Abstract
Pemetrexed-containing chemotherapy has shown promise in the treatment of non-small-cell lung cancer (NSCLC). However, although adenosquamous cell lung cancer (ASCLC) is a type of NSCLC, the availability of studies investigating its response to pemetrexed-containing chemotherapy is limited. A 66-year-old woman was referred to Mito Medical Center, University of Tsukuba with hemoptysis and a chest computed tomography (CT) scan revealed a large cavitary mass in the lower lobe of the left lung. The patient underwent left lower lobectomy and mediastinal lymph node dissection. The tumor was staged as pT2bN2M0. An epidermal growth factor receptor (EGFR) exon 19 deletion was identified in the adenocarcinomatous as well as the squamous cell carcinomatous components. Despite gefitinib therapy for pulmonary metastases, the patient developed cavitary metastases in both lungs. Therefore, treatment with pemetrexed-containing chemotherapy was initiated. A chest CT scan revealed significant regression of the metastatic lesions in both lungs, with thinning of the walls. The patient remains well and recurrence-free 19 months after the initiation of pemetrexed-containing chemotherapy. Therefore, the clinical response of EGFR mutation-positive ASCLC to pemetrexed-containing chemotherapy was promising, suggesting pemetrexed to be one of the key drugs for this subset of ASCLC patients.
Keywords: adenosquamous cell lung cancer, epidermal growth factor receptor, pemetrexed-containing chemotherapy
Introduction
Adenosquamous cell lung cancer (ASCLC) is a type of non-small-cell lung cancer (NSCLC) and it is a morphologically mixed tumor, composed of two cell components, namely adenocarcinoma and squamous cell carcinoma in varying proportions, each representing ≤10% of the whole tumor (1). Due to its rarity, ASCLC has not received significant attention in terms of its clinical aspects, with only a limited number of studies investigating the aggressive biological characteristics of ASCLC (2–5).
Pemetrexed-containing chemotherapy is currently one of the highly promising regimens for the treatment of advanced NSCLC (6). In addition, the effectiveness of pemetrexed-containing chemotherapy for NSCLCs with specific genetic changes has been previously reported (7–9). We herein report a case of recurrent epidermal growth factor receptor (EGFR) mutation-positive ASCLC successfully treated with pemetrexed-containing chemotherapy. Of note, this was a rechallenge with pemetrexed following gefitinib therapy and postoperative adjuvant pemetrexed-containing chemotherapy.
Case report
A 66-year-old woman with no history of smoking presented to Mito Medical Center, University of Tsukuba (Tsukuba, Japan) with a cough and intermittent hemoptysis over the past 8 weeks. Chest radiography and computed tomography (CT) revealed a large cavitary mass in the lower lobe of the left lung. Bronchoscopy revealed a tumor occluding the left lower bronchus, with active bleeding. The patient was initially diagnosed with adenocarcinoma on the basis of the histopathological examination of transbrochial biopsy specimens. Distant metastasis was not detected. The Eastern Cooperative Oncology Group performance status score was 1. To prevent the deterioration of her respiratory condition due to hemoptysis, the patient underwent a lobectomy of the left lower lung and mediastinal lymph node dissection. The resected tumor consisted of adenocarcinomatous as well as squamous cell carcinomatous components, with each comprising ≤10% of the tumor. The final pathological diagnosis was ASCLC (Fig. 1) and the tumor was staged as pT3bN1M1a. The adenocarcinomatous and squamous cell carcinomatous components of the surgically resected tumor were precisely separated by manual microdissection under microscopic observation to avoid contamination of each sample by different components. An EGFR exon 19 deletion was identified in both components. Soon after the surgical resection, the patient received 4 courses of chemotherapy with carboplatin and pemetrexed. Six months after the chemotherapy, the patient developed small cavitary metastases sized ≤10 mm in both lungs. Therefore, treatment with 250 mg of gefitinib therapy once daily was initiated. A chest CT scan revealed regression of the metastatic cavitary tumors bilaterally, which was evaluated as partial response (PR). The patient developed a grade 2 skin rash, according to the 2013 National Cancer Institute Toxicity Criteria (http://ctep.cancer.gov). One year after the initiation of gefitinib therapy, small cavitary metastases sized ≤10 mm were again detected in both lungs (Fig. 2). The patient strongly wished to receive pemetrexed-containing chemotherapy again, due to its mild adverse event profile. Considering the metastases being confined to the lungs and her good general condition, the patient received chemotherapy with carboplatin, pemetrexed and bevacizumab. After receiving 2 cycles of the chemotherapy, she achieved a PR (Fig. 3) and received an additional 2 cycles of the regimen. Subsequently, 15 cycles of maintenance therapy with pemetrexed and bevacizumab were administered. There was no severe hematological or non-hematological toxicity. Treatment with bavacizumab-containing chemotherapy has been continued at the outpatient clinic, without any signs of tumor progression 19 months after the initiation of pemetrexed-containing chemotherapy.
