer. Oncology (Williston Park) 2010;24:1134–1138. [PubMed] [Google Scholar]
- 4.Odze RD. Barrett esophagus: Histology and pathology for the clinician. Nat Rev Gastroenterol Hepatol. 2009;6:478–490. doi: 10.1038/nrgastro.2009.103. [DOI] [PubMed] [Google Scholar]
- 5.Yerian L. Histology of metaplasia and dysplasia in Barrett's esophagus. Surg Oncol Clin N Am. 2009;18:411–422. doi: 10.1016/j.soc.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Papanicolaou GN. Atlas of Exfoliative Cytology. Harvard University Press; Cambridge, MA: 1954. Criteria of malignancy; pp. 13–21. [Google Scholar]
- 7.Awut I, Niyaz M, Huizhong X, Biekemitoufu H, Yan ZH, Zhu Z, Sheyhedin I, Changmin Z, Wei Z, Hao W. Genetic diagnosis of patients with esophageal cancer using FISH. Oncol Lett. 2010;1:809–814. doi: 10.3892/ol_00000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niyaz M, Turghun A, Ping ZH, Zhu Z, Sheyhedin I, Ren C, Awut I. TP53 gene deletion in esophageal cancer tissues of patients and its clinical significance. Mol Med Rep. 2013;7:122–126. doi: 10.3892/mmr.2012.1162. [DOI] [PubMed] [Google Scholar]
- 9.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 10.Chao WR, Lee MY, Lin WL, Koo CL, Sheu GT, Han CP. Assessing the HER2 status in mucinous epithelial ovarian cancer on the basis of the 2013 ASCO/CAP guideline update. Am J Surg Pathol. 2014;38:1227–1234. doi: 10.1097/PAS.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 11.Halling KC, Kipp BR. Fluorescence in situ hybridization in diagnostic cytology. Hum Pathol. 2007;38:1137–1144. doi: 10.1016/j.humpath.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Tafe LJ, Allen SF, Steinmetz HB, Dokus BA, Cook LJ, Marotti JD, Tsongalis GJ. Automated processing of fluorescence in-situ hybridization slides for HER2 testing in breast and gastro-esophageal carcinomas. Exp Mol Pathol. 2014;97:116–119. doi: 10.1016/j.yexmp.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Prins MJ, Ruurda JP, van Diest PJ, van Hillegersberg R, ten Kate FJ. Evaluation of the HER2 amplification status in oesophageal adenocarcinoma by conventional and automated FISH: A tissue microarray study. J Clin Pathol. 2014;67:26–32. doi: 10.1136/jclinpath-2013-201570. [DOI] [PubMed] [Google Scholar]
- 14.Gordon MA, Gundacker HM, Benedetti J, Macdonald JS, Baranda JC, Levin WJ, Blanke CD, Elatre W, Weng P, Zhou JY, et al. Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Ann Oncol. 2013;24:1754–1761. doi: 10.1093/annonc/mdt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Uehara T, Fujimoto M, Tsuruyama T, Date H, Haga H. HER2 status in lung adenocarcinoma: A comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer. 2014;85:373–378. doi: 10.1016/j.lungcan.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Awut I, Niyaz M, Biekemitoufu H, Zhang Z, Sheyhedin I, Hao W. Molecular pathological diagnosis for early esophageal cancer in Kazakh patients. Oncol Lett. 2012;3:549–553. doi: 10.3892/ol.2011.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura H, Saji H, Idiris A, Kawasaki N, Hosaka M, Ogata A, Saijo T, Kato H. Chromosomal instability detected by fluorescence in situ hybridization in surgical specimens of non-small cell lung cancer is associated with poor survival. Clin Cancer Res. 2003;9:2294–2299. [PubMed] [Google Scholar]
- 18.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Ba J, Hao X, Zhang S, Hu Y, Zhang X, Yuan W, Hu L, Cheng T, Zetterberg A, et al. Multi-gene fluorescence in situ hybridization to detect cell cycle gene copy number aberrations in young breast cancer patients. Cell Cycle. 2014;13:1299–1305. doi: 10.4161/cc.28201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura H, Idiris A, Kawasaki N, Taguchi M, Ohira T, Kato H. Quantitative detection of lung cancer cells by fluorescence in situ hybridization: Comparison with conventional cytology. Chest. 2005;128:906–911. doi: 10.1378/chest.128.2.906. [DOI] [PubMed] [Google Scholar]
- 21.Fiegl M, Massoner A, Haun M, Sturm W, Kaufmann H, Hack R, Krugmann J, Fritzer-Szekeres M, Grünewald K, Gastl G. Sensitive detection of tumour cells in effusions by combining cytology and fluorescence in situ hybridisation (FISH) Br J Cancer. 2004;91:558–563. doi: 10.1038/sj.bjc.6601942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritcher EG, Brankley SM, Kipp BR, Voss JS, Campion MB, Morrison LE, Legator MS, Lutzke LS, Wang KK, Sebo TJ, Halling KC. A comparison of conventional cytology, DNA ploidy analysis, and fluorescence in situ hybridization for the detection of dysplasia and adenocarcinoma in patients with Barrett's esophagus. Hum Pathol. 2008;39:1128–1135. doi: 10.1016/j.humpath.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahmanyar S, Ye W. Dietary patterns and risk of squamous-cell carcinoma and adenocarcinoma of the esophagus and adenocarcinoma of the gastric cardia: A population-based case-control study in Sweden. Nutr Cancer. 2006;54:171–178. doi: 10.1207/s15327914nc5402_3. [DOI] [PubMed] [Google Scholar]
- 24.Morita M, Kumashiro R, Kubo N, Nakashima Y, Yoshida R, Yoshinaga K, Saeki H, Emi Y, Kakeji Y, Sakaguchi Y, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophegus: Epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15:126–134. doi: 10.1007/s10147-010-0056-7. [DOI] [PubMed] [Google Scholar]