Abstract
Conventional methods for therapeutic blood-brain barrier (BBB) disruption facilitate drug delivery but are cumbersome to perform. A previous study demonstrated that adenosine receptor (AR) stimulation by 5′-N-ethylcarboxamide adenosine (NECA) increased the extravasation of intravascular tracers into the brain and proposed that AR agonism may be an effective method for therapeutic BBB disruption. We attempted to confirm the extravasation of tracers into the brain and also investigated tracer extravasation into peripheral organs and tracer retention in the blood. We found that NECA not only increased the extravasation of intravascular fluorescein and low molecular weight dextran into the brain of mice but also increased the concentrations of these tracers in the blood. In fact, the brain:blood ratio-normalized BBB permeability for either tracer is actually decreased by NECA administration. Elevated blood urea nitrogen levels in mice following NECA treatment suggested that renal function impairment was a probable cause of tracer retention. Therefore, NECA has almost no effect on the extravasation of intravascular Evans blue dye (EBD), an albumin-binding tracer with little renal clearance. Rather than inducing BBB disruption, our study demonstrated that NECA increased tracer extravasation into the brain by increasing the concentration gradient of the tracer across the BBB.
The blood-brain barrier (BBB) not only protects the brain against unwanted exchanges of chemicals and proteins but also impedes therapeutic agents from entering the brain. Methods to therapeutically disrupt the BBB have been developed to facilitate drug delivery1,2,3,4, and their use has been shown to increase the efficacy of chemotherapy against brain tumors in experimental studies5,6,7,8,9,10 and in clinical trials11,12,13. However, these methods are technically demanding and can cause serious adverse effects14,15,16,17,18.
Adenosine receptors (ARs) have recently been regarded as promising pharmacological targets for inducing BBB disruption19. This notion is based on a study showing that the intravenous administration of 5′-N-ethylcarboxamide adenosine (NECA), a broad spectrum AR agonist, increases brain extravasation of intravascular low molecular weight (LMW) and high molecular weight (HMW) dextrans20. Interestingly, this pharmacological effect of NECA was somewhat dose-specific, with a maximal effect at 0.08 mg/kg but sub-maximal effects at either lower doses of 0.02 and 0.04 mg/kg or higher doses of 0.16 and 0.32 mg/kg20. It was postulated that NECA at doses above 0.08 mg/kg desensitized the ARs, leading to reduced pharmacological responses. Consistent with AR signaling, the efficacy by which NECA induces brain extravasation of intravascular tracers was attenuated in mice lacking either A1 or A2A receptor subtypes and in mice receiving antagonists against the ARs20. These findings could be of tremendous translational value because AR agonists are already clinically approved for other indications, and the intravenous administration of NECA is far less invasive than existing methods for disrupting the BBB.
The discovery that AR agonism may disrupt the BBB is surprising because poor BBB permeability was reportedly a primary obstacle in the development of AR agonists as drug candidates for treating central disorders21,22. For instance, AR agonists cause potent vasodilatation when applied centrally, but often has no effect on cerebral blood flow when administered intravenously21,23. In addition, in vitro studies have yielded controversial results. Although AR agonists decreased trans-endothelial electrical resistance (TEER), which measures paracellular resistance, across an endothelial monolayer20,24,25, it had no effect on TEER or tracer permeability when the endothelial monolayer was co-cultured with astrocytes22, which mimics the BBB inducing microenvironment of the brain26,27. The discrepancy between that in vitro study22 and the recent in vivo findings20 suggests that the increased extravasation of intravascular tracer, as a measure of therapeutic BBB disruption, induced by NECA and other AR agonists did not result from a direct effect on the BBB.
The original aim of this study was to evaluate the efficacy of NECA in inducing BBB disruption to facilitate the transport of neuroprotective agents28 into the brain. However, we found that NECA strongly increased the plasma concentrations of inert tracers commonly used to measure BBB permeability. Thus, rather than acting on the BBB directly, NECA indirectly increased the extravasation of these tracers across the BBB by increasing their concentration gradients.
