Abstract
The aim of the present study was to investigate the overexpression and significance of ribosomal L1 domain containing 1 (RSL1D1) in prostate cancer (PCA). The present study performed immunohistochemical analysis on the tissues of 138 patients with pathologically confirmed PCA. The patients were followed up for a median of 87 months. In addition, 50 patients with benign prostatic hyperplasia (BPH) were enrolled in the present study as a control group. Of the 138 PCA tissue samples, 124 (89.9%) expressed RSL1D1, while 4 out of the 50 (8.0%) BPH tissues expressed RSL1D1. The present study defined a high RSL1D1 expression level as the relative gene expression that was equal to or higher than the median, and low expression as the gene expression lower than the median. The pathological stage of patients with PCA (≥pT3a vs. pT2c) and the Gleason scores of patients (≥7 vs. <7) were associated with RSL1D1 expression (χ2=4.809 and 14.703; P=0.028 and P<0.0001, respectively) and a high expression of RSL1D1 (χ2=10.294 and 17.520; P=0.001 and P<0.0001, respectively). Kaplan-Meier curve analysis demonstrated that the biochemical recurrence (BCR)-free survival rate of the patients was increased in patients without RSL1D1 expression (P=0.0046), in those with low RSL1D1 expression (P<0.0001) and in those without RSL1D1 expression in the mesenchyme (P=0.006) compared with those patients with no expression, low expression and no mesenchymal expression, respectively. A high expression level of RSL1D1 was demonstrated to be an independent prognostic factor of BCR in patients with PCA using Cox regression analysis. Overall, the present study demonstrated that RSL1D1 expression was associated with PCA, and that it may aid in the improvement of diagnosis, prognosis and risk stratification of patients with PCA.
Keywords: prostate cancer, ribosomal L1 domain containing 1, immunohistochemistry, biochemical recurrence-free survival
Introduction
Prostate cancer (PCA) is one of the most common forms of malignancy in men >50 years of age, and there are >200,000 newly diagnosed cases worldwide per year (1). Several clinicopathological variables, including prostate-specific antigen (PSA) level, Gleason score (GS) and clinical stage of PCA have contributed to treatment decision-making prior to the administration of an intervention. However, prescribing an appropriate treatment for men with newly diagnosed PCA remains controversial, since there are no precise molecular biomarkers that are associated with the biological characteristics of the tumors (2). The identification of a biomarker that may be used in clinical practice is therefore required to provide accurate prognostic information.
Ribosomal L1 domain containing 1 (RSL1D1; gene no., 26156), also termed PBK1, L12 and CSIG, is a nucleolar protein that belongs to the L1p/L10e family, and contains a ribosomal L1 domain in the N-terminus and a lysine-rich domain in the C-terminus (3). RSL1D1 has been demonstrated to delay cellular senescence through the inhibition of the translation of phosphatase and tensin homolog protein (3). In addition, it is a novel proapoptotic regulator that is activated in response to DNA damage (4). One of the most significant risk factors associated with PCA is aging, a process which represents an attenuation of antitumorigenic signals (5), such as those in cellular aging (6–9). It is currently widely accepted that cellular aging is critical in tumor suppression and has been associated with the presence of benign prostate lesions (10–12). However, to the best of our knowledge, there are no studies concerning RSL1D1 expression in prostatic tumor tissues and its association with the clinicopathological features of patients with PCA. Therefore, the current study investigated whether prostate tumors may be distinguished from benign prostatic hyperplasia (BPH) using cellular processes associated with aging and senescence, and whether RSL1D1 may be used as a novel biomarker for the diagnosis or risk stratification of patients with PCA.
Materials and methods
Patient characteristics and tissue samples
For the present study, tumor samples were obtained from 138 patients with prostatic adenocarcinoma, who underwent a radical prostatectomy at the Guangxi Medical University (Nanning, China) between October 2004 and July 2008. No patients received adjuvant androgen deprivation therapy prior to surgery. In addition to the PCA samples, the present study obtained 50 corresponding tissue samples, via transurethral resection performed at the same hospital, from patients with BPH to serve as controls. The mean age of the patients with PCA at the time of diagnosis was 68.3 years (range, 51–85 years), and the mean pre-operative PSA level was 18.92 ng/ml (range, 2.14–121.50 ng/ml). The mean age of the 50 patients diagnosed with BPH was 68.8 years, (range, 52–89 years) and the mean PSA was 6.05 ng/ml (range, 0.3–14.4 ng/ml). All patients with PCA were followed-up after radical surgery. Biochemical recurrence (BCR) was defined as PSA levels >0.2 ng/ml occurring two or more times.
