Abstract
Purpose
Nerve growth factor (NGF) is a classic neuroprotective factor that contributes to angiogenesis under pathological conditions, which might be mediated by the upregulation of vascular endothelial growth factor (VEGF). Retinal Müller cells are a critical source of growth factors, including NGF and VEGF, and express the receptor for NGF, indicating the functional significance of NGF signaling in Müller cells. The aim of this study is to explore the effect of NGF on the production of other growth factors and cellular proliferation in Müller cells and to further detect the potential mechanism of these effects.
Methods
Primary Müller cells from C57BL/6J mice were isolated and identified with glutamine synthetase (GS) immunofluorescence (IF), a specific marker for Müller cells. TrkA, a high affinity receptor for NGF, was detected with IF staining in the primary Müller cells. Then, the cultured cells were stimulated with recombinant mouse NGF, and the supernatants and the cellular lysate were collected at different time points. VEGF secretion in the supernatant was detected with an enzyme-linked immunosorbent assay (ELISA). The signaling activation in the Müller cells was accessed by western blot using specific phosphorylated antibodies. In addition, cell proliferation was analyzed with 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Furthermore, K252a, U0126, and LY294002, the inhibitors for TrkA, extracellular signal-regulated kinases 1/2 (ERK1/2), and phosphatidylinositol 3-kinase (PI3K)/AKT, respectively, were used in combination with NGF in the assays analyzing VEGF expression and cell proliferation.
Results
Primary mouse Müller cells were successfully cultured and confirmed with GS positive staining. The IF results showed that the TrkA receptor was abundantly expressed on Müller cells. The ELISA results revealed that NGF significantly promoted the production and secretion of VEGF in Müller cells after 12 or 24 h of stimulation, with more elevation after 24 h. Furthermore, NGF activated ERK1/2 and PI3K/AKT signaling, which was shown by the marked upregulation of phosphorylation in the western blot. As expected, K252a, the inhibitor of TrkA, a high-affinity NGF receptor, suppressed the activation, showing little phosphorylation of ERK1/2 and PI3K/AKT signaling. Importantly, the VEGF levels were decreased after the inhibitors for TrkA, ERK1/2, and PI3K/AKT were used compared with NGF alone. In addition, the MTT assay showed that NGF promoted the proliferation of the Müller cells, which was also blocked by the TrkA, ERK1/2, and PI3K/AKT inhibitors.
Conclusions
The results showed that NGF enhanced the secretion of VEGF and promoted cell proliferation via the ERK1/2 and PI3K/AKT pathways in Müller cells, indicating that NGF is involved in angiogenesis-related factor generation and gliosis in Müller cells.
Introduction
Nerve growth factor (NGF), a classic neuroprotective factor, supports the survival of retinal ganglion cells and photoreceptors, maintaining the development and homeostasis of the retina [1-4]. NGF has been used in clinical trials for treating neural degenerative diseases, such as optic glioma and advanced optic nerve atrophy, Alzheimer disease, hypoxic-ischemic perinatal brain injury, etc. [5,6]. However, NGF did not support an obvious functional improvement over the course of a long therapy. In addition to retinal neural cells, NGF is mostly generated by Müller cells, and its receptors, including TrkA and p75, are also expressed on Müller cells, indicating the functional significance of NGF signaling in Müller cells [7-10]. Müller cell-derived vascular endothelial growth factor (VEGF) is essential for retinal angiogenesis, and Müller cells play a significant role in supporting retinal neurons [11-13], but when over-proliferated, they contribute to retinal gliosis, resulting in neuronal cell death and forming a glial scar at later stages [14]. Therefore, the exact role of NGF in Müller cells must be investigated.