Results
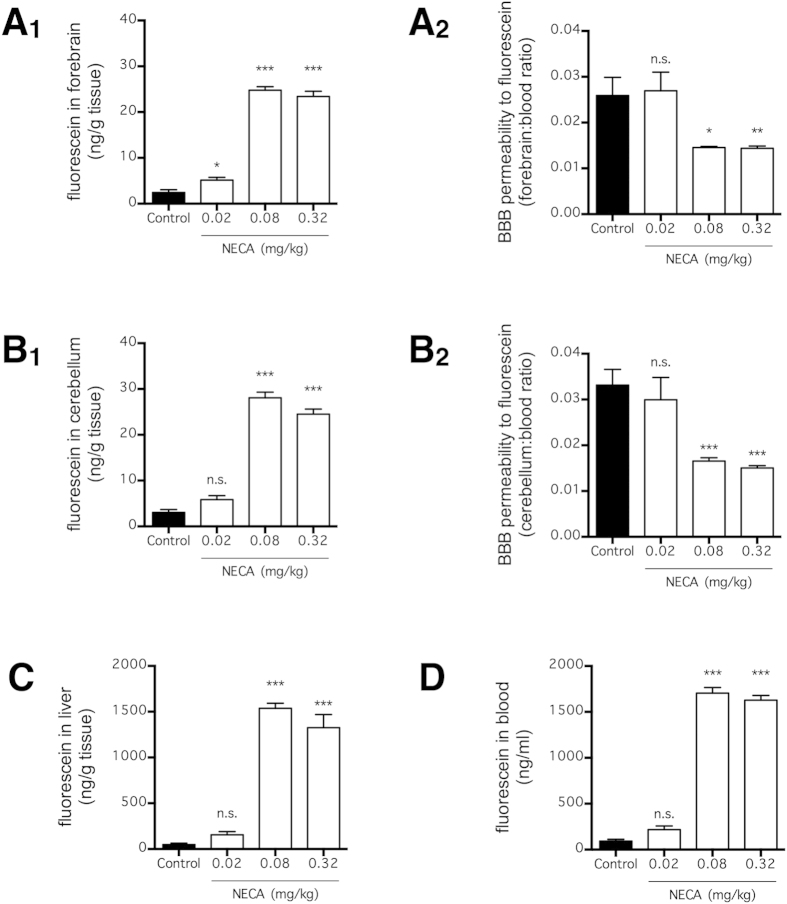
NECA increases brain extravasation of fluorescein without BBB disruption
To determine BBB permeability, mice were injected with fluorescein, a LMW tracer that does not normally cross the BBB29, 3 h after NECA administration (Fig. 1). Thereafter, the amount of fluorescein that extravasated into the brain parenchyma was quantified (Fig. 1A,B). The dose and route of administration for NECA and the time point for assessment of BBB permeability were based on the original study that demonstrated the BBB disrupting effect of AR stimulation20. As discovered in that study, NECA dose-dependently increased the amount of fluorescein extravasated into the forebrain and cerebellum, with 0.08 mg/kg being the most effective dose (p < 0.001; n = 6) (Fig. 1A,B). However, when fluorescein levels in the liver and blood were measured, we also observed a substantial increase in fluorescein concentration (p < 0.001; n = 6) (Fig. 1C,D). Additionally, higher doses of NECA (0.08 and 0.32 mg/kg) actually decreased the normalized BBB permeability (p < 0.001; n = 6), which was calculated by taking the ratio of brain:blood fluorescein concentrations (Fig. 1A,B). These findings suggest that NECA increased the brain extravasation of fluorescein by increasing its plasma concentration, but the effect on fluorescein extravasation was less effective at higher doses (>0.08 mg/kg) due to a gradual decrease in BBB permeability.
Figure 1. NECA increased fluorescein extravasation and concentration in blood.
Mice received fluorescein (i.v. via tail vein) at 3 h after NECA injection (n = 6 per group), and fluorescein concentrations in forebrain (A1), cerebellum (B1), liver (C), and blood (D) were quantified 90 min later. BBB permeability was normalized to fluorescein concentration in blood for fluorescein concentrations in forebrain (A2) and cerebellum (B2). The bars represent means ± SEM. Significant differences were determined by Fisher’s LSD test. *p < 0.05, **p < 0.01, and ***p < 0.001; n.s. indicates no significant difference.
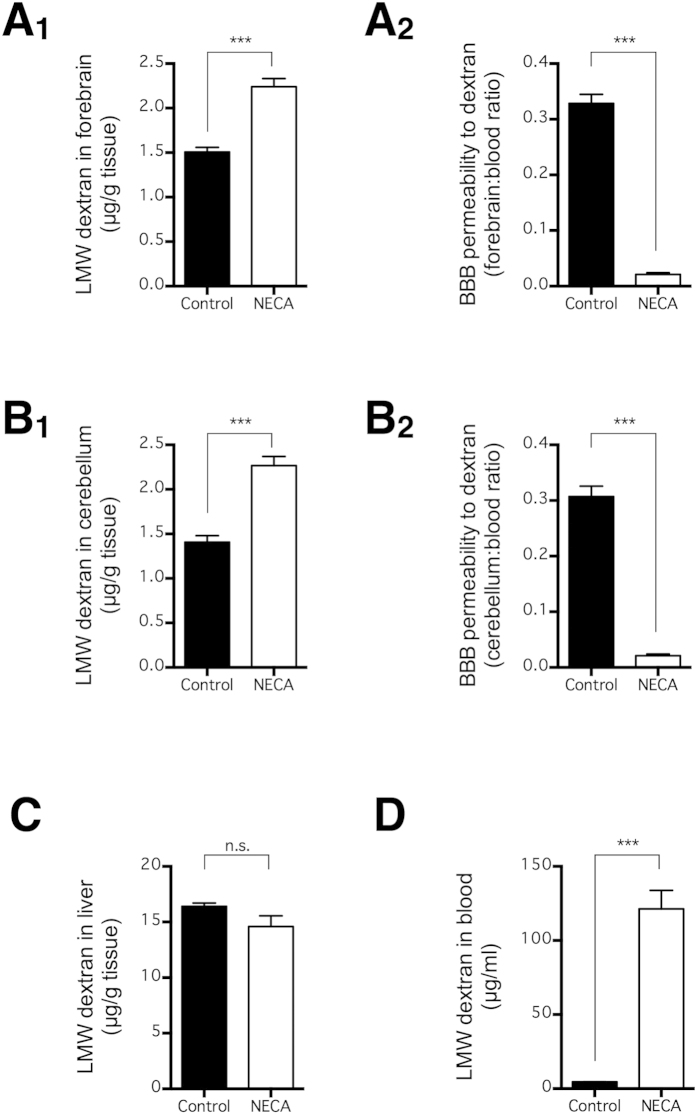
NECA increases brain extravasation of LMW dextran without BBB disruption