The use of tissues was approved by the Institutional Review Board of the Guangxi Medical University and written informed consent was obtained from all patients.
Histological staining and immunohistochemical analysis
Paraffin-embedded, 4-mm thick tissue sections from all the obtained samples were stained with hematoxylin and eosin for histological analysis. RSL1D1 was detected using a goat anti-human RSL1D1 polyclonal antibody (catalog no., BS-0793R; dilution, 1:200; Bioss Inc., Woburn, MA, USA). The antibody was diluted according to the manufacturer's recommendations.
All the tissue sections were de-waxed, rehydrated and incubated in 3% hydrogen peroxide for 10 min at room temperature to halt endogenous peroxidase activity, prior to subsequent incubation overnight with the RSL1D1 antibody at 4°C in phosphate-buffered saline (PBS; ZSGB-BIO, Beijing, China) containing 1% bovine serum albumin (ZSGB-BIO). RSL1D1 staining was detected using an EnVision kit (EnVision™+HRP; Dako, Glostrup, Denmark). The nuclei were counterstained with 4′,6′-diamidino-2-phenylindole dilactate (ZSGB-BIO), and 0.3% hydrogen peroxide (ZSGB-BIO) in PBS was used as the chromogen. Following staining, the tissue sections were counterstained using hematoxylin (ZSGB-BIO), and subsequently dehydrated using ethanol and xylene. Permount (ZSGB-BIO) was then applied to the coverslips. Rat anti-human polyclonal immunoglobulin G (catalog no., CB3560554; dilution, 1:200; Biomeda Corporation, Foster City, CA, USA) was used as the primary antibody in the negative controls.
Histological analysis was performed by two pathologists (Department of Urology, Sixth Affiliated Hospital of Sun Yat-sen University; Department of Nuclear Medicine, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China) who were blinded to the clinical information of the patients. The staining intensity of the cells for RSL1D1 was graded according to the percentage of cells that expressed RSL1D1, as follows: 0, <10% of cells; grade I, 10–25% of cells; grade II, 26–50% of cells; grade III, 51–75% of cells; and grade IV, ≥76% of cells. Strong staining (grade III and IV) of RSL1D1 was considered as the high expression group, whereas light staining (grade I and II) was considered as the low expression group. Overexpression of RSL1D1 was determined as any expression in the PCA samples compared with the BPH samples. Morphological diagnoses were conducted according to the International Union Against Cancer 2009 staging classification guidelines for PCA (13), and histological analyses were performed according to the 2010 Gleason grading system (14).
Statistical analysis
The association between the expression of RSL1D1 and the clinicopathological variables of the patients was analyzed using Fisher's exact or the χ2 test. Variables affecting the BCR of the patients were analyzed using logistic regression. SPSS version 13.0 software (SPSS, Chicago, IL, USA) was used for analysis. Two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
