Abstract
Recurrent miscarriage (RM) is the occurrence of repeated pregnancies that end in miscarriage of the fetus before 20 weeks of gestation. At least 50% of the RM patients are considered idiopathic. High IL-33 levels are critical in early pregnancy and impact the outcome of subsequent pregnancies. However, the association of polymorphisms of IL-33 with idiopathic RM is still unclear. The present study was initiated to investigate whether IL-33 polymorphisms are risk factors for idiopathic RM in Chinese Han population. Study subjects comprised of 321 cases and 384 controls. Five polymorphisms (rs10435816, rs16924159, rs16924171, rs1929992, rs1332290) in IL-33 and serum IL-33 concentrations were assessed. rs16924159 variant exhibits significant association with RM in additive and recessive genetic model (additive model P = 0.015, recessive model P = 0.007). In contrast, rs10435816, rs16924171, rs1929992 and rs1332290 are not significantly associated with RM. Serum IL-33 levels are significantly lower in RM cases than in control (173.51 ± 94.12 versus. 200.97 ± 110.06 (pg/ml), P = 4.57 × 10−4). There are lower levels of serum IL-33 in rs16924159 homozygous mutant (AA) than homozygous wild-type (GG) in this study population, including cases and control groups (172.18 ± 103.01 versus. 205.82 ± 119.01 (pg/ml), P = 0.006). Reduced IL-33 levels and rs16924159 IL-33 variant may contribute to the pathogenesis of idiopathic RM in Chinese Han population.
Recurrent miscarriage (RM) is the loss of three or more consecutive pregnancies before 20 weeks of gestation. RM affects 1–5% of women who attempt to bear children1. Various factors have been identified for miscarriage, including uterine anomaly, chromosomal abnormalities, endocrine dysfunction, thrombophilia, immune disorders, life style factors and maternal infections1. However, At least 50% of the RM patients have no deviations from any applied diagnostic test. The underlying causes remain unknown and are considered idiopathic. Idiopathic RM is characterized by multifactorial etiology. Although much work has been done to identify the cause of idiopathic RM, its reason remains unknown2. There is growing evidence that RM has also genetic susceptibility2,3,4. In most conducted genetic association studies targeting RM, the most frequently addressed genes in the context of RM are associated with immunotolerance, inflammation and changes of maternal metabolism and blood coagulation3.
Successful pregnancy depends on the cytokine environment, which can either be protective or harmful to the conceptus5. The balance of pro-inflammatory and anti-inflammatory cytokines was suggested to be critical for successful pregnancy6. Maternal immunological tolerance of the allogenic fetus in pregnancy is linked with imbalance between T regulatory cells (Tregs) and T helper 17 cells (Th17)7,8. There is an elevation of Treg subset in the normal human pregnancy. However, a decrease in the number of Tregs is associated with miscarriage9,10. In contrast to the Tregs cells, the proportion of Th17 cells was significantly higher in patients with RM than in normal pregnant11,12,13.
IL-33 is a member of the IL-1 family. Members of this family are known to play important roles in host defense, immune regulation, neuronal injury and inflammation14. IL-33 is mainly expressed in epithelial, endothelial cells, activated Th2 cells and mast cells14,15,16,17. IL-33 appears to be a cytokine with dual function, performing both as a traditional cytokine through activation of the IL-33/ST2 pathway and as a nuclear factor involved in transcriptional repression and regulation of genes18,19. IL-33 has been reported to be the specific ligand for suppression of tumorigenicity 2(ST2), which is a member of the IL-1 receptor superfamily. IL-33 significantly stimulated the production of inflammatory cytokines, such as IL-1β, TNF-α, IL-8 and IL-620. IL-33 enhances Tregs cells differentiation. Furthermore, it provides a necessary signal for Tregs cells accumulation and maintenance in inflamed tissues13,21.
Recent research shows that IL-33 expression was found in endothelial and smooth muscle cells in the placenta, chorioamniotic membranes, and umbilical cord22. Human endometrial stromal cells (HESCs) rapidly release IL-33. IL-33 knockdown in undifferentiated HESCs abrogate this pro-inflammatory decidual response23. Further, sequential activation of the IL-33/ST2L/sST2 axis was disordered in decidualizing HESCs from women with recurrent pregnancy loss. Signals from HESC cultures prolonged the implantation window but also caused subsequent pregnancy failure in mice23. Serum IL-33 levels of normal pregnancies were highly variable in first eleven weeks of early pregnancy, with no statistical differences identified between each gestational week24. However, there is a significant increase in serum IL-33 levels from patients who went on to abortion compared with normal control pregnancies24. Above data suggest that dysregulated levels of serum IL-33 indicate miscarriage in live early pregnancies.
