Abstract
upa20 induces cell enlargement and hypertrophy development. In our research, overexpression of SlUPA-like, orthologous to upa20, severely affected the growth of vegetative and reproductive tissues. Wilted leaves curled upwardly and sterile flowers were found in transgenic lines. Through anatomical analysis, palisade and spongy tissues showed fluffy and hypertrophic development in transgenic plants. Gene expression analysis showed that GA responsive, biosynthetic and signal transduction genes (e.g. GAST1, SlGA20OXs, SlGA3OXs, SlGID1s, and SlPREs) were significantly upregulated, indicating that GA response is stimulated by overproduction of SlUPA-like. Furthermore, SlUPA-like was strongly induced by exogenous JA and wounding. Decreased expression of PI-I and induced expression of SlJAZs (including SlJAZ2, SlJAZ10 and SlJAZ11) were observed in transgenic plants, suggesting that JA response is repressed. In addition, SlUPA-like overexpressed plant exhibited more opened stoma and higher water loss than the control when treated with dehydration stress, which was related to decreased ABA biosynthesis, signal transduction and response. Particularly, abnormal developments of transgenic plants promote the plant susceptibility to Xanthomonas campestris pv. campestris. Therefore, it is deduced from these results that SlUPA-like plays vital role in regulation of plant development and stress tolerance through GA, JA and ABA pathways.
Xanthomonas causes multiple of crop diseases, such as vascular wilts, cankers, leaf spots, fruit spots and blights. The susceptible plant often display pustules on the abaxial surface of leaf, such as the enlargement of mesophyll cell1. Palisade and spongy parenchyma cells are strongly enlarged along with the decreased starch contents in chloroplasts, which is similar to the phenotypes induced by AvrBs32. The AvrBs3 family proteins are type III effectors delivered by Xanthomonas. The N-terminal and C-terminal portion of AvrBs3 are highly conserved and their biological activities are related to the particular repetitive region. The conserved C-terminal contains potent nuclear localization signal (NLS). So, AvrBs3 like plant transcription factor locates to the nuclear and modulates the transcriptional expressions of host genes3. Marois, et al.1 reported that 22 induced and 2 repressed genes are identified, of which 13 genes are confirmed to be induced by X. campestris pv. vesicatoria (Xcv). The 13 genes were designed as upa1-13(up-regulated by AvrBs3), which show high homology to auxin-induced genes, expansion-like genes and pectate lyase genes. Among them, a few gene sequences exhibit low homology to gibberellins (GA)-responsive genes1. Therefore, the specific host-gene activation by AvrBs3 is a complex signaling network, of which the function of phytohormones is indispensable.
Phytohormones regulate plant growth and development, but their signaling pathways are also captured by pathogens to defend against plant defenses. Some effectors or phytotoxins secreted by pathogens have evolved to overcome pattern triggered immunity (PTI) of plants and establish the success infections by means of reprogramming plant hormonal pathway4. Coronatine (COR) produced by Pseudomonas syringae, suppresses the SA-mediated defense in planta5,6. Hopl1, one of the effectors of P. syringae, disrupts HSP70 (HEAT SHOCK PROTEIN70) in chloroplasts and alters the biosynthesis of SA7,8. Two effectors secreted by Xanthomonas, XopJ and XopD, suppress SA-dependent defense9,10. Effectors also use the antagonistic effects between JA and SA to colonize host plants. HopX1 from Pseudomonas syringae, promotes the degradation of jasmonate ZIM-domain (JAZ) proteins through its central ZIM domain. The JA-induced defenses are activated to counteract SA-dependent defenses11. In general, SA-mediated signaling pathway are involved in resistance to biotrophic pathogens and activation of JA response leads to defense against necrotrophic pathogens12. In addition, a balance of both JA and GA sustains the antagonistic behavior of defense and plant growth. JA is associated with activation of defense against pathogen ingress and GA promotes plant growth at the expense of defense13. GA was firstly identified in the pathogenic fungus Gibberella fujikuroi, the causal agent of excessive elongation of infected plant. Knockout or overexpression of Elongated uppermost internode (Eui) genes encoding a GA deactivating enzyme was susceptible and resistant to the infection of Xanthomonas and Magnaporthe oryzae in rice14. However, no information is obtained so far that the effector proteins secreted by pathogens directly interfere with the GA signaling pathway.
Stomata are important entry sites for pathogens ingress. Pathogens have evolved effector proteins or phytotoxins to counteract host stomatal defenses by inhibiting stomatal closure or promoting stomatal opening15. The phytotoxin COR can impede pathogen-associated molecular pattern (PAMP)-induced stomatal closure by the ABA-dependent pathway16. HopX1 from virulent Pta11528 bacteria play an important role in maintaining stomatal aperture11. Likewise, expression of HopF2 results in significantly wider stomatal aperture and insensitivity to PAMP-induced stomatal closure17. Besides, it was reported that activation of ABA signaling pathway could inhibit stomatal opening induced by pathogens. Overexpression of RCAR3, one of ABA receptors, inhibits stomatal reopening during Pst DC3000 infection18. Pst DC3000-induced stomatal closure is affected by the mutation of NCED1 gene (notabilis (not) mutant)19. Whether Xanthomonas or AvrBs3 acts on stomatal immunity still needs more evidence.
It is reported that bHLH family gene participates in regulation of GA and JA metabolisms or responses. PHYTOCHROME-INTERACTING FACTOR3-LIKE5 (PIL5) inhibits seed germination by repressing the expressions of GA biosynthetic genes in Arabidopsis20. Phytochrome interacting factors (PIFs) control hypocotyl elongation in Arabidopsis seedlings21. MYCs (MYC2, MYC3 and MYC4) act redundantly on the activation of JA response22. But, upa20, anther bHLH family gene, is not proved to be implicated in alteration of GA or JA response.
upa20 is the direct target of AvrBs3 in pepper. Transient expression of upa20 induces hypertrophy phenotypes and decreased starch contents in chloroplasts. Hypertrophy of mesophyll cells is attributed to enlargement of cell size, whereas cell division in hypertrophic leaves is not mentioned2. There are two homologous genes of upa20 in tobacco and one in tomato genomes23. Overexpression of Nt upp-L results in hyperplasia development. Palisade and spongy parenchyma cells of transgenic leaves are smaller but abundant in number compared with control. The molecular mechanism of hyperplasia development is due to acceleration of cell cycle from G1 phase to mitotic phase, leading to the promotion of cell division23. From these results, upa20 and upp-L in pepper and tobacco participate in different regulatory mechanism. The function of homologous gene is still undetermined in tomato.
Inducing visible cellular changes in transient expression of avrBs3 selects the scope of the AvrBs3-specific effects to solanaceous plants, of which the tomato is selected as our research material. The function of SlUPA-like, homologous gene to Ca upa20, was identified in tomato. Constitutive expression of SlUPA-like induced cell enlargement and reduced cell numbers in leaves of wild type tomato, Ailsa Craig (AC++). Moreover, overexpression of SlUPA-like improved GA response and susceptibility to pathogen infection together with repressed JA and ABA response.
