Abstract
Background. Medicinal plants offer cheaper and safer treatment options to current diabetic drugs. The present study evaluated the effect of aqueous root bark extract of Zanthoxylum chalybeum on oral glucose tolerance and pancreas histopathology in alloxanized rats. Method. Diabetes was induced in rats by administration of alloxan monohydrate. Root extract of Z. chalybeum was administered to rats at 200 and 400 mg/kg BW daily for 28 days. Blood glucose was measured by glucometer and pancreatic histopathology evaluated microscopically. Results. Initial increase was observed in blood glucose of the rats after oral administration of glucose from time zero. Two hours after treatment with Z. chalybeum, a significant reduction in blood glucose was observed within treatment groups (p < 0.05) compared to 0.5 hr and 1 hr. There was no significant difference between treatment group receiving 400mg/Kg BW extract and the normal groups (p = 0.27), implying that the former group recovered and were able to regulate their blood sugar, possibly via uptake of glucose into cells. The reversal in pancreatic histopathology further supports the protective effect of Z. chalybeum extract towards diabetic damage. Conclusion. Extract of Z. chalybeum is effective in controlling blood glucose in diabetes and protecting pancreatic tissues from diabetic damage.
1. Introduction
Diabetes mellitus is a group of metabolic diseases characterized by elevated blood glucose levels resulting from defects in insulin secretion, insulin action, or both [1]. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels [2]. Diabetes is a major risk factor for the development of cardiovascular disease. More than 70% of deaths in diabetic patients are due to vascular disease. One of the greatest factors in the development and progression of the complications of diabetes mellitus is hyperglycemia [3]. Hyperglycemia contributes to majority of diabetic complications by altering vascular cellular metabolism, vascular matrix molecules, and circulating lipoproteins [2].
One method used for testing for diabetes is the oral glucose tolerance test. The oral glucose tolerance test (OGTT) measures the body's ability to use glucose which is the main source of energy for body cells.
Treatment of diabetes involves use of drugs that reduce glucose levels, including insulin and oral antihyperglycemic drugs. Although there is treatment for diabetes mellitus, most drugs in current use are seriously constrained by both their side effects and cost of treatment. Due to these challenges, populations mainly in Sub-Saharan Africa have resorted to cheaper and readily available alternative sources of treatment, such as use of medicinal plants or traditional medicines [4]. The World Health Organization (WHO) estimates that 80% of the worlds' populations use traditional medicine. The continued use of traditional medicines is linked to their low cost and a general belief that they have minimal side effects [5].
Zanthoxylum chalybeum which belongs to the family Rutaceae is a traditional medicinal plant commonly used by some tribes in Eastern Uganda for treatment of malaria and diabetes [6]. It is a deciduous spiny shrub that grows up to a height of 12 m, with a rounded but open crown. The bole has characteristically large, conical, woody knobs with sharp thorns. The branches also bear scattered thorns with conspicuous dark scales. It has compound leaves comprising 3 to 5 pairs of shiny leaflets plus a terminal leaflet, with a strong citrus smell when crushed [6]. Stem bark decoctions or root bark decoctions are widely taken to treat diabetes, malaria, sickle cell disease, ulcers, and tumors. Root bark decoctions are considered stronger than stem bark decoctions [6, 7].
However, although Z. chalybeum is documented to be used in management of diabetes mellitus in traditional medicine, no scientific study has been done to validate its hypoglycemic activity. This study is the first to evaluate the antidiabetic activity of Z. chalybeum using a laboratory rat model in order to provide scientific evidence to support its use by local communities in the management and treatment of diabetes.