However, the association of polymorphisms of IL-33 with idiopathic RM is still unclear. The present study was initiated to evaluate association of the IL-33 gene polymorphisms with idiopathic RM in Chinese Han population. Five polymorphisms (rs10435816, rs16924159, rs16924171, rs1929992, rs1332290) in IL-33 gene were selected for genotyping, and then we analyzed the association between the five SNPs and the risk of idiopathic RM.
Materials and Methods
Subjects
This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The Institutional Review Boards of the Sichuan Provincial People’s Hospital and West China Second University Hospital, Sichuan University approved this study. All subjects provided informed consent before participating in the study.
Cases comprised 321 women from Sichuan Provincial People’s Hospital and West China Second University Hospital between January 2011 and June 2013, who had experienced three or more pregnancy losses with the same partner, and which had occurred from between the beginning of pregnancy to the 20th week of gestation; gestational age was calculated as the time between the first day of the last normal menstrual period and the first signs of pregnancy loss. The work-up included endometrial biopsies for evaluating luteal phase defects;pelvic ultrasound scans to assess ovarian morphology and the uterine cavity; hysteroscopy to evaluate uterine anatomic abnormalities; karyotyping of peripheral blood to evaluate chromosomal aberrations in both patient and partner. These procedures were performed on all patients. In addition, trisomy and triploidy of fetal tissues and were evaluated using fluorescence in situ hybridization (FISH). Data on FISH of fetal tissues were available for 266 (82.87%) patients.
Exclusion criteria included older age (40 years or older at first miscarriage), parental and fetal chromosomal aberrations, Rh incompatibility, anatomical abnormalities, preclinical miscarriages, endocrine disorders (including diabetes), liver function abnormalities, and abnormal thyroid function, thyroid antibodies, hyperprolactinemia prior to luteal phase defects, and fetomaternal alloimmune thrombocytopenia, infections (toxoplasmosis, human cytomegalovirus, rubella, human immunodeficiency virus, Group B streptococci, Chlamydia trachomatis, hepatitis B and C and bacterial vaginosis). Patients were also excluded if they reported systemic autoimmune disease, such as anti-phospholipid syndrome, systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis.
Control subjects composed of 384 women who were recruited from Sichuan Provincial People’s Hospital and West China Second University Hospital between January 2011 and June 2013. Age and ethnical group were matched with patients. All subjects had at least one live birth and no miscarriages, and did not have a family history of RM.
IL-33 genotyping
Blood samples were taken from all participants in EDTA containing tube for total genomic DNA extraction. Total genomic DNA was extracted by silica columns isolation kits (Tiangen BioTech CO.LTD, Beijing China).
Genotype data of IL-33 for the chromosomal region 9:6215149–6257983 were download from the HapMap website (CHB database, Hapmap Data Rel 27 Phase II III, Feb09, on NCBI B36 assembly, dbSNP b126). In total, 37 SNPs with a frequency >0.10 were identified in CHB (Han Chinese in Beijing). The criteria for all the referenced SNPs was that minor allele frequency (MAF) set at >10% and r2 > 0.8. The five tag-SNPs (rs10435816, rs16924159, rs16924171, rs1929992, rs1332290) were selected by running the tagger program in Haploview 4.2. These five SNPs in 5 tests capture 37 of 37 (100%) alleles with a mean r2 of 0.957. rs10435816, rs16924159 and rs16924171 are located in the 5′ upstream region of IL-33 (NM_001199640.1), rs1929992 and rs1332290 are located in the intron region of IL-33 (NM_001199640.1). IL-33 genotyping was performed using real time PCR assays (TaqMan probe). TaqMan® SNP Genotyping Assay kit were from Applied Biosystems. Cat. C__30088929_10 (rs10435816), C__34291196_10 (rs16924159), C__34291189_10 (rs16924171), C__11716827_10 (rs1929992), C___2762144_10 (rs1332290). Replicate blinded quality control samples were included to assess the reproducibility of the genotyping procedure. The successful genotyping rate was 99.0%. In brief, 24 individuals of cases and controls were randomly selected to sequence for confirmation of five SNPs genotyping. The confirmation rate was 100%.