To examine whether our unexpected findings were specific to fluorescein, we repeated the same experiment using LMW dextran, the same tracer used in the original study20 that concluded that NECA opens the BBB. Consistent with the above findings, NECA (0.08 mg/kg) significantly increased the amount of LMW dextran extravasated into the forebrain and cerebellum (p < 0.001; n = 10) (Fig. 2A,B), but it also, even more substantially, increased the concentration of LMW dextran in blood (p < 0.001; n = 10) (Fig. 2D). Therefore, NECA decreased the normalized BBB permeability calculated by taking the ratio of brain:blood LMW dextran concentrations (p < 0.001; n = 10) (Fig. 2A,B). Taken together, NECA increased the brain extravasation of LMW fluorescein and dextran by increasing their plasma concentrations; this was despite a decrease in BBB permeability, determined by normalizing the differences in plasma tracer concentrations.
Figure 2. NECA increased low molecular weight (LMW) dextran extravasation and concentration in blood.
Mice received LMW dextran (i.v. via tail vein) at 3 h after NECA injection (n = 10 per group), and LMW dextran concentrations in forebrain (A1), cerebellum (B1), liver (C), and blood (D) were quantified 90 min later. BBB permeability was normalized to LMW dextran concentration in blood for LMW dextran concentrations in forebrain (A2) and cerebellum (B2). The bars represent means ± SEM. Significant differences were determined by the unpaired t test. ***p < 0.001; n.s. indicates no significant difference.
Increase in tracer concentration is associated with impaired renal function
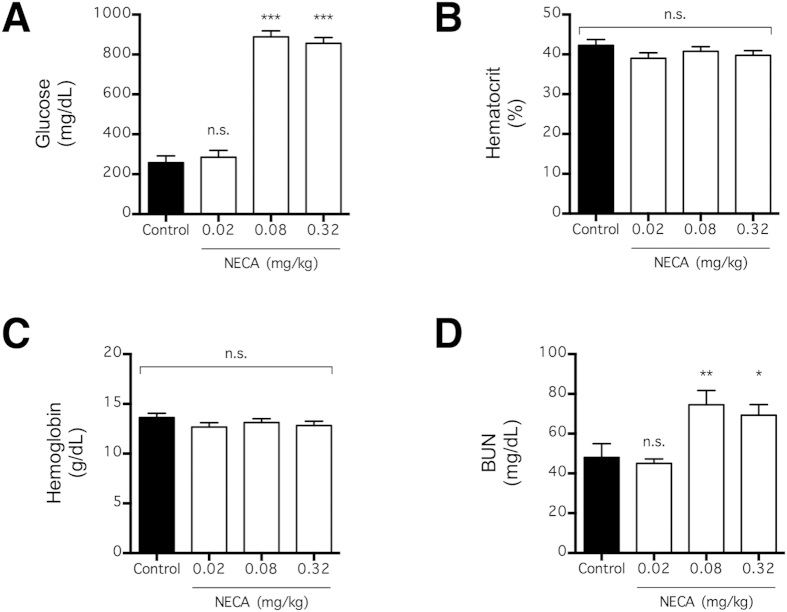
Given that the plasma concentration of intravascular tracer was increased, we examined the blood of NECA-treated mice to understand this pharmacological response (Fig. 3). Consistent with the AR-dependent effect of NECA on blood glucose levels in mice30, NECA (0.08 and 0.32 mg/kg) increased the plasma glucose concentration (p < 0.001; n = 4) in mice used in this study (Fig. 3A). This confirmed that our protocol for preparing and injecting NECA was technically competent.
Figure 3. Systemic effect of NECA on glucose and renal function.
Blood glucose (A), hematocrit (B), blood hemoglobin (C), and blood urea nitrogen (BUN) (D) were determined 4.5 h following NECA injection in mice (n = 4 per group). The bars represent means ± SEM. Significant differences are determined by Fisher’s LSD test. *p < 0.05, **p < 0.01, and ***p < 0.001; n.s. indicates no significant difference.