Results
Cloning and molecular characterization of SlUPA-like
In four distinct subfamilies of Upp/Upa-like transcription factors by the phylogenetic analysis, the gene Sl AW034575 from the group 1of solanaceous plants is orthologous to Ca upa20, whereas not to Nt UPP-L23. We cloned the full-length cDNA of the Sl AW034575 gene from the AC++ by rapid amplification of cDNA ends (RACE) and named it SlUPA-like following the nomenclature of upa (upregulated by AvrBs3). The putative SlUPA-like protein has 330 amino acids with a typical bHLH domain region from amino acid 156 to 214, and an estimated molecular mass of 36.3 kD. Additionally, we searched DNA sequence of SlUPA-like from tomato genome (GenBank accession No.NP_001266190.1). Alignment of the genomic DNA and cDNA showed that the SlUPA-like contained seven exons and six introns.
The expression profiles of SlUPA-like in different tissues of wild type
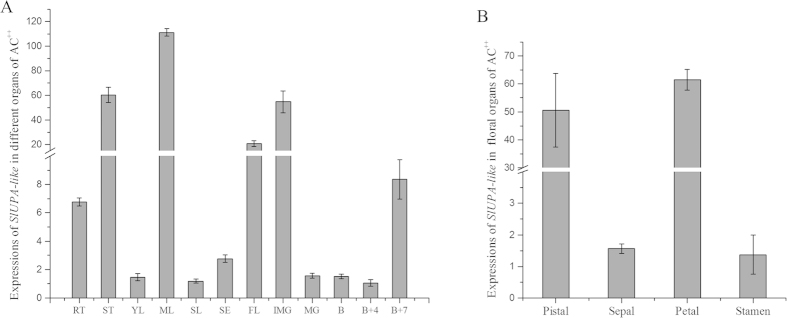
To clarify the role of SlUPA-like in plant development, its transcript accumulation in various tissues was quantified by quantitative RT-PCR. As shown in Fig. 1A, SlUPA-like was high expressed in stem, mature leaves, flowers and immature fruits, whereas low in root, young leaves, senescent leaves, sepals, and ripe fruits. Besides, pistils and petals showed high SlUPA-like accumulation compared to sepals and stamens in flower tissues (Fig. 1B). These results implied that SlUPA-like is expressed in specific organ that grows larger via cell elongation or enlargement.
Figure 1. Expression profiles of SlUPA-like in different tissues of AC++.
The expression level of SlUPA-like in different tissue of AC++ (A) and different organ of flower (B). RT, roots; ST, stems; YL, young leaves; ML, mature leaves; SL, senescent leaves; SE, sepals of flower in anthesis; FL, flower; IMG, immature green fruits; MG, mature green fruits; B, breaker fruits; B + 4, 4 d after breaker fruits; B + 7, 7 d after breaker fruits.
Overexpression of SlUPA-like induces cell enlargement by regulation of cell cycle
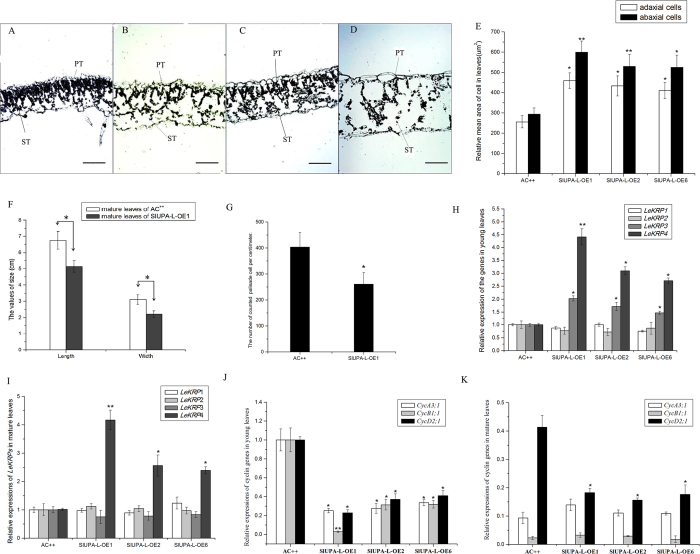
To further analyze the biological function of SlUPA-like in tomato, we generated transgenic tomato plant by overexpressing SlUPA-like. A total of 8 independent transgenic lines were obtained and three of them were selected for further characterization. From Fig. 2A–D, the leaves of SlUPA-like-overexpressed lines (SlUPA-L-OE) showed fluffy and hypertrophic development in both palisade and spongy parenchyma tissues, which leads to thickened blades. From the anatomical analysis, palisade tissue was mostly destroyed in mature leaves in contrast to relative integrity in young leaves of transgenic lines. Moreover, the mean area of adaxial and abaxial pavement cells were simultaneously enlarged in leaves of transgenic lines (Fig. 2E, Fig. S1). However, the length and width of the first leaflet were both significantly reduced compared to the control (Fig. 2F), although the ratio of length to width was almost unaffected (data not shown), indicating that cell numbers in SlUPA-L-OE leaves were decreased. Likewise, the number of palisade cell per centimeter was decreased in transgenic plants (Fig. 2G). The hypertrophy development is due to cell enlargement, which results from the abnormal expressions of E2F-response genes1,23. In Arabidopsis, over-expressions of KRP1 and KRP2 lead to a compensatory increase in cell volume in response to severely reduced cell number and expressing of KRP1 and KRP4, driven by AtML1 promoter, induces leaves curling upward24,25. In this assay, expression levels of cyclin-dependent kinase inhibitor genes KIP-RELATED PROTEIN1/INHIBITOR1 OF CDC2 KINASE (LeKRPs) were determined by RT-PCR26. Figure 2H showed that LeKRP3 and LeKRP4 were significantly induced in young leaves of transgenic plants, especially LeKRP4 that is orthologous to the AtKRP1 (Fig. S2). In mature leaves of transgenic plants, LeKRP4 level was still higher than control (Fig. 2I). Besides, expression level of three cyclin genes were investigated27. CycA3;1, CycB1;1 and CycD2;1 were significantly repressed in young leaves of transgenic plants as well as CycD2;1 in mature leaves (Fig. 2J,K), indicating that overexpression of SlUPA-like represses the progress of cell cycle and promotes cell enlargement. Therefore, these results revealed that overexpression of SlUPA-like induces cell enlargement, which is due to the repressed progress of cell cycle.
Figure 2. Cell enlargement induced by SlUPA-like and expression analysis of LeKRPs and cycle related genes in leaves of transgenic lines and control.
Light micrographs of cross sections of young leaves of AC++ (A), young leaves of SlUPA-L-OE plants (B), mature leaves of AC++ (C), and mature leaves of SlUPA-L-OE plants (D), magnification, 80×. PT, palisade tissue; ST, spongy tissue. (E).The relative average area of adaxial and abaxial cells from control and SlUPA-L-OE leaves were calculated. (F). Statistical analysis of length and width of the first leaflets. (G). Calculated number of palisade cell per centimeter from the first leaflets. Expression analysis of LeKRPs both in young leaves (H) and mature leaves (I). Expression analysis of cell cycle related genes both in young leaves (J) and mature leaves (K). Each value represents the mean ± SD of three replicates. Asterisks indicate a significant difference (**P < 0.01 and *P < 0.05) between WT and transgenic lines.