Serum IL-33 Measurements
Because pregnancy influences IL-33 levels, blood drawing criteria were used for patients and control women. This included interval (4–6 months) from the last event (miscarriage or delivery) and time of blood draw (8:00–10:00 AM). Blood samples were taken from all participants in plain tubes (no preservatives) for serum preparation. Serum was prepared by centrifugation of coagulated blood tubes at 2000 g for 10 min at room temperature, and was stored in 200 μl aliquots at −80 °C. Serum IL-33 were measured using a human IL-33 enzyme-linked immunosorbent assay (catalogue number ADI-900-201; Enzo Life Sciences., Shanghai China) according to the manufacturer’s instructions. Assay sensitivity was 1.7 pg/ml (range: 7.8–500 pg/ml). Samples were tested in triplicate at the same time in the same assay.
Statistical analysis
Statistical analysis was performed by the software SPSS for Windows (version 13.0). Continuous variables were expressed as mean ± SD. Statistical differences were performed using t test or Mann–Whitney U test. Then differences in genotype distribution of the polymorphism between case and control subjects were compared by χ2 test. Three models were used for statistical analysis, including dominant model, the recessive genetic model and the additive model. Logistic regression analysis was used to evaluate the association between genotype of SNPs and idiopathic RM. Subsequently, Pearson χ2 statistics were calculated and P values were computed by comparing the statistic to a χ2 distribution with 1 or 2 degrees of freedom for the allelic and genotypic tests. P value of less than 0.05 was considered statistically significant. Gene variants were tested for Hardy-Weinberg equilibrium using Haploview version 4.2 (http://www.broad.mit.edu/mpg/haploview).
We used CaTS Power Calculator (www.sph.umich.edu/csg/abecasis/cats) to calculate the power to detect an association between IL-33 variants and RM in our cohort. The parameters used were: 321 RM cases and 384 control women, genotypic relative risk for heterozygotes (1/2) and minor allele homozygote (2/2), and the MAF for RM cases and controls for the five tested SNPs, and assumed 5% population prevalence of RM. Assuming these parameters, this sample size provided 82%, 83%, 82%, 82% and 83% power for rs10435816, rs16924159, rs16924171, rs1929992 and rs1332290, respectively.
Results
The clinical characteristics of study participants
The clinical characteristics of patients and controls are listed in Table 1. There are no significant differences in mean age, smoking (%) and mean BMI (kg/m2) between cases and control (P > 0.05). While lower age at menarche, higher irregular menstrual history (%) and number of pregnancies were seen in the RM group. Although they did not constitute strong risk factors of RM, they were selected as the covariates that were controlled for in subsequent analyses.
Table 1. Clinical Characteristics of the Study Population.
| Characteristic | Cases | Controls | P value |
|---|---|---|---|
| Mean age (years) | 31.35 ± 5.04 | 31.95 ± 4.32 | 0.089 |
| Smoking (%) | 25 (7.79) | 33 (8.59) | 0.584 |
| Mean BMI (kg/m2) | 21.31 ± 2.99 | 20.95 ± 3.06 | 0.366 |
| Menarche(years) | 12.88 ± 1.32 | 13.19 ± 1.18 | 0.013 |
| Irregular menstrual history (%) | 39 (12.15) | 28 (7.29) | 0.038 |
| Number of pregnancies | 4.91 ± 1.15 | 3.01 ± 1.28 | <0.0001 |
| Miscarriage | 3.83 ± 0.725 | 0 ± 0 | <0.0001 |
P versus controls; BMI, Body Mass Index.
IL-33 genotype distribution
Genotype distribution of five (rs10435816, rs16924159, rs16924171, rs1929992 and rs1332290) SNPs are in Hardy-Weinberg equilibrium in cases and controls (P > 0.05).