2. Material and Methods
2.1. Material
Zanthoxylum chalybeum root bark was obtained from Katakwi district in Eastern Uganda where it is abundant and locally called Eusuk (Ateso local language). The plant was authenticated by a plant taxonomist at Natural chemotherapeutics Research Institute, Ministry of Health. After collection a voucher specimen was deposited at the National Herbarium, Makerere University, Kampala. The root was washed, debarked, and dried in an air oven at 50°C for 48 h. The bark was then pulverized into powder using a grinder. The powder was extracted by boiling in water for 30 minutes and allowing cooling to room temperature. The extract was then filtered and concentrated using an air oven at 50°C to obtain the crude extract. The crude extract was reconstituted in distilled water and used to evaluate the hypoglycemic property in rats.
2.2. Experimental Animals
Twenty-four male and 24 female Wistar albino rats (aged 12–14 weeks, weighing 180–240 g) were obtained from the College of Veterinary Medicine, Animal Resources and Biosecurity (COVAB), Makerere University, Kampala, and used for evaluation of the antidiabetic property of Z. chalybeum root bark extract in rats.
2.3. Induction of Diabetes in Rats
The animals were allowed to acclimatize for 2 weeks, and then diabetes mellitus was chemically induced in the rats using freshly prepared solution of alloxan monohydrate in distilled water at a dose of 150 mg Kg−1 BW injected intraperitoneally. Since alloxan caused fatal hypoglycemia due to massive insulin release by the pancreas, the rats were in addition orally given 20% (w/v) glucose solution (10 mL) after 6 h. They were further kept for 24 h on 5% (w/v) glucose solution to prevent hypoglycemia. Rats which developed diabetes mellitus observed by glycosuria and hyperglycemia (i.e., blood glucose concentration >250 mgdL−1) were selected for the subsequent experimental tests. This study was approved by Graduate Research Committee, College of Natural Sciences, Makerere University, Kampala.
2.4. Experimental Design
Animals were kept under standard laboratory conditions (25 ± 3°C, 12 h light/dark cycle) and had free access to food and clean tap water ad libitum for 28 days of experimental period. The procedures were of animal experiments in accordance with the Organization for Economic Cooperation and Development (OECD) guidelines for testing of chemicals [8]. All the procedures were in accordance with the International Council for Laboratory Animal Science (ICLAS) and Ethical Guideline for Researchers. The experimental study was conducted on four groups of animals each with twelve rats. Six male and six female Wistar albino rats were randomly allocated to each of the four groups. The groups were treated as follows: Group I consisted of diabetic rats orally given water, food, and metformin by gavage (10 mg/Kg BW) once daily for 28 days. Group II consisted of diabetic rats orally given water, food, and Z. chalybeum extract by gavage (200 mg/Kg BW) once daily for 28 days. Group III consisted of diabetic rats orally given water, food, and Z. chalybeum extract by gavage (400 mg/Kg BW) once daily for 28 days. Group IV consisted of normal rats, orally given water and food with no treatment administered, that is, the normal control group.
2.5. Effect of Zanthoxylum chalybeum on Oral Glucose Tolerance in Alloxan-Induced Diabetic Rats
After 28 days of repeated treatment but before sacrifice, two male and two female Wistar albino rats were randomly selected from each of the four groups and blood samples were collected from tail vein of the rats of control and treated groups after an overnight fast to obtain baseline blood glucose levels. Subsequently, rats of both control and treated groups were orally given glucose (2 g/Kg BW) by gavage. Blood samples were collected from tail vein of the rats at intervals of 30 min up to 2 h for estimation of glucose concentrations using a glucometer.
2.6. Histopathological Analysis of Rat Tissues following Treatment
The animals were anesthetized and the pancreas was removed and fixed in a 10% solution of formaldehyde. The tissues were dehydrated because the reagents used at a later stage were immiscible with water. Varying concentration of isopropyl alcohol, that is, 70%, 80%, 90%, 96%, and 100%, was used for the dehydration. The minimum time for dehydration between two different concentrations was 1 h. The fixed tissues were then cleared in xylene and embedded in paraffin wax. The sections (5 μm) from each of the tissues were examined using a light microscope (×40) after staining with hematoxylin and eosin dye.