Overexpression of SlUPA-like interferes with the growth and development of plants
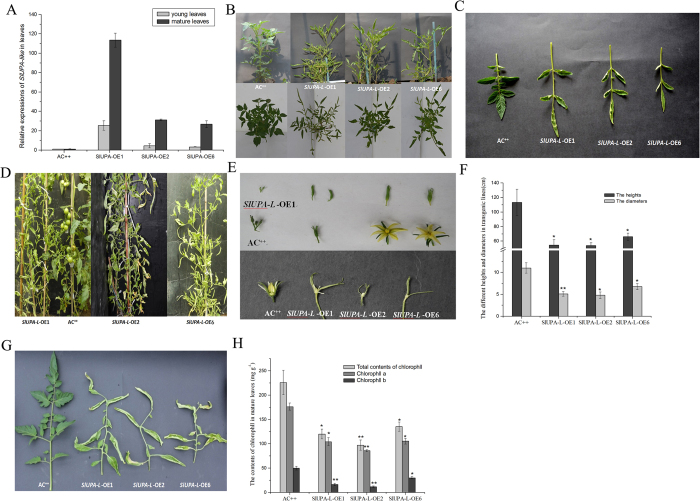
In addition to inducing the cell enlargement, overexpression of SlUPA-like severely interfered with plant growth and development. At seedling stage, the expression of SlUPA-like in transgenic lines was 2 to 25-fold higher than control (Fig. 3A). Compound leaves of SlUPA-L-OE lines bear sparse leaflets, especially fewer intercalary leaflets. All of the leaflets were curled upwardly, leading to the whole plant looked slender (Fig. 3B,C). In adult plants, the transcripts of SlUPA-like in mature leaves of transgenic lines were 20 to 120-folds higher than AC++ (Fig. 3A). We observed that SlUPA-L-OE lines cannot bloom and set up fruits (Fig. 3D). So, we could not get any seeds. When the flowers of transgenic plants were opened artificially, the petals, stamens and pistils were much smaller than control (Fig. 3E). The vegetative organs of transgenic lines also showed obvious alterations. The average height of transgenic lines was shorter than control as well as the average diameter (Fig. 3F). The average diameter of plants was measured at 10 cm above the ground. Furthermore, all mature leaves of transgenic lines were curled upwardly and wilted under the normal conditions (Fig. 3G), and the leaf vein was thicker and wider than wide type. From the cross-sectional view, the structure of vascular bundle in leaf veins was disrupted. Nearly all the cells were enlarged except a few phloem cells and even no apparent xylem cells were found (Fig. S3). Meanwhile, the contents of total chlorophyll, chlorophyll a and b were significantly reduced in mature leaves (Fig. 3H). Moreover, axillary buds furthest from the apex of plant could not form (Fig. S4), which resembles pro mutant carrying a point mutation in GRAS region28.
Figure 3. The aberrant developments showed in SlUPA-L-OE plants.
(A). Expression analysis of SlUPA-like in young and mature leaves. The phenotypes of young plants (B), young leaves (C), and adult plants (D). (E). The flowers of transgenic plants (the top half of this picture) and opened flowers artificially (the bottom half of this picture). (F). The mean height and diameter in adult plants. The phenotypes (G) and chlorophyll content (H) of mature leaves of transgenic lines and wild type. Each value represents the mean ± SD of six replicates. Asterisks indicate a significant difference (**P < 0.01 and *P < 0.05) between WT and transgenic lines.
Excessive accumulated transcripts of SlUPA-like promote GA response
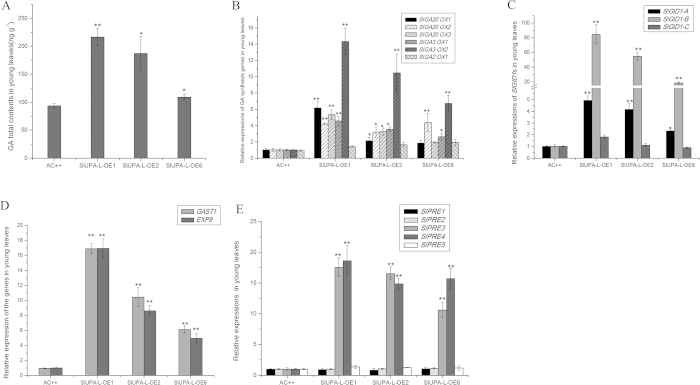
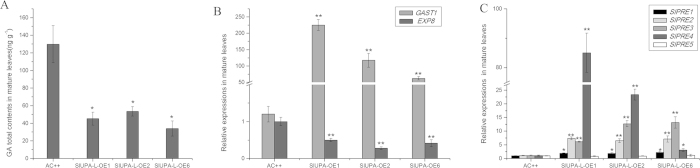
Palisade and spongy parenchyma cells were strongly enlarged, which is similar to GA-induced phenotypes28. To determine the reason of hypertrophy phenotype, the endogenous content of GA in control and transgenic lines were investigated. Figure 4A showed that total GA contents in young leaves of transgenic lines were 1.2-to 2-fold higher than control. To reveal the molecular mechanism of GA accumulation in transgenic lines, the transcripts of GA biosynthetic and catabolic genes were examined. Figure 4B showed that expressions of GA synthetic genes were significantly increased, especially SlGA3OX2, whereas no obvious change was found in GA-deactivation gene SlGA2OX1. In tomato genome, there are three putative GA receptor genes, GIBBERELLIN INSENSITIVE DWARF1s (GID1s), which were tentatively termed: SlGID1-A~C (Fig. S5). Two SlGID1s were expressed at extremely high level in young leaves of the transgenic plants, especially SlGID1-B (Fig. 4C), indicating activating GA response may be due to the over-accumulated GA receptors. The abundance of GAST1 mRNA (gibberellic acid stimulated transcript 1) is induced by GA3 treatment at transcriptional level29. EXP8 is proposed to induce cell expansion and EXP8 expression is induced by exogenous GA30. In this assay, the transcriptional levels of both GAST1 and EXP8 in young leaves were significantly increased (Fig. 4D), indicating that overexpression of SlUPA-like promotes GA response. It was reported that increased GA contents and two SlGID1s gene expressions facilitate the formation of GA-GID1-DELLA protein complex, which downregulates the repression of DELLA proteins31. Therefore, hindering inhibitory effects of DELLA proteins may be one of strategies to enhance the GA response.
Figure 4. Expression analysis of GA biosynthetic and signal transduction genes along with detection of endogenous GA contents in young leaves.
(A). Expression analysis of GA biosynthentic genes in young leaves. (B). Detection of endogenous GA contents in young leaves. Expression analysis of SlGID1s (C), GA responsive genes (D) and SlPREs (E) in young leaves. Each value represents the mean ± SD of three replicates. Asterisks indicate a significant difference (**P < 0.01 and *P < 0.05) between WT and transgenic lines.
Paclobutrazol resistance1 (PRE1), a helix-loop-helix protein, alters various aspects of gibberellin-dependent response, such as elongation of petioles32. In tomato, there are five putative PREs that were tentatively termed: SlPRE1~5 (Fig. S6). In our research, expression levels of two SlPREs in young leaves of transgenic lines were significantly higher than wild type (Fig. 4E), suggesting that over-accumulated transcripts of two SlPREs contribute to GA response.