Table 2 summarizes the distribution of IL-33 genotypes between patients and control. Significant differences in the distribution of rs16924159 genotype are observed between patients and controls. rs16924159 variant exhibits significant association with RM in additive and recessive genetic model (additive model P = 0.015, recessive model P = 0.007). In contrast, rs10435816, rs16924171, rs1929992 and rs1332290 are not significantly associated with RM in any of three genetic models. As shown in Table 3, after adjustments for menarche age, irregular menstrual history and number of pregnancies by Binary Logistic Regression, rs16924159 showed significant association with RM in two genetic models (adjusted odds ratio (OR) = 2.037, 95% confidence interval (CI) = 1.135–3.656, P = 0.017 in an additive model; OR = 1.975, 95% CI = 1.161–3.357, P = 0.012 in a recessive model; OR = 1.926, 95% CI = 1.232–3.012, P = 0.014 in a homozygous model; OR = 1.199, 95% CI = 0.863–1.667, P = 0.299 in a heterozygous model).
Table 2. Association between SNPs and risk for idiopathic recurrent miscarriage in Chinese Han population.
| SNP | Genotype | Case (n = 321) | Control (n = 384) | Additive |
Dominant |
Recessive |
|||
|---|---|---|---|---|---|---|---|---|---|
| P value | Corrected P | P value | Corrected P | P value | Corrected P | ||||
| rs10435816 | AA | 103 (0.321) | 118 (0.307) | ||||||
| AG | 142 (0.442) | 174 (0.453) | |||||||
| GG | 76 (0.237) | 92 (0.240) | 0.926 | 1 | 0.699 | 1 | 0.93 | 1 | |
| rs16924159 | GG | 104 (0.324) | 151 (0.393) | ||||||
| GA | 152 (0.474) | 184 (0.479) | |||||||
| AA | 65 (0.202) | 49 (0.128) | 0.006 | 0.03 | 0.057 | 0.285 | 0.007 | 0.035 | |
| rs16924171 | AA | 91 (0.283) | 115 (0.299) | ||||||
| AT | 159 (0.495) | 183 (0.477) | |||||||
| TT | 71 (0.221) | 86 (0.224) | 0.867 | 1 | 0.93 | 1 | 0.642 | 1 | |
| rs1929992 | AA | 67 (0.209) | 98 (0.255) | ||||||
| AG | 172 (0.536) | 185 (0.482) | |||||||
| GG | 82 (0.255) | 101 (0.263) | 0.264 | 1 | 0.819 | 1 | 0.147 | 0.735 | |
| rs1332290 | AA | 58 (0.181) | 71 (0.185) | ||||||
| AC | 148 (0.461) | 189 (0.492) | |||||||
| CC | 115 (0.358) | 124 (0.323) | 0.602 | 0.324 | 0.886 | ||||
P versus controls; P value by χ2 test. Corrected P = P value × 5 (the number of genotyped SNPs).
Table 3. Associated between rs16924159 variant with risk for idiopathic recurrent miscarriage after adjustment for Binary Logistic Regression.
| P value | Wald value | OR | 95% CI | |
|---|---|---|---|---|
| Additive | 0.017 | 5.69 | 2.037 | 1.135–3.656 |
| Dominant | 0.288 | 1.127 | 1.244 | 0.831–1.862 |
| Recessive | 0.012 | 6.33 | 1.975 | 1.161–3.357 |
| Homozygous | 0.014 | 5.87 | 1.926 | 1.232–3.012 |
| Heterozygous | 0.299 | 1.036 | 1.199 | 0.863–1.667 |
Adjustments for menarche age, irregular menstrual history and number of pregnancies by Binary Logistic Regression; OR, odds ratio; CI, confidence interval.