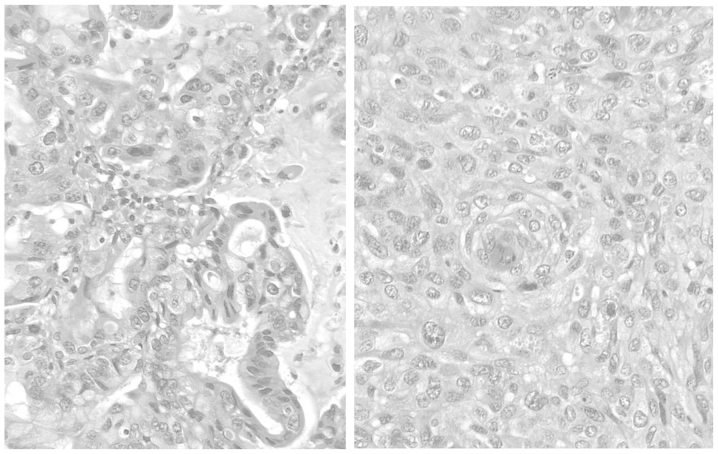
Figure 1.
The resected tumor was composed of adenocarcinomatous (right) and squamous cell carcinomatous (left) components, with each comprising at least 10% of the tumor.
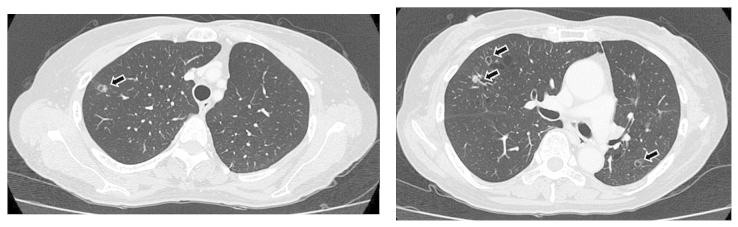
Figure 2.
Chest computed tomography (CT) scan 6 months after the initiation of chemotherapy, showing small cavitary metastases sized ≤10 mm in both lungs (arrows). CT imaging slice at the level of the sub-carina (right panel), and at the level of the upper part of the trachea (left panel).
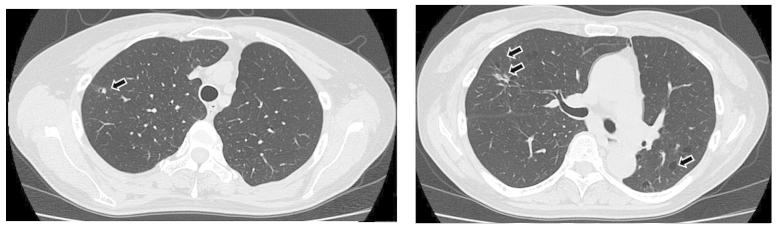
Figure 3.
Chest computed tomography (CT) scan on day 28 of pemetrexed-containing chemotherapy, showing significant regression of the metastatic cavitary tumors in both lungs, with thinning of the walls (arrows). CT imaging slice at the level of the sub-carina (right panel), and at the level of the upper part of the trachea (left panel).
Discussion
Due to rarity of ASCLC, its clinical and prognostic aspects have not been extensively investigated. The majority of available studies report an aggressive biological behavior of ASCLC (2–5). In ASCLC, EGFR mutations appear to be important predictive markers for the response to EGFR-tyrosine kinase inhibitors (TKIs), similar to the observed favorable outcome in other cell types of NSCLC (10). However, the frequency of EGFR abnormalities and the effectiveness of TKIs in EGFR-mutated ASCLC have not yet been completely evaluated. In patients with resectable ASCLC, postoperative platinum-based chemotherapy has significantly improved patient survival and reduced the risk of developing distant metastases (2). Similar to other cell types of NSCLC, it is now widely accepted that postoperative platinum-based chemotherapy is the standard medical treatment in stage IB-IIIA ASCLC (2). However, EGFR-mutated ASCLCs have not been extensively investigated in terms of the effectiveness of chemotherapy with cytotoxic drugs; moreover, whether EGFR-positive ASCLCs have a more favorable outcome with such treatment compared with their EGFR-negative counterparts, remains unknown.