Interestingly, endogenous GA contents in mature leaves of SlUPA-L-OE lines were reduced as well as the decreased expression of EXP8 (Fig. 5A,B), whereas GAST1 and four SlPREs (including SlPRE1-SlPRE4, particularly SlPRE4) were remarkably induced (Fig. 5B,C), suggesting that GA response was still stimulated in mature leaves.
Figure 5. Expression analysis of GA biosynthetic and signal transduction genes along with detection of endogenous GA contents in mature leaves.
(A). The detection of endogenous GA contents in mature leaves. Expression analysis of GA responsive genes (B) and SlPREs (C) in mature leaves. Each value represents the mean ± SD of three replicates. Asterisks indicate a significant difference (**P < 0.01 and *P < 0.05) between WT and transgenic lines.
Overexpression of SlUPA-like represses JA response
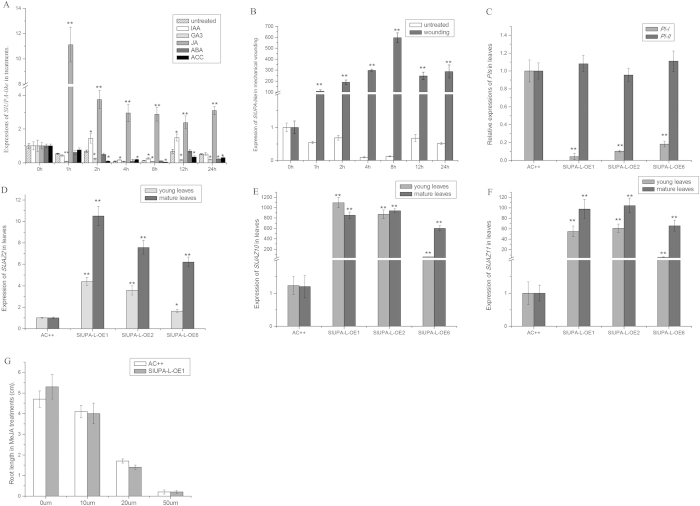
In order to characterize the function of SlUPA-like in hormonal response, its expression was investigated under five phytohormonal treatments. The transcripts of SlUPA-like were substantially accumulated within 1hour under JA treatment (Fig. 6A). JA also has an important role in wounding induction, which will rapidly increase within a few minutes33. Besides, the expressions of JAZ genes are simultaneously increased as primary responsive genes to JA treatment and mechanical wounding34. In our experiment, SlUPA-like expression was rapidly induced within 1 h and peaked at 8 h, followed by a decline but maintained at relatively high level until 24 h when treated with wounding (Fig. 6B), indicating that SlUPA-like is regarded as a primary responsive gene in response to JA. In transgenic plants, expression of PI-I was significantly repressed by 85~90% compared to control (Fig. 6C). It was reported that suppressed JA response is due to the JAZ proteins binding to MYC transcription factors35,36,37. In our experiment, SlJAZ2, SlJAZ10 and SlJAZ11 were severely induced in young and mature leaves of transgenic lines, especially SlJAZ10 and SlJAZ11 (Fig. 6D–F). Phylogenetic analysis showed that AtJAZ7, AtJAZ8, SlJAZ9, SlJAZ10 and SlJAZ11 belong to one clade and lack the conserved LPIAR motif, which makes AtJAZ8 stable in inhibition of JA response38. So, these results suggested that overexpression of SlUPA-like represses JA response by overexpressions of three SlJAZ genes. However, the inhibitory effects of three SlJAZs were limited. The length of root was repressed by exogenous MeJA in dose-dependent manner, which was similar to wild type (Fig. 6G). Therefore, the repressed JA response induced by overexpression of SlUPA-like can be overpassed by the application of exogenous JA, which may be due to the functional redundancy between SlJAZs.
Figure 6. Overexpressions of SlUPA-like repressed JA response.
Expression of SlUPA-like under phytohormonal treatments (A) and mechanical wounding treatments (B). (C). Expressions of two PIs in young and mature leaves of transgenic lines and control. Expressions of SlJAZ2 (D), SlJAZ10 (E) and SlJAZ11 (F) in young and mature leaves of transgenic lines and control. (G). Root growth treated with MeJA in AC++ and transgenic seedlings. Each value represents the mean ± SD of three replicates. Asterisks indicate a significant difference (**P < 0.01 and *P < 0.05) between WT and transgenic lines.
Overexpression of SlUPA-like stimulates stomata opening under dehydration stress
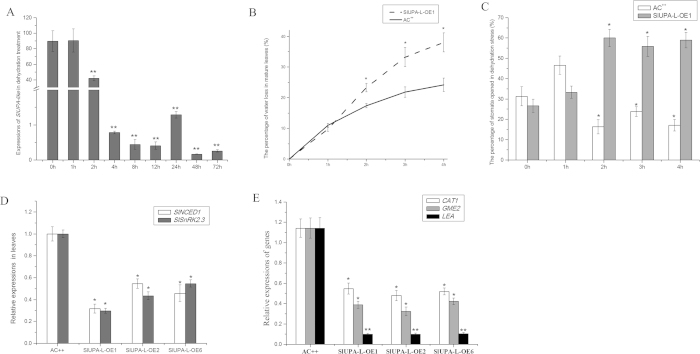
Stomata controls gas exchange between leaves and environment along with water loss during transpiration. It is known that wide stomatal aperture leads to high water loss. In wild type plants, SlUPA-like was downregulated after 4 hours of dehydration treatment (Fig. 7A). Moreover, the leaves of SlUPA-L-OE lines exhibited higher water loss than control under dehydration stress (Fig. 7B). In general, stomatal behavior is the vital index for assessing water loss. Under dehydration stress, the percentage of opened stoma in control was obviously decreased after 2 hours, whereas the percentage was increased in transgenic lines (Fig. 7C), implying that SlUPA-like promotes stomatal opening and decreases water retention under dehydration stress.
Figure 7. The dehydration treatments and related stomatal behavior in plants.
(A). Expression analysis of SlUPA-like under dehydration stress. The percent of water loss (B) and the percentage of opened stomata in mature leaves (C) of control and SlUPA-L-OE1 under dehydration stress. Expression analysis of ABA signal transduction (D) and responsive genes in mature leaves. Each value represents the mean ± SD of three replicates. Asterisks indicate a significant difference (**P < 0.01 and *P < 0.05) between WT and transgenic lines.
ABA is known for regulation of stomatal closure in response to abiotic stress and pathogen-associated molecular pattern (PAMP)-triggered immunity39. In order to discover the reason of stomatal behavior in transgenic lines, the expression levels of ABA biosynthetic, signal-transduction and responsive genes were monitored. SlNCED1 was primarily involved in ABA biosynthesis in tomato40 and activation of SnRK2 by ABA can phosphorylate downstream effectors, leading to activation of ABA response41. In mature leaves of SlUPA-L-OE plants, expression of SlNCED1 was remarkably repressed as well as SlSnRK2.3, implying that ABA biosynthesis and signal-transduction may be both suppressed (Fig. 7D). Meantime, three ABA responsive gene expressions, CAT1, GME2 and LEA, were obviously repressed by 54–92% in transgenic plants, particularly LEA, indicating that ABA response is suppressed in transgenic plants (Fig. 7E)42,43,44. So, stimulating stomatal open in transgenic lines may be due to the inhibition of ABA response.