Because AR signaling is known to affect urine volume31,32, the increased plasma fluorescein concentration could be secondary to a change in blood volume. In this experiment, NECA (0.02–0.32 mg/kg) failed to have any effect on the hematocrit or hemoglobin concentration, two indicators of acute change in blood volume, of the treated mice at 4.5 h following intravenous injection (p > 0.05; n = 4) (Fig. 3B,C). Thus, any effect of NECA on blood volume is unlikely to affect plasma fluorescein concentration in the time course of this study.
The previous study reported that NECA-induced BBB leakage was substantially attenuated in A1-knockout mice20. Interestingly, AR signaling via the A1 receptor subtype is also known to contribute to acute renal failure induced by cytotoxic agents33,34,35. Consistent with acute renal impairment, mice that received NECA (0.08 and 0.32 mg/kg) in this study showed increased BUN (p < 0.001; n = 4), an indicator of renal deficit, within the time course of the experiments (Fig. 3D). Therefore, the NECA-induced acute increase in plasma concentrations of the tracers (Figs 1D and 2D), which increased their extravasation into the brain (Figs 1A,B and 2A,B) indicating a perceptual disruption of the BBB, could result from the decreased renal excretion of these tracers.
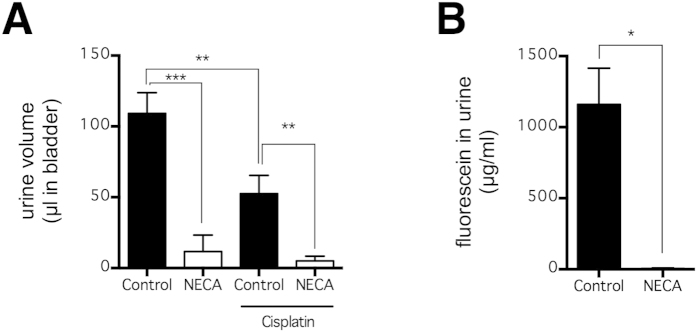
To directly assess whether NECA can reduce urine volume and renal clearance of fluorescein, mice again received NECA (0.08 mg/kg) and fluorescein (3 h later), and this time the volume and fluorescein concentration of their urine remaining in bladder 4.5 h after NECA injection were determined (Fig. 4). As a mild positive control, cisplatin (16 mg/kg), a chemotherapeutic agent that impairs kidney function, was injected in a subset of mice 3 days prior to the experiment. Cisplatin moderately decreased urine volume (p < 0.01; n = 6) (Fig. 4A), but had no effect on fluorescein concentration in urine (p > 0.05; n = 6). In comparison, NECA is much more effective in impairing kidney function; it substantially decreased urine volume in either control mice (p < 0.001; n = 6) or cisplatin-treated mice (p < 0.01; n = 6) (Fig. 4A). In addition, NECA also substantially decreased fluorescein concentration in urine (p < 0.05; n = 3–12) (Fig. 4B). Notably, the concentration of fluorescein is much higher in urine (Fig. 4B) than it is in the blood (Fig. 1D), suggesting that renal clearance is a primary route of fluorescein excretion. Taken together, our data demonstrates that NECA increased fluorescein in blood by impairing renal function.
Figure 4. NECA decreased urine volume and fluorescein excretion.
Mice received fluorescein (i.v. via tail vein) at 3 h after NECA injection (n = 6 per group), and urine volume (A) and fluorescein concentration (B) were quantified 90 min later. A subset of mice also received cisplatin, a chemotherapy agent known to induce kidney injury, 3 d prior to the experiment. The bars represent means ± SEM. Significant differences are determined by Fisher’s LSD test. *p < 0.05, **p < 0.01, and ***p < 0.001; n.s. indicates no significant difference.
NECA-induced tracer extravasation is attenuated when tracer is bound to plasma albumin
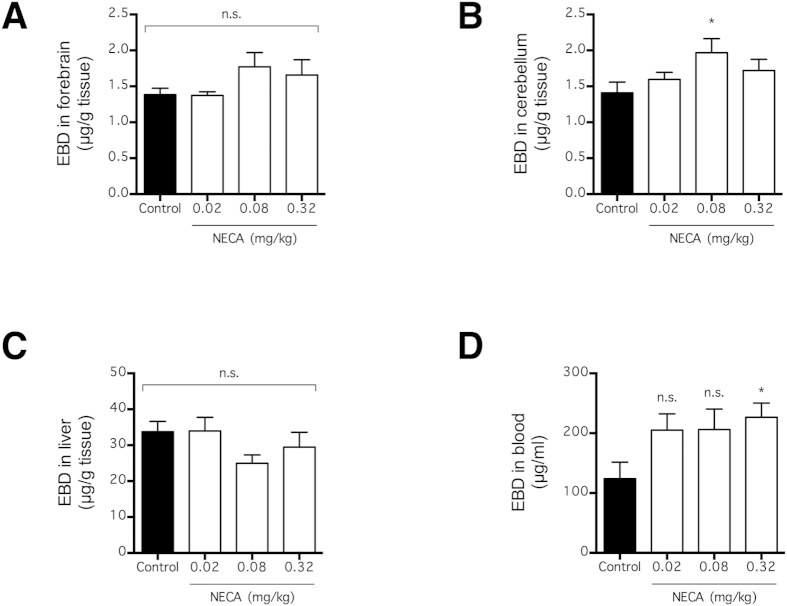
Our data were based on the systemic distribution of intravenous tracers and suggests that NECA increased the extravasation of intravascular tracers by preventing their renal clearance from blood (Figs 1 and 2). Therefore, NECA is expected to be less effective at inducing the extravasation of a tracer that is retained in the blood regardless of renal performance. To test this possibility, mice were injected with the Evans blue dye (EBD), a tracer that binds strongly to plasma albumin and hence, exhibits more plasma retention than an equivalent dose of fluorescein29, 3 h after NECA administration (Fig. 5). As with fluorescein and LMW dextran, the amounts of EBD that extravasated into the brain and liver parenchyma were quantified 90 min following tracer injection. Consistent with our hypothesis, the NECA-induced tracer extravasation into the brain and the liver was strongly attenuated (Fig. 5A–C). In fact, there was no significant increase in EBD extravasation into the forebrain or the liver (p > 0.05; n = 10) (Fig. 5A,C), and only a moderate