Retinal Müller cells, the principal glia of the retina, link neurons and vessels through their processes that completely ensheathe the retinal vasculature [15]. These cells have a vital role in forming and maintaining the blood–retinal barrier and regulating retinal glutamate levels and blood flow [16]. Müller cells have been regarded as an important source of vascular endothelial growth factor (VEGF), NGF, basic fibroblast growth factor-2 (bFGF2), tumor necrosis factor, etc. [8,11,17]. Interestingly, the receptor for NGF can be found in Müller cells, indicating the involvement of NGF signaling in the physiologic and pathological processes of Müller cells. In addition to a neuroprotective role, NGF exerts a proangiogenic role in various pathological conditions, such as ischemia-induced retinal neovascularization and a hindlimb ischemic model, by activating the TrkA and VEGFR-2 pathways in endothelial cells [18,19]. In cultured human umbilical vein endothelial cells (HUVECs), NGF activates TrkA, triggering a mitogenic response and exerting an autocrine role in HUVECs [20]. Our previous study also demonstrated that NGF promoted angiogenesis via the TrkA receptor in the ischemic retina, and Müller cell activation is required in inflammation-induced retinal neovascularization [21]. However, little is known about the potential of NGF to induce VEGF generation in Müller cells.
Müller cells are active players in nearly all forms of retinal injury and disease [22,23]. They undergo reactive gliosis, presented by the proliferation of Müller cells and the expression of glial fibrillary acidic protein (GFAP) in response to neuronal damage and other insults in an attempt to protect the tissue from further damage [24-26]. However, lasting gliosis releases many inflammatory cytokines, resulting in secondary injury and accelerating disease progress. For example, Müller cell proliferation is required in the occurrence and development of proliferative vitreoretinopathy and may be regarded as a limiting factor in the recovery of vision after reattachment [27]. Previous studies reported that many growth factors, such as bFGF and endothelin-2, induce the hypertrophy and proliferation of Müller cells in detached retinas [28-30]. However, the role of the classic neuroprotective factor NGF in the proliferation of Müller cells is still unknown and must be investigated.
In this study, we investigated the effect of NGF on VEGF generation in Müller cells and their proliferation and explored the underlying signaling pathway. We provide direct evidence that NGF promotes the generation and secretion of VEGF in Müller cells via extracellular signal-regulated kinases 1/2 (ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/AKT signaling. In addition, NGF enhances the proliferation of Müller cells in a time-dependent manner. The results will help us better understand the role of NGF in VEGF production derived from Müller cells and the molecular mechanisms of Müller cell proliferation, which is involved in many retinal angiogenic and proliferative diseases.
Methods
Primary Müller cell culture
C57BL/6J pups were sacrificed at postnatal 4–7 days, and their eyes were removed. Then, the retinas were isolated with care to avoid contamination from the RPE and the ciliary epithelium. They were chopped into approximately 1 mm2 pieces and cultured in DMEM/F12 (1:1 ratio of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium; Life Technologies, Grand Island, NY) with 10% fetal bovine serum (FBS; Life Technologies, Grand Island, NY) in a humidified environment of 5% (vol./vol.) CO2 at 37 °C for 3–4 days. Half of the floating retinal aggregates and debris was removed, and the dish was replenished with complete medium. After 3 days, the retinal aggregates were washed by vigorous rinsing, and the cells were kept in and fed with 90% DMEM/F12 (Life Technologies) plus 10% FBS, leaving a purified flat cell population of Müller cells attached to the bottom of the dish. After the second passage, the cultured cells were validated as being Müller cells with positive immunocytochemical staining using antibody against glutamine synthetase (GS), which is specifically localized in retinal Müller cells. Non-serum DMEM/F12 medium was added to the dishes, and the culture was incubated overnight before further analysis.
Cell treatment
The cells were stimulated with recombinant NGF after serum starvation overnight (PeproTech, Rocky Hill, NJ). According to the manufacturer’s recommendation, the NGF was reconstituted and stored in a buffer containing 0.1% bovine serum albumin (BSA) at a concentration of 1 mg/ml. The working solution concentration of NGF was 100 ng/ml, which contained 100 ng/ml of BSA. Thus, the control cells were treated with 100 ng/ml of BSA. Serum-free DMEM/F12 medium was used when the Müller cells were treated with NGF and the inhibitors. For the inhibitor treatments, the cells were pretreated with the inhibitors, including 10 nM and 20 nM K252a (tyrosine inhibitor of TrkA receptor; Calbiochem, San Diego, CA), 10 μM and 20 μM U0126 (ERK1/2 pathway inhibitor; CST), or 10 μM and 20 μM LY294002 (PI3K/AKT inhibitor; Calbiochem) for 30 min and were then stimulated with NGF. The concentration of these inhibitors was chosen according to previous reports, which showed that 10 μM and 20 μM U0126 or LY294002 were widely used in Müller and astrocyte glia cell experiments without toxic effects. After 5, 15, or 30 min treatment, the cell lysate was used for western blot. After 12 and 24 h, the supernatants were collected for ELISA, and after 24, 48, and 72 h, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed in the cells as described [31,32].