Overexpression of SlUPA-like improves plant susceptibility to pathogens
We have shown that GA response was excessively improved, instead JA response was repressed, and the percentages of opened stoma of SlUPA-L-OE plants were increased under stress condition. Given that GAs negatively regulate disease resistance of host plants14, and deficient JA level positively regulates the susceptibility of plant to X. campestris45, and stomatal opening provides access for infections of Xcv46, we logically speculate that SlUPA-like overexpressed plants may be susceptible to pathogens. To test this hypothesis, leaflets of transgenic and control plants were inoculated with Xanthomonas campestris pv. campestris (Xcc). As expected, the leaflets of transgenic lines showed yellow spot after 3 days of inoculation and spread out most parts of the blades after 8 days, whereas the control leaflets were unaffected all the time (Fig. 8). To further determine Xcc infection, a 253 bp DNA fragment from genome of Xcc was amplified in leaves of both AC++ and transgenic lines. The PCR products were only accumulated in transgenic lines (Fig. S7). Additionally, Fig. S8 showed that SlUPA-L-OE plants were more susceptible to plant disease than AC++ when exposed to infected plants. These data demonstrated that overexpression of SlUPA-like improves the susceptibility of tomato plants to pathogens.
Figure 8.
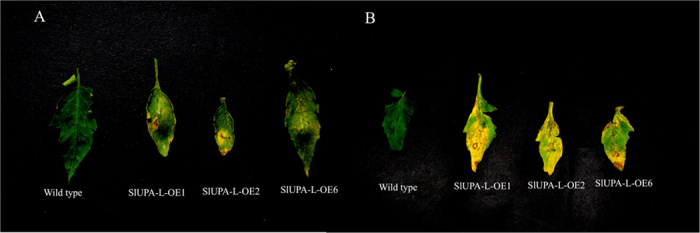
Leaflets from control and transgenic lines showed the phenotype after inoculation of Xcc for 3 days (A) and 8 days (B).
Discussion
GA-GID1-DELLA protein complex may contribute to the cell enlargement of young leaves. Hypertrophy development is attributed to cells enlargement along with decreased contents of starch2, which are similar to phenotypes of excessive GA accumulation. Loss-of-function of eui mutant shows elongated internode and reduced development of starch granule47. Experiment confirmed that overexpression of SlUPA-like induced cell enlargement and GA response (Figs 2 and 5). LeKRP genes were remarkably increased in both young and mature leaves of transgenic plants, together with the suppressed expressions of cell cycle related genes (Fig. 2), suggesting that overexpression of SlUPA-like facilitates the DNA endo-replication and inhibition of cell cycle. Meantime, both transcripts of SlGID1-A and SlGID1-B were significantly increased along with increased GA contents (Fig. 4C). Through phylogenetic analysis, AtGID1-b, SlGID1-A and SlGID1-B belong to the same clade (Fig. S5). AtGID1b-overexpressions can rescue GA response by forming the GA-GID1-DELLA protein complex31. In young leaves of transgenic plants, the key elements for formation of GA-GID1-DELLA protein complex coexisted. However, in mature leaves of transgenic lines, GA content were decreased (Fig. 5A), implying that this kind of protein complexes is probably destroyed. Therefore, the GA-GID1-DELLA protein complex probably contributes to the enhanced GA response and cell enlargement in young leaves of transgenic lines.
Expressions of GAST1 are probably induced by overexpression of SlPREs independent of GA synthesis. In the young leaves of transgenic lines, expression levels of SlPRE3 and SlPRE4 were significantly increased compared to wide type (Fig. 4E). Similarly, transcripts of four SlPREs, SlPRE1-SlPRE4, were upregulated in the mature leaves and particularly the expression of SlPRE4 was further magnified compared to young leaves (Fig. 5C). Meanwhile, GAST1 expression was both increased in young and mature leaves, especially in mature leaves (Figs 4 and 5), indicating that expression of GAST1 was sequentially induced by SlPREs. However, the expression of EXP8 was suppressed in mature leaves (Fig. 5B), implying that the GAST1 was regulated by different upstream signaling pathway. It is reported that EXP8 does not respond to ABA and water, but specially responds to GA30. By contrast, expression of GAST1 was response to both GA and ABA29. These results suggested that GAST1 locates further downstream in GA signaling pathway. Moreover, it was proved that GA stimulates cell elongation through activation of PREs, which locate downstream point of GA biosynthesis48. Therefore, continuous GA response in transgenic lines was attributed to overexpression of SlPRE, which may be independent of GA biosynthesis.
Repressed JA response may not result in female sterility of flowers in transgenic lines. Plant protease inhibitors (PIs) can be regarded as defensive proteins to minimize the adverse effects from the attack of phytophagous insects or pathogens. In our experiment, the expression of PI-I, JA induced gene, was strongly repressed in SlUPA-like-overexpressed lines (Fig. 6C), suggesting that JA response is suppressed. However, the expression of PI-II was unaffected by overexpression of SlUPA-like (Fig. 6C), although its expression can be induced by JA49. Therefore, it need further exploration that JA response can be monitored by PI-I expression.
jasmonic acid–insensitive1(jai1) mutant is defective in JA signaling of tomato, exhibiting female sterility50. jai1 mutants can set up fruit upon pollination, although the stigmas of flowers protrude from the anther cone during flower development50. That is, the anther filaments are not capable of reaching the position above the stigma at the time of flower blooming51. However, flowers in SlUPA-L-OE lines were sterile and their phenotypes were not similar to jai1 (Fig. 3E). Moreover, the sterile flowers of transgenic lines didn’t have the ability to parthenocarpy when treated with 2, 4-D (data not shown). The reason of infertility of SlUPA-L-OE lines needs further research.
SlUPA-like may directly regulate the expressions of SlJAZs. SlGIDs, SlPREs, SlJAZs and GAST1 were extremely induced by SlUPA-like (Figs 4, 5, 6). It was hypothesis that part or all of these genes contain the binding site for bHLH transcription factors within their promoters. The conserved recognition sequence of bHLH family is CANNTG (where N can be any nucleotide), in which the canonical sequence is CACGTG motif52. Within 1.5 kb upstream of the initiator-ATG, the numbers of putative binding site for bHLH were counted (Table S1). The numbers of binding sites in promoters of SlGIDs and SlPREs were not accordance with their expression levels, indicating that overexpression of two SlGIDs and four SlPREs may be indirectly regulated by SlUPA-like. The expression patterns of three SlJAZs (SlJAZ2, SlJAZ10, and SlJAZ11) largely accorded with the distribution of binding sites in their promoters (Table S1), revealing that these genes may be direct targets of SlUPA-like. Besides, the expression of SlJAZ9, homolog to SlJAZ11, is not induced by SlUPA-like. Previous works proved that G-rich DNA element is capable of formatting secondary structure and functions as repressor elements53,54. Within the promoter of SlJAZ9, Poly (G) motif from −1453 to −1733 bp makes it hard to be bonded by transcription factors. In addition, the promoter of GAST1 contained no classical binding site, implied that excessive expression of GAST1 may be mediated by other factors. Nevertheless, all these results from bioinformatics analysis were not sufficient to make conclusion, which need to be validated by chromatin immunoprecitation.