significant increase in EBD extravasation into the cerebellum (p < 0.05; n = 10) (Fig. 5B). NECA also moderately increased the plasma EBD concentration (p < 0.05 for 0.32 mg/kg; n = 10) (Fig. 5D), but not as much as the plasma fluorescein and LMW dextran concentrations (Figs 1D and 2D). These observations agreed with our data suggesting that NECA increased the tracers’ plasma concentrations by impairing their clearance rather than changing plasma volume (Fig. 3). Because NECA was less effective at facilitating the extravasation of EBD into the central and peripheral tissues (Fig. 5A–C), its effect on plasma tracer concentration is likely crucial for its effect on tracer extravasation across the BBB.
Figure 5. NECA has little effect on EBD extravasation and concentration in blood.
Mice received EBD (i.v. via tail vein) at 3 h after NECA injection (n = 10 per group), and EBD concentrations in forebrain (A), cerebellum (B), liver (C), and blood (D) were quantified 90 min later. The bars represent means ± SEM. Significant differences are determined by Fisher’s LSD test. *p < 0.05; n.s. indicates no significant difference.
Discussion
Recent progress in understanding the cellular mechanism of neuronal death led to the development of compounds that are effective in animal models of neurodegeneration28. Given that several of these compounds have poor BBB permeability, their translational value can be greatly augmented with a safe and reliable method for disrupting the BBB. Existing methods to disrupt the BBB include the intracarotid administration of hyperosmolar solutions1,2 and ultrasound stimulation3,4. The former requires extensive surgery to cannulate the external carotid artery, and the latter method requires expensive equipment that is not widely available. In addition, these methods carry risks for serious adverse effects14,15,16,17,18. Based on the findings that NECA increased the extravasation of intravascular tracers into the brain20, AR agonism is widely regarded to be a feasible strategy for disrupting the BBB19,36. Unlike intracarotid administration of hyperosmolar solutions and ultrasound stimulation, AR agonism requires no invasive surgery, no expensive equipment, and can be formulated with neuroprotective agents.
We aimed to take advantage of AR agonism in the delivery of neuroprotective agents into the brain in our animal models of neurodegenerative diseases. Unexpectedly, however, we found that AR agonism by NECA, the prototypical broad-spectrum AR agonist best characterized for BBB disruption20,36, did not increase BBB permeability for fluorescein (Fig. 1A,B) and LMW dextran (Fig. 2A,B). Rather, NECA increased the extravasation of these intravascular tracers into the brain by increasing the plasma concentrations of the tracer (Figs 1D and 2D). This result was in marked comparison to BBB disruption by an intra-carotid infusion of hyperosmolar mannitol, which has been shown to increase tracer extravasation into the brain without affecting tracer concentration in the blood37. Unfortunately, the previous studies that investigated the effect of AR agonism on BBB disruption only measured tracer concentration in the brain parenchyma and not that in the blood20,24,36.
Consistent with the data reported by Carman et al.20, we found that NECA was most effective at inducing tracer extravasation at a dose of 0.08 mg/kg, compared with lower or higher doses (Figs 1 and 5). To explain this peculiar dose-response relationship, Carman et al. postulated that higher doses of NECA were less effective due to a desensitization of ARs20. In the present study, when the BBB permeability was normalized based on the brain:blood ratio of the tracer, NECA appeared to have no effect on BBB permeability at a lower dose (0.02 mg/kg) (Fig. 1A,B) and substantially decreased the permeability at higher doses (0.08 and 0.32 mg/kg) (Fig. 1A,B). In contrast to the observed BBB permeability trend, a NECA-induced increase in the plasma tracer concentration was most effective at 0.08 mg/kg (Fig. 1D). Based on our data, we proposed that NECA increased tracer extravasation at lower doses (<0.08 mg/kg) by increasing plasma tracer concentration, but decreased tracer extravasation at higher doses (>0.08 mg/kg) by a distinct mechanism that decreased BBB permeability.