RSL1D1 expression in PCA and BPH tissue samples
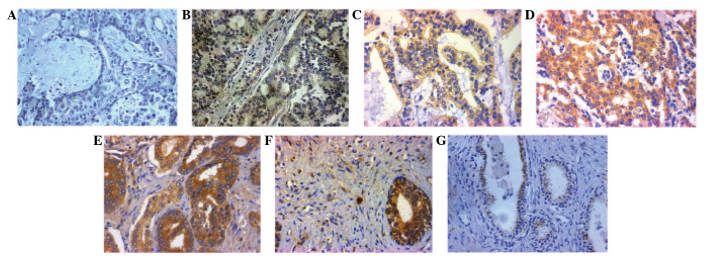
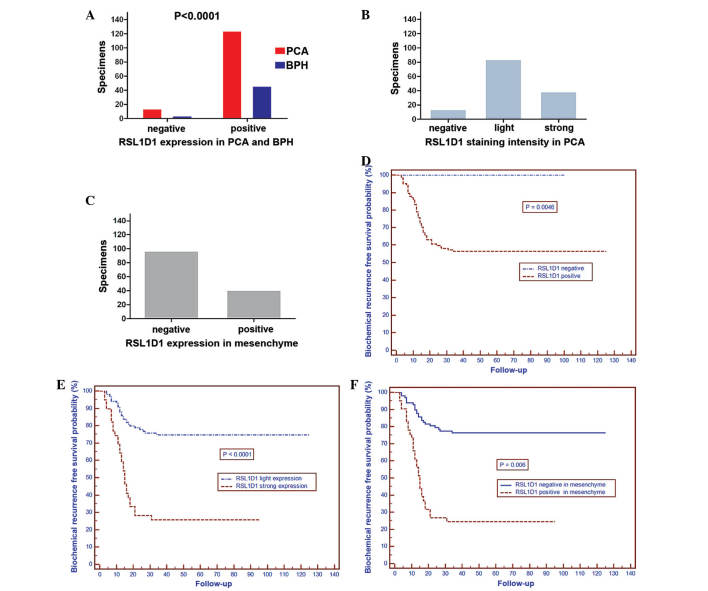
Immunohistochemical analysis demonstrated that RSL1D1 expression was localized to the nucleoli, with low to high expression in PCA cells. There was also expression in the mesenchyme of PCA cells (Fig. 1A–G). Positive expression of RSL1D1 was observed in 124 out of 138 (89.9%) patients with PCA and 4 out of 50 (8.0%) patients with BPH. Of these 124 patients, 18 patients (13.0% of total) exhibited grade I staining, 67 patients (48.6%) grade II staining, 25 patients (18.1%) grade III staining and 14 patients (10.1%) grade IV staining. Furthermore, RSL1D1 expression was more likely to be observed in the mesenchyme of PCA cells (41 out of 138 patients; 29.7%) (Fig. 2A–C).
Figure 1.
Immunohistochemistry of the RSL1D1 protein. (A) Negative control. (B-E) Representative images of RSL1D1 expression in PCA tissues with (B) grade I, (C) grade II, (D) grade III and (E) grade IV staining. (F) RSL1D1 in the mesenchyme of PCA tissues. (G) RSL1D1 light staining in benign prostate tissue. Magnification, x200. RSL1D1, ribosomal L1 domain containing 1; PCA, prostate cancer.
Figure 2.
(A) Distribution of RSL1D1 staining in tissues from patients with PCA and BPH. (B) RSL1D1 staining intensity in tissues from patients with PCA. (C) RSL1D1 staining in the mesenchyme of tissues from patients with PCA. (D and E) Association between RSL1D1 protein expression and BCR of patients with PCA: (D) BCR was increased in patients that did not express RSL1D1 compared with patients that did express RSL1D1 (P=0.0046); (E) RSL1D1 light vs. strong expression (P<0.0001); and (F) mesenchyme expression of RSL1D1, negative vs. positive (P<0.0001). PCA, prostate cancer; BPH, benign prostatic hyperplasia; NS, nucleostemin.
Association between RSL1D1 overexpression and clinicopathological variables of patients with PCA
The present study observed that the overexpression rate of RSL1D1 was significantly associated with the pathological GS of the patients with PCA (<7 vs. >7; 80.3 vs. 100%; P<0.0001). Similarly, the pathological stage of the cancer was associated with the overexpression rate of RSL1D1 [organ-confined (≤pT2c) vs. extraprostatic (≥pT3a); 85.7 vs. 97.8%; P=0.028]. There was no significant association identified between age (P=0.604), PSA level (P=0.166) and lymph node status (P=0.126) of patients with the overexpression rate of RSL1D1. A high expression rate of RSL1D1 was significantly associated with the GS of patients with PCA (>7 vs. <7; 44.8 vs. 12.7%; P<0.0001). There was a significant association between a strong expression rate of RSL1D1 and the pathological stage of the patients with PCA (≤pT2c vs. ≥pT3a; 21.9 vs. 45.7%; P=0.001). However, there was no significant association with the PSA level (<20 vs. ≥20 ng/ml; 26.0 vs. 33.3%; P=0.381), the age of the patients (≥70 and <70 years; 31.7 vs. 25.6%, P=0.436) or the presence of lymph node metastasis (yes vs. no; 39.1 vs. 26.1%; P=0.31) (Table I).