SlUPA-like may participate in the crosstalk between GA and JA response. In transgenic plants, expression levels of three SlJAZs were extremely up-regulated in transgenic leaves along with repressed JA response (Fig. 6). Meantime, SlPREs were significantly induced in transgenic plants with the activation of GA response. These data suggested that SlUPA-like may mediate the crosstalk of GA and JA response. Overexpression of AtJAZs has GA-hypersensitivity phenotypes, particularly AtJAZ9 whose overaccumulation could be resistant to exogenous paclobutrazol55. In our research, extremely upregulated expressions of SlJAZ9/10 (homologous to AtJAZ7/AtJAZ8)38 were found in transgenic plants. Moreover, overexpression of SlPREs shows GA response (Figs 4 and 5). So, stimulated GA response in transgenic plant may be due to the over-accumulation of both JAZ and PRE proteins. It was reported that JAZ9 interferes with DELLA-PIF3 interactions and competes with DELLA targets55. So, overexpression of SlJAZ9/10 may destroy the inhibitory effect of DELLA proteins. However, DELLA proteins don’t interact with AtJAZ2, AtJAZ7 and AtJAZ8 in vitro55. So, whether the interactions between three SlJAZs and DELLA proteins mediated by other factors still needs to be tested. In addition, ILI1 and PRE1 inactivate the inhibitory function of IBH1 (another bHLH protein) through hetero-dimerization56. Therefore, it was possible that SlUPA-like protein is another target of PREs in tomato.
Stimulating stomatal opening may be mediated by ABA response in transgenic lines. Locating the epidermis of leaves, stoma is primary organ to lose water during transpiration and is also the key channel for pathogen infection. Plant have evolved stomatal defense to fight against the infection of pathogens. Certainly, plant pathogens secreted toxin or effectors to counteract stomatal defense to promote the stomatal reopening16. In this assay, when treated with severe water loss, most of stomatal pores closed in control plant, whereas more stoma in transgenic plants reopened (Fig. 7C). Meanwhile, expressions of ABA biosynthetic, signal-transduction and responsive genes were significantly suppressed in transgenic lines (Fig. 7D,E). To some extent, the stomatal behavior under infection of pathogen could be reproduced through dehydration treatments16. Thus, stimulated stomatal opening by overexpression of SlUPA-like may be mediated by suppressed ABA response.
Aberrant development facilitates the infections of pathogens in transgenic lines. Increased GA response, suppressed JA response and stimulated stomatal opening give us clues why overexpression of SlUPA-like leads to aberrant development (including curled and wilted leaves, sterile flowers and so on). It was reported that alterations of hormonal pathways improved plants susceptibility to Xanthomonas, which infection was mediated by stomatal behaviors16. Plant pathogens firstly gain entry into plant tissue via wounds or natural openings such as stomata. In order to counteract stomatal defense of host plant, pathogens can evade host immunity by suppressing the stomatal closure16. For these reasons, stomatal opening promoted by overexpression of SlUPA-like together with reprogrammed GA and JA response enhanced infection efficiency (Fig. 8), although the Xcc is not specific pathogen for solanaceous plants. This study revealed that SlUPA-like can be regard as a landmark gene in monitoring the disease tolerance of plants.
Methods
Plant Materials and Growth Conditions
In our experiment, Solanum lycopersicum Mill. cv. Ailsa Craig (AC++), a near-isogenic tomato line, was used as model plant grown in greenhouse (16-h-day/8-h-night cycle, 25 °C/18 °C day/night temperature, 80% humidity, and 250 μmol m−2s−2 light intensity). Plants of the first generation (T0) came from tissue culture. Tissue culture plantlets from transgenic lines and control with no construct were planted in greenhouse. The transgenic and control plants were synchronously planted in greenhouse. When the height of control plants reached 20–30 cm, the young leaves from middle part of plant were collected. Each line included 3–5 plants. When control plants set up fruits, mature leaves of 8 independent transgenic lines and control were harvested from top, middle and low part of each plant, and immediately frozen with liquid nitrogen, mixed, and stored at −80 °C until RNA extraction. The statistical analyses were performed. Each line included equal or greater than three plants.
Sequence, structure and phylogenetic analysis
The exons and introns of genomic DNA were analyzed by GENESCAN (http://genes.mit.edu/GENSCAN.html). The theoretical molecular weight was calculated with the ExPASy compute Mw tool (http://expasy.org/tools/protparam.html). Multiple sequence alignment was conducted using the ClustalX 1.81 and DNAMAN 5.2.2 programs.
Plant treatments
(a). Phytohormones treatments. 35-day-old AC++ seedlings were used for treatments. Tomato seedlings were sprayed with different concentration of phytohormones (50 uM IAA, 50 uM GA3, 50 uM MeJA, 100 uM ABA and 50 uM ACC)57 respectively until the water dripped, and then immediately enclosed in transparent bags with some small holes (about 0.5 cm in diameter) on the surface. After treatments for 0, 1, 2, 4, 8, 12 and 24 hours, the leaves of seedlings were harvested for analysis. All the samples were immediately frozen and stored at −80 °C until RNA extraction. Each treatment repeated with two times. (b). Dehydration treatments. 35-day-old AC++ seedlings were carefully pulled out from soil, washed with water and left on a piece of dry filter paper with 60% humidity at 25 ± 1 °C. After 0, 1, 2, 4, 8, 12, 24, 48 and 72 hours, the leaves were collected, frozen in liquid nitrogen and stored at −80 °C. The transgenic plants and wild type were planted in greenhouse and each line contains 3–5 plants. When the control set up fruits, mature leaves of transgenic lines and control were harvested from top, middle and lower part of each plant, and left on a piece of dry filter paper with 60% humidity at 25 ± 1 °C. After different hours, the statistical analysis of opened stoma in mature leaves was performed. (c). Mechanical wounding treatments. 35-day-old AC++ seedlings were used for mechanical wounding treatment. The leaflets were cut with scalpels into small pieces and left on a piece of wetted filter paper in sealed pots for 0, 1, 2, 4, 8, 12 and 24 hours, then the pieces of leaves were collected, respectively.
Construction of SlUPA-like overexpression vector and plant transformation
Total RNA of AC++ leaf was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. Then, 0.5 μg of total RNA was used to synthesize first-strand cDNA through reverse transcription-PCR using Moloney murine leukemia virus reverse transcriptase (Takara) with tailed oligo(dT)18 primer. 2 μL of cDNA was used to clone the full-length cDNA with primers of SlUPA-like-F and dT-R through high-fidelity PCR (Prime START mix DNA polymerase; Takara). The amplified products were tailed by using the DNA-Tailing kit (Takara) and linked with pMD18-T vector (Takara). Positive clones were picked out via Escherichia coli transformation and confirmed by sequencing (Invitrogen). Then, a 993 bp specific DNA fragment of SlUPA-like was amplified with primers SlUPA-like-F and SlUPA-like-R, which had been tailed with BamHI and SacI restriction sites at the 5′ end, and linked into the pBI121 plasmid in the sense orientation to form RNA expression unit. Subsequently, the generated binary plasmid was transformed into Agrobacterium tumefaciens LBA4404 strain, following the previously described protocols58. Positive transgenic plants were selected on kanamycin medium and screened by polymerase chain reaction analysis with primers NPTII-F and NPTII-R. Then, T0 plants from each transgenic line were propagated through apex culture in vitro propagation.