2.7. Statistical Data Analysis
The data was analyzed using Graphpad software. The glucose concentration results were expressed as mean ± Standard Error of the Mean (SEM). The mean and SEM of the treatment groups were generated by use of the analysis of variance (ANOVA) test. The significant difference between and within the treatment groups was considered significant at set p value < 0.05 using Dunnett's multiple comparison tests.
3. Results
3.1. Effect of Z. chalybeum Root Bark Extract on Oral Glucose Tolerance in Diabetic Rats
Oral glucose tolerance test was carried out 28 days after repeated doses of the aqueous root extract. There was a significant increase in blood sugar level after oral dosing within the same treatment group compared to treatment at 0 hr (Figure 1). Two hours following treatment with the extract, there was a significant reduction in blood glucose within the same treatment group compared to that after 0.5 h and 1 hr. However, there was no significant difference between the treatment group receiving 400 mg/Kg BW and the control groups after two-hour treatment. The results further show that diabetic rats receiving Z. chalybeum aqueous root extract (400 mg/Kg BW) made some recovery and were able to regulate their sugar levels (Figure 1).
Figure 1.
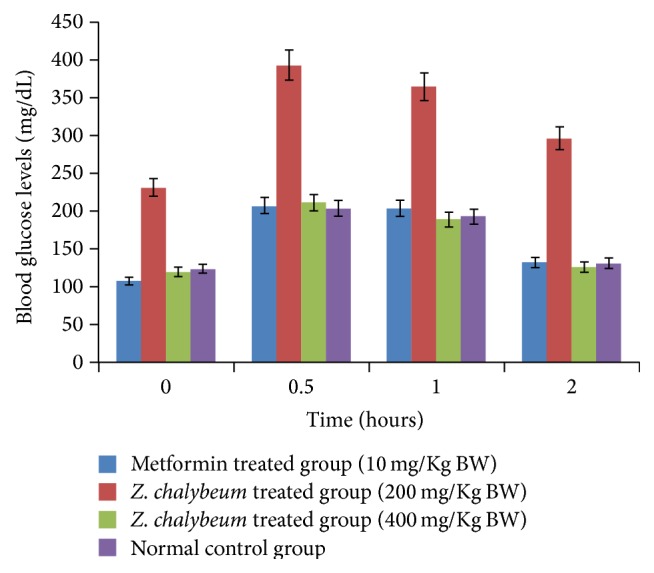
Effect of Z. chalybeum root bark extract on oral glucose tolerance in diabetic rats.
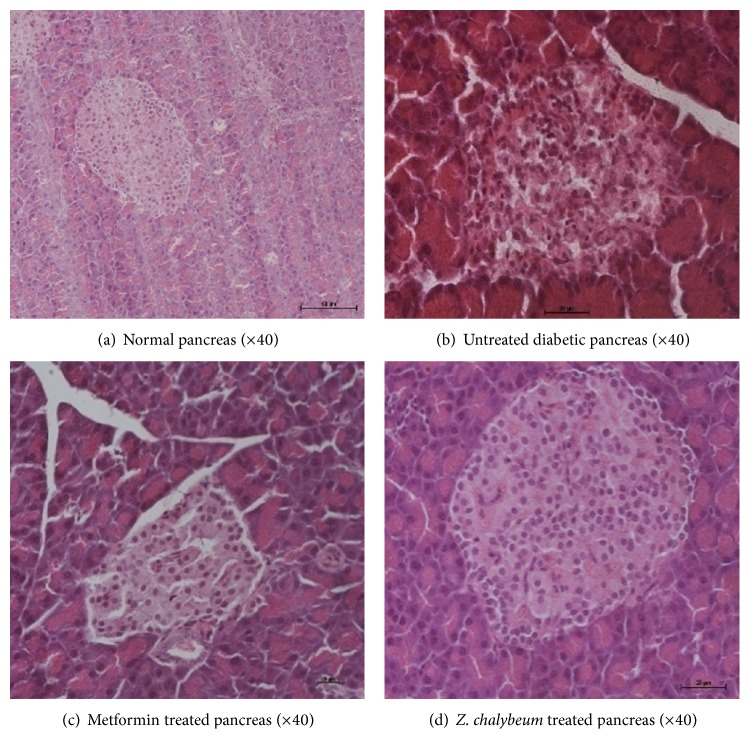
Induction of diabetes with alloxan resulted in severe damage to the β-cells of the islets of Langerhans (Figure 2(a)). A month after treatment with Z. chalybeum (400 mg/Kg BW), there was regeneration of the central β-cells (Figure 2(d)). The normal structure of the cells and the structure of the islets were restored. There was also increase in the number of secretive β-cells. Beta cells are epithelial cells and have the ability to regenerate. The Z. chalybeum extract stimulates the regeneration of β-cells of the islets. However the number of β-cells was still low and yet the animals were able to maintain glucose levels near normal. This therefore suggests that the Z. chalybeum extract also increases the sensitivity of the insulin receptors to the effects of insulin.
Figure 2.
Effect of Z. chalybeum aqueous root bark extract on the histology of the pancreas in diabetic rats.
4. Discussion