Mean serum IL-33 levels
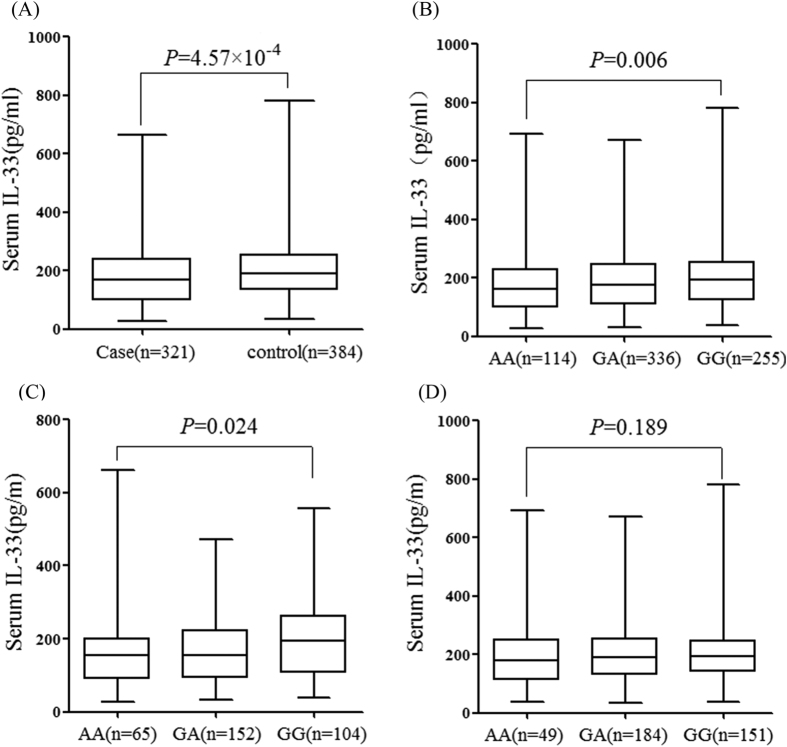
As shown in Fig. 1A, serum IL-33 levels are significantly lower in RM cases than in control (173.51 ± 94.12 versus 200.97 ± 110.06 pg/ml, P = 4.57 × 10−4). As shown in Fig. 1B, there are lower levels of serum IL-33 in rs16924159 homozygous mutant (AA) than homozygous wild-type (GG) in this study population, including cases and control groups (172.18 ± 103.01 versus 205.82 ± 119.01 pg/ml, P = 0.006). However, There is no significantly difference in homozygous wild-type (GG), compared with homozygous wild-type (GG) and heterozygous (A/G) genotype carriers (172.18 ± 103.01 versus 191.61 ± 103.91 pg/ml, P = 0.067).
Figure 1. Comparison of serum IL-33 levels among IL-33 genotypes.
(A) Serum IL-33 concentrations in cases and controls. IL-33 levels were significantly lower in patients with RM than in controls. (B) Serum IL-33 levels according to rs16924159 genotypes among cases and controls. Women with AA genotype showed significantly lower IL-33 levels than GG genotypes. (C) Serum IL-33 levels according to rs16924159 genotypes among cases. Idiopathic RM patients with the AA genotype showed significantly lower serum IL-33 levels than those with GG genotypes. (D) Serum IL-33 levels according to rs16924159 genotypes among controls. No statistical difference of serum IL-33 levels was found between women with those with GG genotypes.
Moreover, there is a significant decline in serum IL-33 levels according to rs16924159 genotypes in RM cohorts (AA versus GG, 160.57 ± 100.03 versus 196.90 ± 102.12 pg/ml, P = 0.024) (Fig. 1C). However, no significantly difference is observed in serum IL-33 levels according to rs16924159 genotypes among controls (AA versus GG, 187.57 ± 105.90 versus 211.96 ± 129.34 pg/ml, P = 0.189) (Fig. 1D).
Haplotype analysis
Five-locus (IL-33 rs10435816, rs16924159, rs16924171, rs1929992, rs1332290) IL-33 haplotypes were constructed based on the prevalence of individual SNPs and linkage disequilibrium between them. Nine haplotypes were found to be common and were included in subsequent analysis. No haplotypes exhibits significant association with RM (Table 4).
Table 4. IL-33 Haplotypes.
| Haplotypea | Totalb | Casesb | Controlb | P value |
|---|---|---|---|---|
| GGTAC | 0.376 | 0.389 | 0.36 | 0.2637 |
| AGAGA | 0.269 | 0.272 | 0.266 | 0.8109 |
| AAAGA | 0.187 | 0.182 | 0.192 | 0.6504 |
| AATAC | 0.034 | 0.031 | 0.037 | 0.5395 |
| AAAAC | 0.023 | 0.022 | 0.024 | 0.8519 |
| GAAGA | 0.017 | 0.017 | 0.017 | 0.9973 |
| GGAAC | 0.016 | 0.013 | 0.02 | 0.276 |
| AGTAC | 0.015 | 0.016 | 0.015 | 0.8443 |
| AGAGC | 0.012 | 0.011 | 0.013 | 0.7764 |
Note: aIL-33 rs10435816/rs16924159/rs16924171/rs1929992/rs1332290 haplotypes.
bHaplotype frequency.