Table I.
Association between clinicopathological variables and expression of RSL1D1 in 138 patients with prostate cancer.
| Variables | Total, n | RSL1D1+ overexpression, n (%) | P-value | RSL1D1 strong expression, n (%) | P-value |
|---|---|---|---|---|---|
| Age, years | 0.6040 | 0.4360 | |||
| <70 | 78 | 71 (91.0) | 20 (25.6) | ||
| ≥70 | 60 | 53 (88.3) | 19 (31.7) | ||
| PSA, ng/ml | 0.1660 | 0.3810 | |||
| <20 | 96 | 84 (87.5) | 25 (26.0) | ||
| ≥20 | 42 | 40 (95.2) | 14 (33.3) | ||
| Pathological stage | 0.0280 | 0.0010 | |||
| T2c | 92 | 79 (85.7) | 18 (19.6) | ||
| ≥T3a | 46 | 45 (97.8) | 21 (45.7) | ||
| Post-operative GS | <0.0001 | <0.0001 | |||
| <7 | 71 | 57 (80.3) | 9 (12.7) | ||
| ≥7 | 67 | 67 (100.0) | 30 (44.8) | ||
| LN metastasis | 0.1260 | 0.3100 | |||
| No | 115 | 101 (87.8) | 30 (26.1) | ||
| Yes | 23 | 23 (100.0) | 9 (39.1) |
RSL1D1, ribosomal L1 domain containing 1; PSA, prostate-specific antigen; GS, Gleason score; LN, lymph node.
Association between RSL1D1 expression and BCR
Cox analysis was performed to analyze whether RSL1D1 expression was significantly associated with the BCR of the patients. The results are shown in Table II. A GS of ≥7, PSA ≥20 ng/ml, RSL1D1 strong expression, extraprostatic cases (≥pT3a cancer stage), lymph node metastasis and RSL1D1-positive staining in the mesenchyme were significant predictors of BCR using Cox univariate analysis, whereas multivariate analysis demonstrated that GS and a strong expression of RSL1D1 were significant predictors of BCR.
Table II.
Prognostic factors for biochemical recurrence of 138 patients with prostate cancer using Cox regression analysis.
| Variables | HR | 95% CI | P-value |
|---|---|---|---|
| Univariate analysis | |||
| Age, years (<70 vs. ≥70) | 1.059 | 0.621–1.807 | 0.833 |
| PSA, ng/ml (<20 vs. ≥20) | 5.276 | 3.041–9.155 | <0.0001 |
| Pathological GS (<7 vs. ≥7) | 30.922 | 9.659–98.998 | <0.0001 |
| Pathological stage (OC vs. EPE) | 11.403 | 6.040–21.527 | <0.0001 |
| Lymph node status (neg vs. pos) | 7.000 | 4.018–12.197 | <0.0001 |
| RSL1D1 expression (neg vs. pos) | – | – | – |
| RSL1D1 staining (light vs. strong) | 4.405 | 2.570–7.549 | <0.0001 |
| RSL1D1 in mesenchyme (neg vs. pos) | 2.079 | 1.215–3.559 | 0.008 |
| Multivariate analysis | |||
| Age, years (<70 vs. ≥70) | 1.116 | 0.639–1.948 | 0.700 |
| PSA, ng/ml (<20 vs. ≥20) | 1.895 | 0.950–3.780 | 0.069 |
| Pathological GS (<7 vs. ≥7) | 11.498 | 3.155–41.910 | 0.0001 |
| Pathological stage (OC vs. EPE) | 1.885 | 0.798–4.452 | 0.148 |
| Lymph node status (neg vs. pos) | 1.157 | 0.536–2.498 | 0.711 |
| RSL1D1 expression (neg vs. pos) | – | – | – |
| RSL1D1 staining (light vs. strong) | 1.915 | 1.094–3.350 | 0.023 |
| RSL1D1 in mesenchyme (neg vs. pos) | 1.193 | 0.585–2.433 | 0.628 |
PSA, prostate-specific antigen; RSL1D1, ribosomal L1 domain containing 1; GS, Gleason score; OC, organ-confined; EPE, extra-prostatic extension; neg, negative; pos, positive; HR, hazards ratio; CI confidence interval.