Diabetes mellitus causes disturbances in the uptake of glucose by cells as well as glucose metabolism. Thus, alloxan-induced hyperglycemia is a very useful experimental way of studying and demonstrating the activity of new hypoglycemic agents [9]. Oral glucose tolerance tests were used to analyze blood glucose levels taken at different regular intervals after repeated treatments with Z. chalybeum aqueous root extracts. Results of the oral glucose tolerance test, using aqueous root extract of Z. chalybeum (400 mg/Kg BW), indicate significant decrease (p < 0.05) in blood glucose levels of the alloxan-induced diabetic rats (Figure 1). This suggests that the aqueous root extracts of Z. chalybeum enhance glucose utilization and hence improve glucose tolerance in diabetic rats. This also reaffirms that glucose tolerance test (GTT) is a suitable measure and indicator for the cells ability to use glucose, the body's main source of energy [10]. OGT test can thus be used to diagnose prediabetic and diabetic conditions. Similarly, oral administration of aqueous root extract of Z. chalybeum (400 mg/Kg BW) had a significant glucose lowering effects in alloxan-induced diabetic rats (p < 0.05).
Other species in the genus Zanthoxylum have been studied experimentally and reported to have significant antidiabetic activity. For example, various parts of Z. zanthoxyloides including the roots, bark, and leaves have been used for medicinal purposes, including the treatment of diabetes mellitus. Z. zanthoxyloides has been reported to significantly (p < 0.05) lower blood glucose in treated animals in comparison to nontreated groups [11]. Other species in the genus that are used traditionally to treat diabetes in India are Z. armatum and Z. nitidum [12, 13]. The beneficial effects of Z. chalybeum treatment in diabetic rats in this study were likely due to improved insulin release and glucose uptake in remnant β-cells.
The induction of diabetes using alloxan resulted in severe damage of β-cells of the islets of Langerhans (Figure 2(b)). However, after repeated treatment with Z. chalybeum (400 mg/Kg BW), for 28 days, there was regeneration of the central β-cells (Figure 2(d)). There was also a notable increase in the number of secretive β-cells which are epithelial cells with ability to regenerate. The Z. chalybeum extract appeared to stimulate the regeneration of β-cells of the islets of Langerhans. Albeit the still low number of the β-cells, the animals were able to maintain glucose levels close to the normal. This therefore also implies that the Z. chalybeum extract increases the sensitivity of the insulin receptors to insulin.