It was recently indicated that pemetrexed-containing chemotherapy exhibits superior efficacy for NSCLCs with specific genetic changes (7–9). Although this is only a case report of an ASCLC patient with EGFR mutation, the outcome in our patient was consistent with the results of other cell types of NSCLC (10).
Of note, response to pemetrexed-containing chemotherapy following acquired resistance to EGFR-TKIs in a patient with advanced NSCLC was recently reported (11): That case was a metastatic adenocarcinoma of the lung with exon 21 L858R mutation. The patient was treated with pemetrexed and cisplatin and achieved a PR. After recurrence, the patient received erlotinib for 6 months. Due to regrowth in the pleura, the patient underwent pemetrexed rechallenge, considering the slow progression, good tolerance and good efficacy of the drug. Due to the satisfactory response, the authors reported that rechallenge with pemetrexed-containing chemotherapy should be one of the treatment choices for a proportion of patients with acquired resistance to TKIs, one of whom had shown response to previous chemotherapy (11). However, our patient had received postoperative chemotherapy with platinum and pemetrexed. Six months after the adjuvant chemotherapy, the patient received gefitinib due to multiple pulmonary metastases bilaterally, which were well controlled for 10 months. Pemetrexed rechallenge was selected in our case, as the patient strongly wished to receive pemetrexed-containing chemotherapy again, as she had experienced no severe adverse events with this chemotherapy. Therefore, the patient received chemotherapy with pemetrexed and bevacizumab for re-recurrence following gefitinib therapy. The mechanisms underlying the effectiveness of pemetrexed rechallenge have not been fully elucidated, but the fact that the patient achieved a good response may be of value and must be reported. Our results suggest that, in adition to TKIs, pemetrexed-containing chemotherapy may be one of the key regimens for the treatment of EGFR mutation-positive ASCLC patients.
References
- 1.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization Classification of Tumours. Lyon: IARC Press; 2004. Pathology and genetics, Tumours of the lung, pleura, thymus and heart. [Google Scholar]
- 2.Filosso PL, Ruffini E, Asioli S, Giobbe R, Macri L, Bruna MC, Sandri A, Oliaro A. Adenosquamous lung carcinomas: A histologic subtype with poor prognosis. Lung Cancer. 2011;74:25–29. doi: 10.1016/j.lungcan.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu J, Oda M, Hayashi Y, Nomomura A, Watanabe Y. A clinicopathologic study of resected cases of a denosquamous carcinoma of the lung. Chest. 1996;109:989–994. doi: 10.1378/chest.109.4.989. [DOI] [PubMed] [Google Scholar]
- 4.Riquet M, Perrotin C, Lang-Lazdunski L, Hubsch JP, Dujon A, Manac'h D, Le Pimpec Barthes F, Briere J. Do patients with adenosquamous carcinoma of the lung need a more aggressive approach? J Thorac Cardiovasc Surg. 2001;122:618–619. doi: 10.1067/mtc.2001.114629. [DOI] [PubMed] [Google Scholar]
- 5.Gawrychowski J, Bruliński K, Malinowski E, Papla B. Prognosis and survival after radical resection of primary adenosquamous lung carcinoma. Eur J Cardiothorac Surg. 2005;27:686–692. doi: 10.1016/j.ejcts.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Lee SH, Keam B, Kim TM, Kim DW, Yang SC, Kim YW, Heo DS. EGFR mutations as a predictive marker of cytotoxic chemotherapy. Lung Cancer. 2012;77:433–437. doi: 10.1016/j.lungcan.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Noh KB, Lee JS, Lee EJ, Min KH, Hur GY, Lee SH, Lee SY, Kim JH, Lee SY, et al. Thymidylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer. 2013;81:102–108. doi: 10.1016/j.lungcan.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez Hernández A, José Juan O, Martínez Vidal J, Blanco R, Maciá S, Galiana Esquerdo G, Aparisi Aparisi F, Noguera Garde J, Catot S, Gaspá Losa F, García-Piñon F. Quantification of circulating endothelial cells as a predictor of response to chemotherapy with platinum and pemetrexed in patients with advanced non-squamous non-small cell lung carcinoma. Clin Transl Oncol. 2015;17:281–288. doi: 10.1007/s12094-014-1223-5. [DOI] [PubMed] [Google Scholar]
- 10.Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Zhou F, Ren S, Zhou C. Response to pemetrexed rechallenge after acquired resistance of EGFR-TKI in a patient with advanced NSCLC. Lung Cancer. 2014;84:203–205. doi: 10.1016/j.lungcan.2014.02.010. [DOI] [PubMed] [Google Scholar]