The mean follow-up time of the patients was 87 months (range, 61–136 months). Of the 138 cases analyzed immunohistochemically, 54 patients had BCR with PSA levels of ≥0.2 ng/ml ≥3 times, and 23 patients succumbed to the disease. The present results demonstrated that 5-year BCR-free survival rate was increased in patients that did not express RSL1D1 compared with patients that did express RSL1D1 (P=0.0046; Fig. 2D), in patients with light RSL1D1 expression compared with patients with strong expression (P<0.0001; Fig. 2E) and in patients that did not have mesenchymal RSL1D1 staining compared with patients that did have mesenchymal RSL1D1 staining (P=0.006; Fig. 2F).
Discussion
The identification of a novel biomarker is required for PCA, since it may be used by physicians and surgeons to identify patients who require radical surgery or active surveillance. Furthermore, a biomarker leads to improved screening, diagnosis and clinical outcome prediction prior to surgery (15). As a consequence of the high global incidence of PCA, the demand for the identification of dependable biomarkers is strong. PSA remains the most widely used biomarker for PCA diagnosis and screening; however, it has a number of limitations, including false-positive diagnoses and may lead to excessive treatment, due to its poor sensitivity and specificity (16–18).
The present study analyzed the association between RSL1D1 protein expression and the pre-operative clinicopathological characteristics of patients with PCA. It was observed that the levels of RSL1D1 protein were significantly increased in PCA tissues compared with the expression levels in the tissues of patients with BPH. In addition, the expression of the RSL1D1 protein was observed to be significantly associated with the pathological stage and increased GS of patients with PCA. In survival analysis, the BCR-free survival rate of PCA patients with high RSL1D1 protein expression was significantly decreased compared with patients with low RSL1D1 expression. Similarly, this result was also observed in patients that expressed RSL1D1 and in those that expressed RSL1D1 in the mesenchyme compared with patients with no expression and no mesenchymal expression, respectively. Univariate analyses also demonstrated that high RSL1D1 protein expression in patients with PCA was significantly associated with the BCR-free survival rate. Furthermore, multivariate analysis showed that a high expression level of RSL1D1 protein is an independent risk factor in the prognosis of patients with PCA. These results indicate that the detection of increased RSL1D1 protein may aid in identifying patients with an aggressive PCA phenotype and a poor prognosis. Therefore, RSL1D1 may be a promising prognostic marker for patients with PCA, although the precise molecular mechanisms behind the high expression of RSL1D1 in patients with PCA remains unknown.
In addition, the present study demonstrated that a GS of ≥7 was an independent predictor for the BCR of patients. It is widely accepted that the GS of patients is one of the most critical predictors of PCA progression and survival regardless of the therapy used, and it is also one of the most influential factors used to determine treatment for PCA (19). An increased GS was associated with recurrence and metastasis in a previous study that consisted of 450 patients with PCA with ≥8 years follow-up (20). In the study, the lowest risk of BCR and metastases at 10 years was found in patients with a GS of ≤7. In addition, another study demonstrated that the GS may be used to assess the risk of progression post-operatively in patients primarily treated with radiotherapy that subsequently develop local recurrence (21). In that study, the patients that had the most favorable outcome had a GS of ≤7 and PSA levels of <4 ng/ml. These findings were also confirmed by other studies, which demonstrated that patients with a GS of ≥7 and a higher GS of the tumor were more likely to have BCR compared with patients with a lower GS (22–24).
RSL1D1 can be identified using a yeast two-hybrid screen and is a cellular aging regulatory gene, whose expression is associated with senescence; however, its role in malignant tumors remains unknown (25). The present pilot study has demonstrated that there was a significant association between RSL1D1 protein overexpression and patients with PCA, suggesting that it may be used as a novel biomarker for the diagnosis and risk stratification of patients with PCA. The present pilot study has certain limitations, including the small sample size; therefore, additional studies to investigate the molecular mechanisms of RSL1D1 expression in the tumorigenesis or progression of PCA are essential.
In summary, the present results demonstrated that RSL1D1 protein overexpression is an independent prognostic factor for 5-year BCR-free survival and suggest that the RSL1D1 protein may be a novel biomarker for the risk stratification and prognosis of patients with PCA.