A plausible mechanism by which NECA decreased extravasation of tracers across the BBB is by increasing the expression of transporters that exports these tracers out of the brain. For instance, the multidrug resistance-associated proteins (MRPs) are transporters expressed in cerebral endothelial cells that selectively binds to anionic molecules, including fluorescein38,39,40. Although genetic knockout of MRP-1, a functional MRP subtype expressed in cerebral endothelial cells, in mice has no effect on BBB permeability of fluorescein41, general inhibition of MRPs by a number of inhibitors increased extravasation of fluorescein into the mouse brain41,42. Indeed, MRP-mediated transport of fluorescein is increased by direct glucose application43, and this partially explains why BBB permeability to fluorescein is poor in diabetic animals44. Given that NECA substantially increased blood glucose concentration (Fig. 3A), it is possible that glucose-mediated increased in MRP expression contributed to decreased BBB permeability to fluorescein (Fig. 1A,B). In any case, given that NECA was administered systemically and that it stimulates all known AR subtypes, the net effect of NECA was likely multifactorial.
In conclusion, the two most widely used methods for disrupting the BBB, the osmotic disruption of BBB by intra-carotid hyperosmolar mannitol and microbubble-assisted focused ultrasound, have been shown to be effective in the brain delivery of therapeutic compounds. However, both methods are technically demanding and associated with serious adverse effects when performed incompetently. Despite our failed attempt at using AR agonism for BBB disruption, we continue to be hopeful for the development of new methods of BBB disruption that have the ease of AR agonism and without the adverse effects associated with existing methods.
Methods
Mice and in vivo procedures
Male C57BL/6 mice (21–27 g) were used in this study, and experiments were carried out in accordance to the Institutional Guidelines of the China Medical University for the Care and Use of Experimental Animals (IGCMU-CUEA) and were approved by the Institutional Animal Care and Use Committee (IACUC) of China Medical University (Taichung, Taiwan) (Protocol No. 103-224-NH). The mice had free access to rodent chow and water prior to the experiment. On the day of the experiment, mice were briefly anesthetized by isoflurane (2%, carried by air) and received either vehicle (10 ml/kg, 0.3% DMSO in saline) or NECA (0.02–0.32 mg/kg) via tail vein injection. At 3 h following NECA injection, each mouse was briefly re-anesthetized to receive a bolus injection of either tracer (i.v. through tail vein), sodium fluorescein (4% solution in saline; 2 ml/kg), LMW (10 kDa; FITC-labeled) dextran (10 mg/ml solution in PBS; 4 ml/kg), or EBD (4% solution in saline; 2 ml/kg). After another 90-min period to allow for tracer extravasation (or 4.5 h following NECA injection), the mice were euthanized by an overdose of urethane. Their dye-stained blood were collected from the heart and immediately placed on ice to slow down hemolysis. The mice were then perfused thoroughly with saline to remove residual blood from the vascular lumen, and the perfused brain and liver tissues were collected. In all experiments, NECA was prepared and randomly coded by a research assistant and blinded to the investigators until the end of all data collection.
Tissue processing and tracer quantitation
Extraction and quantitation of the tracers were carried out using a recently reported method45. Briefly, brain and liver tissues were homogenized for 10 min in 1:3 ratios (wt:vol) with 50% TCA, and the tissue debris and protein precipitates were removed by centrifugation at 10,000 × g for 20 min. The blood samples were mixed in 1:3 volume ratios of 50% TCA, and centrifuged to remove protein and cellular precipitates. For the LMW dextrans, the TCA solution was pH adjusted to 7–8. The extracted dye from brain, liver, and blood were added to a clear 96-well plate at 30 μl per well. Each well was supplemented with 90 μl of 95% ethanol to intensify fluorescent signal45, and the dye concentrations were quantified by spectroscopy. In a subset of mice that did not receive an intravascular tracer, blood samples were collected 4.5 h following NECA injection and directly subjected to analysis by a blood electrolyte analyzer (Stat Profile Critical Care Xpress, Nova Biomedical) to determine the concentrations of blood glucose, hematocrit, hemoglobin, blood urea nitrogen (BUN), and elemental electrolytes.
Urine volume and fluorescein concentration
Mice received NECA and sodium fluorescein as described earlier, and urine was collected from the bladder thereafter (4.5 h after NECA). Kidney injury was induced in a subset of mice by cisplatin (16 mg/kg, i.p.) pre-treated 3 d prior to the experiment. The volume of urine was measured using a syringe, and fluorescein concentration was quantified. Because only 3 out of 12 NECA-treated mice had sufficient urine for fluorescein quantification, comparison of fluorescein concentration was made between 12 control mice and 3 NECA-treated mice.
Data presentation and analysis
Data were presented as means ± SEM. Significant differences between treatment groups were determined by the unpaired t test or the Fisher’s least significant difference (LSD) test.
Additional Information
How to cite this article: Cheng, C.-C. et al. Adenosine receptor agonist NECA increases cerebral extravasation of fluorescein and low molecular weight dextran independent of blood-brain barrier modulation. Sci. Rep. 6, 23882; doi: 10.1038/srep23882 (2016).