Previous reports indicate that medicinal plants that possess hypoglycemic activity act through various mechanisms including improvement in the sensitivity of target cells to the effects of insulin, augmenting glucose-dependent insulin secretion, and stimulating the regeneration of β-cells of islets of Langerhans in pancreas of alloxan-induced diabetic rats [14]. Some of the medicinal plants seem to regulate enzymes of glycolysis, gluconeogenesis, and other pathways [15]. Active phytochemical compounds act through a variety of mechanisms; however, in the present study, identification of the mechanism of action of the extract was not done; thus the suggestions made are only hypothetical.
Earlier studies on phytochemical analysis of aqueous roots extract of Z. chalybeum reported presence of the following compounds: tannins, reducing sugars, saponins, alkaloids, coumarin derivatives, flavonoides, steroid glycosides, triterpenes, and anthocyanosides [16]. Presence of flavonoids, steroids, terpenoids, and phenolic acids has been suggested by several authors to be responsible for antidiabetic activity [17]. Flavonoids have also been known to regenerate the damaged beta cells in alloxan-induced diabetic rats and act as insulin secretagogues [18]. Thus, the hypoglycemic activity of aqueous roots extract of Z. chalybeum may be due to the presence of hypoglycemic flavonoids, terpenes, or saponins; however, this also requires further investigation. The antihyperglycemic effect of Z. chalybeum may be attributed to the potentiation of insulin from existing β-cells of the islets of Langerhans. The blood glucose lowering effect of Z. chalybeum was compared with that of metformin, a standard drug which has been in use for many years for treatment of diabetes and acts by stimulating insulin secretion from pancreatic β-cells [18].
5. Conclusion
Study has shown that oral administration of aqueous root bark extract of Z. chalybeum to alloxan-induced diabetic rats improves their glucose tolerance, an important finding in the control of diabetes. This suggests that Z. chalybeum is useful in protection and amelioration of diabetic complications through enhancement of regeneration of β-cells of the islets of Langerhans. Therefore, the effects of Z. chalybeum appear to be curative rather than palliative. Further studies are needed to define the active agents present and their mode of activity.
Acknowledgments
The authors thank RISE-AFNNET project for providing funds for this study, Natural Chemotherapeutics Research Institute, Ministry of Health, Uganda, for providing lab facilities, and Joint Animal Disease Diagnostic Centre, Central Diagnostic Laboratory, COVAB, Makerere University, for supplying rats for the research work and histopathological examination of the rat tissues.
Abbreviations
- OGTT:
Oral glucose tolerance test
- WHO:
World Health Organization
- COVAB:
College of Veterinary Medicine, Animal Resources and Biosecurity
- OECD:
Organization for Economic Cooperation and Development
- ICLAS:
International Council for Laboratory Animal Science
- SEM:
Standard Error of the Mean
- ANOVA:
Analysis of variance
- Kg BW:
Kilogram body weight.
Conflict of Interests
All authors agreed with the content of the paper and do not have any conflict of interests.
Authors' Contributions
M. S. Agwaya has made significant contribution throughout the study starting from collection of Z. chalybeum roots to the completion of the study. A. M. Nandutu participated in drafting the paper and P. C. Vuzi participated in critical revision and intellectual content. Both A. M. Nandutu and P. C. Vuzi secured funding for this study and were also involved in academic supervision of this study. All authors listed read and approved the final paper.