Discussion
The role of IL-33 polymorphisms in idiopathic RM pathogenesis remains unknown. In this case–control study, we selected five tag SNPs in IL-33 to investigate the association of IL-33 polymorphisms with idiopathic RM. We found that a tag SNP rs16924159, located in the regulatory region of IL-33, was associated with idiopathic RM. The homozygous mutant (AA) genotype of rs16924159 is risk factor in idiopathic RM patients. This is the first study to assess the contribution of IL-33 polymorphisms in hereditary susceptibility of idiopathic RM.
We found that serum IL-33 levels are significantly lower in idiopathic RM cases than in control. Recently, it has been reported that activation of the IL-33/ST2 pathway in human endometrial stromal cells (HESCs) is critical for a successful pregnancy23. Autocrine IL-33 signaling in HESCs facilitates embryo implantation in mice23. However, IL-33 knockdown in decidualizing HESCs impairs embryo implantation23. Women experiencing with RM have deficit in Treg cell number and function, compared with normal pregnant women25. These studies suggest that high IL-33 levels are critical in early pregnancy and impact the outcome of subsequent pregnancies23. However, compared with normal control pregnancies, there is a significant increase in serum IL-33 levels at six weeks’ gestation in patients who destined to abort24. The biological significance of a rise of serum IL-33 levels is uncertain in pregnant woman destined to miscarry. As IL-33 is a crucial role in promoting Tregs cells differentiation and adaptation to the inflammatory environment. One possible explanation is that the pregnancy is failing due to sensitization of the maternal immune system, and that the rise is a compensatory response to attempting to rescue the pregnancy24. Although IL-33 plays an exact role in embryo-maternal interactions, especially during early stages of implantation, our data support the notion that miscarriage may be attributed, at least in part, to low level of IL-33 expression and secretion. In this study, serum IL-33 measurements were performed in non pregnant women. We did not compare serum IL-33 levels between pre-pregnancy with post-pregnancy in the same case. In later study, more experiments are also needed to elucidate the association of serum IL-33 levels between pre-pregnancy with post-pregnancy in idiopathic recurrent miscarriage patients.
Our data also indicate that there are lower levels of serum IL-33 in rs16924159 homozygous mutant (AA) than homozygous wild-type (GG) in this study population, including cases and control groups. Moreover, there is a progressive decline in serum IL-33 levels according to rs16924159 genotypes in RM cohorts. rs16924159 variant, located in the regulatory region of IL-33, was associated with reduced serum IL-33 levels. This may be linked with defective transcriptional processing of IL-33 mRNA.
To our knowledge, we first found that genetic variant in IL-33 is associated with idiopathic RM, and the AA genotype of rs16924159 is associated with lower serum IL-33 levels. However, our study has some limitations. It was performed only in Chinese Han population. Additional more large-scale population studies from different ethnic backgrounds are needed to confirm the association of IL-33 variant rs16924159 with RM. Functional experiments are also needed to elucidate the association.
In conclusion, our study demonstrated that a tag SNP rs16924159 was associated with idiopathic RM and serum IL-33 levels are significantly lower in idiopathic RM cases than in control. There are lower levels of serum IL-33 in rs16924159 homozygous mutant (AA) than homozygous wild-type (GG) in this study population. It may be concluded that IL-33 acts as a risk factor in the pathogenesis of idiopathic RM.
Additional Information
How to cite this article: Yue, J. et al. Genetic variant in IL-33 is associated with idiopathic recurrent miscarriage in Chinese Han population. Sci. Rep. 6, 23806; doi: 10.1038/srep23806 (2016).
Acknowledgments
This work has been supported by National Natural Science Foundation of China (Grant No. 81271048, To JY.Y.), Foundation for Transfer of Scientific and Technological Achievements of Sichuan Province (Grant No. 15010118, To JY.Y.).
Footnotes
Author Contributions Conceived and designed the experiments: JY.Y. and J.Y. Performed the experiments: Y.T. Collected samples: J.Y., L.X. and T.M. Analyzed the data: JY.Y., J.Y. and Y.T. Drafted the manuscript: JY.Y. and J.Y. Revised the manuscript and gave final approval of the version to be published: JY.Y.