Acknowledgments
C.C.C. is supported by an undergraduate student research award from the Ministry of Science and Technology (104-2815-C-039-006-B). This study is supported by research grants from China Medical University Hospital (DMR-101-100), the National Health Research Institute (NHRI-EX104-10412NC), the Ministry of Science and Technology (MOST103-2321-B-039-002), and the Ministry of Health and Welfare: Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019). The authors are grateful for the aid of lab members, especially Hwai-Lee Wang for drug preparation.
Footnotes
Author Contributions C.C.C. performed most of the experiments, analyzed the data, and assisted with manuscript preparation. Y.L.Y. assisted with drug preparation and experimental conduction. K.H.L. performed the blood electrolyte analysis, and assisted with equipment setups. T.W.L. conceived the study, supervised the experiments, and wrote the manuscript.
References
- Steinwall O. An improved technique for testing the effect of contrast media and other substances on the blood-brain barrier. Acta radiol 49, 281–284 (1958). [DOI] [PubMed] [Google Scholar]
- Rapoport S. I. & Thompson H. K. Osmotic opening of the blood-brain barrier in the monkey without associated neurological deficits. Science 180, 971 (1973). [DOI] [PubMed] [Google Scholar]
- Vykhodtseva N. I., Hynynen K. & Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol 21, 969–979 (1995). [DOI] [PubMed] [Google Scholar]
- Hynynen K., McDannold N., Vykhodtseva N. & Jolesz F. A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220, 640–646, doi: 10.1148/radiol.2202001804 (2001). [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A. et al. Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest 64, 684–688, doi: 10.1172/JCI109509 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., McDannold N., Jolesz F. A. & Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci USA 103, 11719–11723, doi: 10.1073/pnas.0604318103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treat L. H. et al. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer 121, 901–907, doi: 10.1002/ijc.22732 (2007). [DOI] [PubMed] [Google Scholar]
- Liu H. L. et al. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 255, 415–425, doi: 10.1148/radiol.10090699 (2010). [DOI] [PubMed] [Google Scholar]
- Wei K. C. et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One 8, e58995, doi: 10.1371/journal.pone.0058995 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. L. et al. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS One 9, e114311, doi: 10.1371/journal.pone.0114311 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E. A., Balaban E., Diehl J., Hill S. & Frenkel E. Successful treatment of primary central nervous system lymphomas with chemotherapy after osmotic blood-brain barrier opening. Neurosurgery 12, 662–671 (1983). [DOI] [PubMed] [Google Scholar]
- Doolittle N. D. et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 88, 637–647 (2000). [DOI] [PubMed] [Google Scholar]
- Angelov L. et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol 27, 3503–3509, doi: 10.1200/JCO.2008.19.3789 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport S. I., Bachman D. S. & Thompson H. K. Chronic effects of osmotic opening of the blood-brain barrier in the monkey. Science 176, 1243–1245 (1972). [DOI] [PubMed] [Google Scholar]
- Hicks J. T., Albrecht P. & Rapoport S. I. Entry of neutralizing antibody to measles into brain and cerebrospinal fluid of immunized monkeys after osmotic opening of the blood-brain barrier. Exp Neurol 53, 768–779 (1976). [DOI] [PubMed] [Google Scholar]
- Miller D. L. & Quddus J. Diagnostic ultrasound activation of contrast agent gas bodies induces capillary rupture in mice. Proc Natl Acad Sci USA 97, 10179–10184, doi: 10.1073/pnas.180294397 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M. et al. Blood-brain barrier disruption by low-frequency ultrasound. Stroke 37, 1546–1548, doi: 10.1161/01.STR.0000221813.27519.0b (2006). [DOI] [PubMed] [Google Scholar]
- Baseri B., Choi J. J., Tung Y. S. & Konofagou E. E. Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol 36, 1445–1459, doi: 10.1016/j.ultrasmedbio.2010.06.005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpass K. New methods to permeabilize the blood-brain barrier. Nat Rev Neurol 7, 597, doi: 10.1038/nrneurol.2011.161 (2011). [DOI] [PubMed] [Google Scholar]
- Carman A. J., Mills J. H., Krenz A., Kim D. G. & Bynoe M. S. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci 31, 13272–13280, doi: 10.1523/JNEUROSCI.3337-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Yoshikawa T., Kang Y. S. & Miller L. P. Blood-brain barrier transport and brain metabolism of adenosine and adenosine analogs. J Pharmacol Exp Ther 268, 14–18 (1994). [PubMed] [Google Scholar]