The percentages of open stomata under dehydration stress
Adult plants of AC++ and SlUPA-like overexpressed lines were planted in field. At 10:00 am, the mature leaves were sampled at the same position from the apex of compound leaves and immediately abaxial stomata of detached leaves were inspected with microscopy. The percentage of open stomata was calculated. Then, the detached leaves were placed in wetted filter paper. After 1, 2, 3 and 4 hours of dehydration treatments, the percentage of opened stomata was calculated, respectively. All experiments were repeated 3 to 5 times.
The root growth inhibition by MeJA
The aseptic axirllay buds with approximately same size from AC++ and transgenic lines were planted in rooting MS medium. The different concentration of methyl jasmonate (MeJA), 0 uM, 10 uM, 20 uM and 50 uM, was added into rooting medium, respectively. After 25 days of cultivation, the lengths of roots were calculated. Three biological replicates were used for each treatment.
Quantitative real-time PCR analysis
Total RNAs from different tissues of AC++ and transgenic lines were extracted. Quantitative real-time PCR was performed using the SYBR Premix Ex Taq II kit (Promega) in a 10 μL total sample volume (5.0 μL of 2 × SYBR Premix Ex Taq, 0.5 μL of primers, 1.0 μL of cDNA, and 3.5 μL of deionized water) under the following conditions: 95 °C for 2 min, followed by 40 cycles of 98 °C for 15 s and annealing temperature for 30 s. To remove the interference of genomic DNA and the template from the environment, no-template control and no-reverse transcription control experiments were performed. Additionally, three replications for each sample were used, and standard curves were run simultaneously. The tomato SlCAC and SlEF1a genes were used as internal standards59. Primers used for quantitative RT-PCR are shown in supplementary Table S2.
Microscopy
The detached leaves sampled from AC++ and transgenic lines were immediately fixed by formalin–acetic acid–alcohol fixative (FAA). Then, steps of dehydration, fixation, sectioning and dewaxing were operated on the fixed materials. Finally, the treated materials were observed under the microscope.
Chlorophyll quantification in tomato leaves
For chlorophyll measurement, leaves were sampled from control and transgenic plants at the same height and simultaneously weighed. Samples were grinded into powder with liquid nitrogen, extracted with 10 mL 80% aqueous acetone (v/v). The extracts were centrifuged at 4, 000 g for 5 min and the absorbances of the supernatant were measured at 646 and 663 nm, using a lambda 900 scanning spectrophotometer (PerkinElmer). Total chlorophyll contents were calculated by the following equation: Chl (mg/mL) = 20.29(OD645) + 8.02(OD663), Chla (mg/mL) = 12.7(OD663)−2.69(OD645), and Chlb (mg/mL) = 22.9 (OD645)−4.677 (OD663). OD represents the absorbance, while 645 and 663 represent the wavelength. Chl, Chla and Chlb represent total chlorophyll, chlorophyll a and chlorophyll b, respectively.
Quantification of Gas
In this assay, quantity of endogenous GAs was determined by GA ELISA Kit (Sangon Biotech, Shanghai, China). The fresh samples including young and mature leaves from AC++ and SlUPA-L-OE lines were grinded in liquid nitrogen. About 1 g treated sample was extracted with 80% methanol and centrifuged at 1000 g for 15 min. The supernatant was partitioned against ethyl acetate and purified by Sephadex chromatography and C18 cartridges60. Standards or samples were then added to the appropriate microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for GA and Avidin conjugated to Horseradish Peroxidase (HRP) was added to each microplate well and incubated. Then a TMB substrate solution was added to each well. Only those wells that contain GA, biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction was terminated by the addition of a sulphuric acid solution and the color change was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm by Multimode Reader. The concentration of GA in the samples was determined from the standard curve.
Infection experiments
In consideration of infectivity of Xanthomonas campestris pv. campestris (Xcc), mechanical damages were caused on leaflets of AC++ and transgenic lines by toothpick. Immediately, 1 ul inoculums were placed into wounds. Then, the leaflets were sealed by one transparent plastic bag. A few days later, bacterial growth in leaflets was monitored. After 3 and 8 days of inoculation, the inoculated leaves were sampled for DNA extraction by CTAB. A 253-bp genomic DNA fragment of Xcc was amplified with primers (Table S2). A 253-bp fragment of M. oryzae 28S rDNA was amplified from fungal genomic DNA using the primers 5′–TCGCCTACCGAGAAATCCC–3′ and 5′–CCGAACCCTTGACCAGCAT–3′, to monitor the infections of Xcc and the products were detected by agarose gel electrophoresis.
Additional Information
How to cite this article: Cui, B. et al. Overexpression of SlUPA-like induces cell enlargement, aberrant development and low stress tolerance through phytohormonal pathways in tomato. Sci. Rep. 6, 23818; doi: 10.1038/srep23818 (2016).
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (no. 31572129), and the Natural Science Foundation of Chongqing of China (cstc2015jcyjA80026), and the Fundamental Research Funds for the Central Universities (No. 106112015CDJZR235504).
Footnotes
Author Contributions G.C. and Z.H. designed and managed the research work and improved the manuscript. B.C., J.H., Y.Z., W.Y., Z.Z. and Y.F. performed the experiments. B.C. wrote the manuscript.