Acknowledgements
The authors would like to thank everyone from the Department of Pathology at Guangxi Medical University.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Gleason DF. Histologic grading of prostate cancer: A perspective. Hum Pathol. 1992;23:273–279. doi: 10.1016/0046-8177(92)90108-F. [DOI] [PubMed] [Google Scholar]
- 3.Ma L, Chang N, Guo S, Li Q, Zhang Z, Wang W, Tong T. CSIG inhibits PTEN translation in replicative senescence. Mol Cell Biol. 2008;28:6290–6301. doi: 10.1128/MCB.00142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N, Zhao G, Chen T, Xue L, Ma L, Niu J, Tong T. Nucleolar protein CSIG is required for p33ING1 function in UV-induced apoptosis. Cell Death Dis. 2012;3:e283. doi: 10.1038/cddis.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narita M, Lowe SW. Senescence comes of age. Nat Med. 2005;11:920–922. doi: 10.1038/nm0905-920. [DOI] [PubMed] [Google Scholar]
- 7.Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J. Aging, tumor suppression and cancer: High wire-act! Mech Ageing Dev. 2005;126:51–58. doi: 10.1016/j.mad.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Choi J, Shendrik I, Peacocke M, Peehl D, Buttyan R, Ikeguchi EF, Katz AE, Benson MC. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56:160–166. doi: 10.1016/S0090-4295(00)00538-0. [DOI] [PubMed] [Google Scholar]
- 11.Castro P, Giri D, Lamb D, Ittmann M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003;55:30–38. doi: 10.1002/pros.10204. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumors (UICC) 7th. Vol. 1. Wiley-Blackwell; Hoboken, NY: 2009. [Google Scholar]
- 14.Epstein JI. An update of the Gleason grading system. J Urol. 2010;183:433–440. doi: 10.1016/j.juro.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Prensner JR, Rubin MA, Wei JT, Chinnaiyan AM. Beyond PSA: The next generation of prostate cancer biomarkers. Sci Transl Med. 2012;4:127rv3. doi: 10.1126/scitranslmed.3003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 17.Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ, Brooks DD, Dash C, Guessous I, Andrews K, et al. American Cancer Society Prostate Cancer Advisory Committee: American cancer society guideline for the early detection of prostate cancer: Update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 18.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21:383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 19.Gleason DF, Mellinger GT. Veterans Administration Cooperative Urological Research Group: 1974. J Urol. 2002;167:953–958. doi: 10.1016/S0022-5347(02)80309-3. [DOI] [PubMed] [Google Scholar]
- 20.Antonarakis ES, Feng Z, Trock BJ, Humphreys EB, Carducci MA, Partin AW, Walsh PC, Eisenberger MA. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: Long-term follow-up. BJU Int. 2012;109:32–39. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chade DC, Shariat SF, Cronin AM, Savage CJ, Karnes RJ, Blute ML, Briganti A, Montorsi F, van der Poel HG, Van Poppel H, et al. Salvage radical prostatectomy for radiation-recurrent prostate cancer: A multi-institutional collaboration. Eur Urol. 2011;60:205–210. doi: 10.1016/j.eururo.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–523. doi: 10.1016/S0022-5347(05)63946-8. [DOI] [PubMed] [Google Scholar]
- 23.Miyake M, Tanaka N, Asakawa I, Morizawa Y, Anai S, Torimoto K, Aoki K, Yoneda T, Hasegawa M, Konishi N, Fujimoto K. Proposed salvage treatment strategy for biochemical failure after radical prostatectomy in patients with prostate cancer: A retrospective study. Radiat Oncol. 2014;9:208. doi: 10.1186/1748-717X-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao D, Kibel AS, Gao F, Tao Y, Humphrey PA. The Gleason score of tumor at the margin in radical prostatectomy is predictive of biochemical recurrence. Am J Surg Pathol. 2010;34:994–1001. doi: 10.1097/PAS.0b013e3181e103bf. [DOI] [PubMed] [Google Scholar]
- 25.Meng L, Yasumoto H, Tsai RY. Multiple controls regulate nucleostemin partitioning between nucleolus and nucleoplasm. J Cell Sci. 2006;119:5124–5136. doi: 10.1242/jcs.03292. [DOI] [PMC free article] [PubMed] [Google Scholar]