- Schaddelee M. P. et al. Functional role of adenosine receptor subtypes in the regulation of blood-brain barrier permeability: possible implications for the design of synthetic adenosine derivatives. Eur J Pharm Sci 19, 13–22 (2003). [DOI] [PubMed] [Google Scholar]
- Berne R. M., Knabb R. M., Ely S. W. & Rubio R. Adenosine in the local regulation of blood flow: a brief overview. Fed Proc 42, 3136–3142 (1983). [PubMed] [Google Scholar]
- Gao X. et al. Overcoming the blood-brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist. ACS Nano 8, 3678–3689, doi: 10.1021/nn5003375 (2014). [DOI] [PubMed] [Google Scholar]
- Kim D. G. & Bynoe M. S. A2A Adenosine Receptor Regulates the Human Blood-Brain Barrier Permeability. Mol Neurobiol 52, 664–678, doi: 10.1007/s12035-014-8879-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. A. & Wiley M. J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol 84, 183–192 (1981). [DOI] [PubMed] [Google Scholar]
- Janzer R. C. & Raff M. C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325, 253–257, doi: 10.1038/325253a0 (1987). [DOI] [PubMed] [Google Scholar]
- Lai T. W., Zhang S. & Wang Y. T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog Neurobiol 115C, 157–188, doi: 10.1016/j.pneurobio.2013.11.006 (2014). [DOI] [PubMed] [Google Scholar]
- Yen L. F., Wei V. C., Kuo E. Y. & Lai T. W. Distinct patterns of cerebral extravasation by evans blue and sodium fluorescein in rats. PLoS One 8, e68595, doi: 10.1371/journal.pone.0068595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figler R. A. et al. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes 60, 669–679, doi: 10.2337/db10-1070 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto H. & Karasawa A. Effects of adenosine A1-agonist and -antagonist on urinary volume and Na excretion in IAP-treated and non-treated rats. Jpn J Pharmacol 63, 257–259 (1993). [DOI] [PubMed] [Google Scholar]
- Mizumoto H., Karasawa A. & Kubo K. Diuretic and renal protective effects of 8-(noradamantan-3-yl)-1,3-dipropylxanthine (KW-3902), a novel adenosine A1-receptor antagonist, via pertussis toxin insensitive mechanism. J Pharmacol Exp Ther 266, 200–206 (1993). [PubMed] [Google Scholar]
- Nagashima K., Kusaka H. & Karasawa A. Protective effects of KW-3902, an adenosine A1-receptor antagonist, against cisplatin-induced acute renal failure in rats. Jpn J Pharmacol 67, 349–357 (1995). [DOI] [PubMed] [Google Scholar]
- Gill A. et al. Protective effect of tonapofylline (BG9928), an adenosine A1 receptor antagonist, against cisplatin-induced acute kidney injury in rats. Am J Nephrol 30, 521–526, doi: 10.1159/000248762 (2009). [DOI] [PubMed] [Google Scholar]
- Bhat S. G. et al. Cisplatin up-regulates the adenosine A(1) receptor in the rat kidney. Eur J Pharmacol 442, 251–264 (2002). [DOI] [PubMed] [Google Scholar]
- Boursereau R., Donadieu A., Dabertrand F., Dubayle D. & Morel J. L. Blood brain barrier precludes the cerebral arteries to intravenously-injected antisense oligonucleotide. Eur J Pharmacol 747, 141–149, doi: 10.1016/j.ejphar.2014.11.027 (2015). [DOI] [PubMed] [Google Scholar]
- Chen K. B. et al. Intravenous mannitol does not increase blood-brain barrier permeability to inert dyes in the adult rat forebrain. Neuroreport 24, 303–307, doi: 10.1097/WNR.0b013e32835f8acb (2013). [DOI] [PubMed] [Google Scholar]
- Huai-Yun H. et al. Expression of multidrug resistance-associated protein (MRP) in brain microvessel endothelial cells. Biochem Biophys Res Commun 243, 816–820, doi: 10.1006/bbrc.1997.8132 (1998). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Han H., Elmquist W. F. & Miller D. W. Expression of various multidrug resistance-associated protein (MRP) homologues in brain microvessel endothelial cells. Brain Res 876, 148–153 (2000). [DOI] [PubMed] [Google Scholar]
- Roberts L. M. et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 155, 423–438, doi: 10.1016/j.neuroscience.2008.06.015 (2008). [DOI] [PubMed] [Google Scholar]
- Sun H. et al. Transport of fluorescein in MDCKII-MRP1 transfected cells and mrp1-knockout mice. Biochem Biophys Res Commun 284, 863–869, doi: 10.1006/bbrc.2001.5062 (2001). [DOI] [PubMed] [Google Scholar]
- Sun H., Miller D. W. & Elmquist W. F. Effect of probenecid on fluorescein transport in the central nervous system using in vitro and in vivo models. Pharm Res 18, 1542–1549 (2001). [DOI] [PubMed] [Google Scholar]
- Legen I. & Kristl A. D-glucose triggers multidrug resistance-associated protein (MRP)-mediated secretion of fluorescein across rat jejunum in vitro. Pharm Res 21, 635–640 (2004). [DOI] [PubMed] [Google Scholar]
- Hawkins B. T., Ocheltree S. M., Norwood K. M. & Egleton R. D. Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci Lett 411, 1–5, doi: 10.1016/j.neulet.2006.09.010 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. L. & Lai T. W. Optimization of Evans blue quantitation in limited rat tissue samples. Sci Rep 4, 6588, doi: 10.1038/srep06588 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]