References
- Marois E., Van den Ackerveken G. & Bonas U. The Xanthomonas type III effector AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol. Plant Microbe In. 15, 637–646 (2002). [DOI] [PubMed] [Google Scholar]
- Kay S., Hahn S., Marois E., Hause G. & Bonas U. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 318, 648–651 (2007). [DOI] [PubMed] [Google Scholar]
- Gurlebeck D., Thieme F. & Bonas U. Type III effector proteins from the plant pathogen Xanthomonas and their role in the interaction with the host plant. J. Plant Physiol. 163, 233–255 (2006). [DOI] [PubMed] [Google Scholar]
- Dou D. & Zhou J. M. Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe 12, 484–495 (2012). [DOI] [PubMed] [Google Scholar]
- Uppalapati S. R. et al. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe In. 20, 955–965 (2007). [DOI] [PubMed] [Google Scholar]
- Zhao Y. et al. Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 36, 485–499 (2003). [DOI] [PubMed] [Google Scholar]
- Jelenska J. et al. A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr. Biol. 17, 499–508 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenska J., van Hal J. A. & Greenberg J. T. Pseudomonas syringae hijacks plant stress chaperone machinery for virulence. Proc. Natl. Acad. Sci. USA 107, 13177–13182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun S., Bartetzko V. & Bornke F. The Xanthomonas campestris type III effector XopJ targets the host cell proteasome to suppress salicylic acid mediated plant defence. PLoS Pathog. 9, e1003427 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonne J. et al. The Xanthomonas type III effector XopD targets the Arabidopsis transcription factor MYB30 to suppress plant defense. Plant Cell 23, 3498–3511 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gimenez-Ibanez S. et al. The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. Plos Biol. 12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell J. M. & Dangl J. L. Signal transduction in the plant immune response. Trends in Biochem. Sci. 25, 79–82 (2000). [DOI] [PubMed] [Google Scholar]
- Navarro L. et al. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 18, 650–655 (2008). [DOI] [PubMed] [Google Scholar]
- Yang D. L. et al. Altered disease development in the eui mutants and Eui overexpressors indicates that gibberellins negatively regulate rice basal disease resistance. Mol. Plant. 1, 528–537 (2008). [DOI] [PubMed] [Google Scholar]
- Arnaud D. & Hwang I. A sophisticated network of signaling pathways tegulates stomatal defenses to bacterial pathogens. Mol. Plant 8, 566–581 (2015). [DOI] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K. & He S. Y. Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980 (2006). [DOI] [PubMed] [Google Scholar]
- Hurley B. et al. The Pseudomonas syringae type III effector HopF2 suppresses Arabidopsis stomatal immunity. PLos One 9, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. W., Luan S. & Lee S. C. A prominent role for RCAR3-mediated ABA signaling in response to Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis. Plant Cell Physiol. 55, 1691–1703 (2014). [DOI] [PubMed] [Google Scholar]
- Du M. M. et al. Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell 26, 3167–3184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. et al. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19, 1192–1208 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. J. et al. Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J. Plant Physiol. 168, 1771–1779 (2011). [DOI] [PubMed] [Google Scholar]
- Fernandez-Calvo P. et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23, 701–715 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukhovitskaya N. I. et al. An RNA virus-encoded zinc-finger protein acts as a plant transcription factor and induces a regulator of cell size and proliferation in two tobacco species. Plant Cell 25, 960–973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L. et al. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13, 1653–1667 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis S. M. & Torii K. U. Autonomy of cell proliferation and developmental programs during Arabidopsis aboveground organ morphogenesis. Dev. Biol. 304, 367–381 (2007). [DOI] [PubMed] [Google Scholar]
- Bisbis B. et al. Cyclin-dependent kinase (CDK) inhibitors regulate the CDK-cyclin complex activities in endoreduplicating cells of developing tomato fruit. J. Biol. Chem. 281, 7374–7383 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang T. et al. Genome-wide analysis of the cyclin gene family in tomato. Int. J. Mol. Sci. 15, 120–140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski S. et al. PROCERA encodes a DELLA protein that mediates control of dissected leaf form in tomato. Plant J. 56, 603–612 (2008). [DOI] [PubMed] [Google Scholar]
- Shi L. F. & Olszewski N. E. Gibberellin and abscisic acid regulate GAST1 expression at the level of transcription. Plant Mol. Biol. 38, 1053–1060 (1998). [DOI] [PubMed] [Google Scholar]
- Chen F., Dahal P. & Bradford K. J. Two tomato expansin genes show divergent expression and localization in embryos during seed development and germination. Plant Physiol. 127, 928–936 (2001). [PMC free article] [PubMed] [Google Scholar]
- Ariizumi T., Murase K., Sun T. P. & Steber C. M. Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1. Plant Cell 20, 2447–2459 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. et al. Overexpression of PRE1 and its homologous genes activates Gibberellin-dependent responses in Arabidopsis thaliana. Plant Cell Physiol. 47, 591–600 (2006). [DOI] [PubMed] [Google Scholar]
- Glauser G. et al. Spatial and temporal dynamics of jasmonate synthesis and accumulation in Arabidopsis in response to wounding. J. Biol. Chem. 283, 16400–16407 (2008). [DOI] [PubMed] [Google Scholar]
- Chung H. S. et al. Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 146, 952–964 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A. et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–U664 (2007). [DOI] [PubMed] [Google Scholar]
- Thines B. et al. JAZ repressor proteins are targets of the SCFCO11 complex during jasmonate signalling. Nature 448, 661–U662 (2007). [DOI] [PubMed] [Google Scholar]
- Yan Y. X. et al. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19, 2470–2483 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. Q., Jiang H. L. & Li C. Y. Systemin/Jasmonate-mediated systemic defense signaling in tomato. Mol. Plant 4, 607–615 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang W., He S. Y. & Assmann S. M. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 56, 984–996 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K. et al. SlNCED1 and SlCYP707A2: key genes involved in ABA metabolism during tomato fruit ripening. J. Exp. Bot. 65, 5243–5255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. et al. Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 44, 939–949 (2005). [DOI] [PubMed] [Google Scholar]
- Guan L. Q. M., Zhao J. & Scandalios J. G. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 22, 87–95 (2000). [DOI] [PubMed] [Google Scholar]
- Conklin P. L. & Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 27, 959–970 (2004). [Google Scholar]
- Zegzouti H. et al. ER5, a tomato cDNA encoding an ethylene-responsive LEA-like protein: characterization and expression in response to drought, ABA and wounding. Plant Mol. Biol. 35, 847–854 (1997). [DOI] [PubMed] [Google Scholar]
- Thaler J. S., Owen B. & Higgins V. J. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol. 135, 530–538 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L. J. & Volin R. B. Role of stomatal opening and frequency on infection of Lycopersicon spp by Xanthomonas campestris pv vesicatoria. Phytopathology 77, 1311–1317 (1987). [Google Scholar]
- Zhang Y. Y. et al. Gibberellin homeostasis and plant height control by EUI and a role for gibberellin in root gravity responses in rice. Cell Res. 18, 412–421 (2008). [DOI] [PubMed] [Google Scholar]
- Bai M. Y., Fan M., Oh E. & Wang Z. Y. A triple helix-loop-helix/basic helix-loop-helix cascade controls cell elongation downstream of multiple hormonal and environmental signaling pathways in Arabidopsis. Plant Cell 24, 4917–4929 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Johnson R. R. & Ryan C. A. regulation of expression of proteinase-inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol. 98, 995–1002 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16, 783–783 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandaokar A. et al. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 46, 984–1008 (2006). [DOI] [PubMed] [Google Scholar]
- Chaudhary J. & Skinner M. K. Basic helix-loop-helix proteins can act at the E-box within the serum response element of the c-fos promoter to influence hormone-induced promoter activation in Sertoli cells. Mol. Endocrinol. 13, 774–786 (1999). [DOI] [PubMed] [Google Scholar]
- Siddiqui-Jain A., Grand C. L., Bearss D. J. & Hurley L. H. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl. Acad. Sci. USA 99, 11593–11598 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Lipsett M. N. & Davies D. R. Helix formation by guanylic acid. Proc. Natl. Acad. Sci. USA 48, 2013-& (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. L. et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 109, E1192–E1200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Y. et al. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21, 3767–3780 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. et al. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 39, 863–876 (2004). [DOI] [PubMed] [Google Scholar]
- Chen G. et al. Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol. 136, 2641–2651 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Rodriguez M., Borges A. A., Borges-Perez A. & Perez J. A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 8, 131 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrani J. C., Sanjuan R., Ruiz-Rivero O., Fos M. & Garcia-Martinez J. L. Gibberellin regulation of fruit set and growth in tomato. Plant Physiol. 145, 246–257 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.