References
- Branch D. W., Gibson M. & Silver R. M. Clinical practice. Recurrent miscarriage. N Engl J Med 363, 1740–1747, doi: 10.1056/NEJMcp1005330 (2010). [DOI] [PubMed] [Google Scholar]
- George L., Granath F., Johansson A. L., Olander B. & Cnattingius S. Risks of repeated miscarriage. Paediatr Perinat Epidemiol 20, 119–126, doi: 10.1111/j.1365-3016.2006.00703.x (2006). [DOI] [PubMed] [Google Scholar]
- Sierra S. & Stephenson M. Genetics of recurrent pregnancy loss. Semin Reprod Med 24, 17–24, doi: 10.1055/s-2006-931797 (2006). [DOI] [PubMed] [Google Scholar]
- Rull K., Nagirnaja L. & Laan M. Genetics of recurrent miscarriage: challenges, current knowledge, future directions. Front Genet 3, 34, doi: 10.3389/fgene.2012.00034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P., Chiasson V. L., Bounds K. R. & Mitchell B. M. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front Immunol 5, 253, doi: 10.3389/fimmu.2014.00253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. K. & Kwak-Kim J. Cytokine gene polymorphisms in recurrent spontaneous abortions: a comprehensive review. Am J Reprod Immunol 60, 91–110, doi: 10.1111/j.1600-0897.2008.00602.x (2008). [DOI] [PubMed] [Google Scholar]
- Lee S. K. et al. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod 26, 2964–2971, doi: 10.1093/humrep/der301 (2011). [DOI] [PubMed] [Google Scholar]
- Aluvihare V. R., Kallikourdis M. & Betz A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5, 266–271, doi: 10.1038/ni1037 (2004). [DOI] [PubMed] [Google Scholar]
- Somerset D. A., Zheng Y., Kilby M. D., Sansom D. M. & Drayson M. T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112, 38–43, doi: 10.1111/j.1365-2567.2004.01869.x (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y. et al. Decidual and peripheral blood CD4+ CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 10, 347–353, doi: 10.1093/molehr/gah044 (2004). [DOI] [PubMed] [Google Scholar]
- Wang W. J. et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol 84, 164–170, doi: 10.1016/j.jri.2009.12.003 (2010). [DOI] [PubMed] [Google Scholar]
- Liu Y. S. et al. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol 65, 503–511, doi: 10.1111/j.1600-0897.2010.00921.x (2011). [DOI] [PubMed] [Google Scholar]
- Schiering C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568, doi: 10.1038/nature13577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancais E. et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA 111, 15502–15507, doi: 10.1073/pnas.1410700111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balato A. et al. IL-33 is regulated by TNF-alpha in normal and psoriatic skin. Arch Dermatol Res 306, 299–304, doi: 10.1007/s00403-014-1447-9 (2014). [DOI] [PubMed] [Google Scholar]
- Stojkovic S. et al. Interleukin-33 induces urokinase in human endothelial cells–possible impact on angiogenesis. J Thromb Haemost 12, 948–957, doi: 10.1111/jth.12581 (2014). [DOI] [PubMed] [Google Scholar]
- Tung H. Y., Plunkett B., Huang S. K. & Zhou Y. Murine mast cells secrete and respond to interleukin-33. J Interferon Cytokine Res 34, 141–147, doi: 10.1089/jir.2012.0066 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsen G., Balogh J., Pollheimer J., Sponheim J. & Kuchler A. M. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol 30, 227–233, doi: 10.1016/j.it.2009.03.003 (2009). [DOI] [PubMed] [Google Scholar]
- Carriere V. et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA 104, 282–287, doi: 10.1073/pnas.0606854104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490, doi: 10.1016/j.immuni.2005.09.015 (2005). [DOI] [PubMed] [Google Scholar]
- Morita H. et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity 43, 175–186, doi: 10.1016/j.immuni.2015.06.021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping V. et al. Interleukin-33 in the human placenta. J Matern Fetal Neonatal Med 26, 327–338, doi: 10.3109/14767058.2012.735724 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salker M. S. et al. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS One 7, e52252, doi: 10.1371/journal.pone.0052252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitu’u-Lino T. J., Tuohey L. & Tong S. Maternal serum interleukin-33 and soluble ST2 across early pregnancy, and their association with miscarriage. J Reprod Immunol 95, 46–49, doi: 10.1016/j.jri.2012.06.003 (2012). [DOI] [PubMed] [Google Scholar]
- La Rocca C., Carbone F., Longobardi S. & Matarese G. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 162, 41–48 (2014). [DOI] [PubMed] [Google Scholar]