References
- 1.Khan A., Zaman G., Anderson R. A. Bay leaves improve glucose and lipid profile of people with Type 2 diabetes. Journal of Clinical Biochemistry and Nutrition. 2009;44(1):52–56. doi: 10.3164/jcbn.08-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(supplement 1):S62–S69. doi: 10.2337/dc10-s062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiorentino T. V., Prioletta A., Zuo P., Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Current Pharmaceutical Design. 2013;19(32):5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 4.Modak M., Dixit P., Londhe J., Ghaskadbi S., Devasagayam T. P. A. Indian herbs and herbal drugs used for the treatment of diabetes. Journal of Clinical Biochemistry and Nutrition. 2007;40(3):163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somani R., Kasture S., Singhai A. K. Antidiabetic potential of Butea monosperma in rats. Fitoterapia. 2006;77(2):86–90. doi: 10.1016/j.fitote.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Tabuti J. R. S. Zanthoxylum chalybeum Engl. [Internet] record from PROTA4U. In: Schmelzer G. H., Gurib-Fakim A., editors. Plant Resources of Tropical Africa. Wageningen, The Netherlands: PROTA; 2011. [Google Scholar]
- 7.Ogwang P. E., Nyafuono J., Agwaya M. S., Omujal F., Tumusiime R. H., Kyakulaga A. H. Preclinical efficacy and safety of herbal formulation for management of wounds. Journal of African Health Sciences. 2011;11(3):524–529. [PMC free article] [PubMed] [Google Scholar]
- 8.OECD. Organization for Economic Co-Operation and Development. OECD Guidelines for the Testing of Chemicals/Section 4: Health Effects Test No. 423; Acute Oral Toxicity-Acute Toxic Class Method. Paris, France: OECD; 2014. [Google Scholar]
- 9.Srinivasan K., Ramarao P. Animal models in type 2 diabetes research: an overview. Indian Journal of Medical Research. 2007;125(3):451–472. [PubMed] [Google Scholar]
- 10.Ali M. A., Sultana M. C., Rahman B. M., Khatune N. A., Wahed M. I. I. Antidiabetic activity of ethanolic extract of Semecarpus anacardium (linn.) Stem barks in normal and alloxan induced diabetic rats. International Journal of Pharmaceutical Science and Research. 2015;3(8):2680–2685. [Google Scholar]
- 11.Aloke C., Nwachukwu N., Ugwuja E. I., et al. Effects of Zanthoxylum zanthoxyloides leaves on blood glucose, lipid profile and some liver enzymes in alloxan induced diabetic rats. International Journal of Science and Nature. 2012;3(3):497–501. [Google Scholar]
- 12.Singh T. P., Singh O. M. Phytochemical and pharmacological profile of Zanthoxylum armatum DC.—an overview. Indian Journal of Natural Products and Resources. 2011;2(3):275–285. [Google Scholar]
- 13.Arun K., Paridhavi M. An ethno botanical, phytochemical and pharmacological utilization of widely distributed species Zanthoxylum: a comprehensive over view. International Journal of Pharmaceutical Invention. 2012;2(1):24–35. [Google Scholar]
- 14.Ayodhya S., Kusum S., Anjali S. Hypoglycemic activity of different extracts of various herbal plants. International Journal of Research in Ayurveda & Pharmacy. 2010;1(1):212–224. [Google Scholar]
- 15.Arya A., Cheah S. C., Looi C. Y., Taha H., Mustafa M. R., Mohd M. A. The methanolic fraction of Centratherum anthelminticum seed down regulates pro-inflammatory cytokines, oxidative stress and hyperglycemia in STZ-nicotinamide-induced type 2 diabetic rats. Food and Chemical Toxicology. 2012;50:4209–4220. doi: 10.1016/j.fct.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Nalule A. S., Mbaria J. M., Kimenju J. W. In vitro anti-helminthic potential and phytochemical composition of ethanolic and aqueous crude extracts of Zanthoxylum chalybeum . African Journal of Pharmacy and Pharmacology. 2013;7(23):1605–1614. [Google Scholar]
- 17.Alagammal M., Agnel R. A., Mohan V. R. Anti-diabetic and anti-hyperlipidaemic effect of Polygala javana DC on alloxan induced diabetic rats. International Research Journal of Pharmacy. 2012;3:231–234. [Google Scholar]
- 18.Mao-Ying Q.-L., Kavelaars A., Krukowski K., et al. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0100701.e100701 [DOI] [PMC free article] [PubMed] [Google Scholar]