GS-Müller cell identification and TrkA receptor expression by immunofluorescence staining
The Müller cells were washed with PBS (1X; 135 mM NaCl, 4.7 mM KCl, 10 mM Na2HPO4, 2 mM NaH2PO4, pH 7.4) twice and fixed for 20 min in 4% paraformaldehyde at room temperature. Then, the cells were blocked with 3% BSA in PBS for 1 h and incubated with 1:100 mouse monoclonal anti-GS antibody (Abcam, Cambridge, UK) and 1:100 rabbit anti-TrkA (Cell Signaling Technology, Beverly, MA) antibody overnight at 4 °C. After washing with PBS/0.1% Tween-20 three times, the cells were incubated with 1:200 Alexa 555-conjugated donkey ant-mouse/Alexa 488-conjugated donkey anti-rabbit antibodies (Cell Signaling Technology) at room temperature for 1 h. The nuclei were stained with 1:1,000 4’,6-diamidino-2-phenylindole (DAPI) for 5 min, and the cells were analyzed under a confocal microscope (LSM 510 META; Carl Zeiss, Oberkochen, Germany). Fluorescence pictures were taken with identical exposure settings. For the negative control, the cells were stained without primary antibodies and showed no signals.
AKT and ERK1/2 phosphorylation by western blotting
Cellular protein was harvested at the indicated time points and homogenized in lysis buffer (RIPA; Biocolors, Shanghai, China) containing a protease (Roche, Indianapolis, IN) and the phosphatase inhibitor phosSTOP (Roche). The protein concentration was determined with bicinchoninic acid (BCA) protein assay. Equal amounts of protein were run on a 10% (w/v) reducing sodium dodecyl sulfate (SDS) polyacrylamide electrophoresis gel. The gel was transferred to polyvinylidene fluoride (PVDF) membrane (Millipore Corporation, Billerica, MA) and blocked in 5% (w/v) BSA (MP Biomedicals, Santa Ana, CA) in TBST (1X; 20 mM Tris-HCl, 150 mM NaCl and 0.1% Tween-20, pH 7.5) for 60 min. The membrane was incubated with primary antibody at 4 °C overnight. The primary antibodies included mouse anti-β-actin antibody (1:2,000 dilution; Abcam, Cambridge, UK), rabbit anti-ERK1/2 antibody (1:1,000 dilution; CST), rabbit anti-p-ERK1/2 antibody (1:1,000 dilution; CST), rabbit anti-AKT antibody (1:1,000 dilution; CST) and rabbit anti-p-AKT antibody (1:1,000 dilution; CST). Then, the membranes were washed with TBST three times. Next, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody at a concentration of 1:5,000 for 1 h at room temperature. The signals were developed with Super Signal West Dura extended duration substrate (Thermo Scientific, Rockford, IL), and the images were captured by an image station. The phosphorylated protein signal was normalized to the corresponding total protein signal after it was quantified using ImageJ software (National Institutes of Health). β-actin was also used as a loading control.
VEGF detection with ELISA and Müller cell proliferation with MTT assay
The Müller cells seeded on 24-well or 96-well plates (Corning Inc., Corning, NY) were cultured in serum-free DMEM/F12 overnight and then treated with 100 ng/ml BSA, 100 ng/ml of recombinant NGF with or without K252a, U0126, and LY294002 for 12, 24, 48, and 72 h. At the appropriate time points, the supernatants of the treated Müller cells were collected, and VEGF levels were detected with a mouse VEGF ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. In addition, an MTT assay (MP Biomedicals) was performed to detect cell viability using a microplate reader set to 570 nm, and the wavelength correction was set to 630 nm as described.
Statistical analysis
There were six samples in each group for the MTT assay and ELISA. A one-way analysis of variance (ANOVA) was used to compare the expression of VEGF and cell survival between the different treatment groups. Differences in VEGF secretion according to the ELISA between NGF treatment and the BSA control were analyzed with the Student t test. The statistical analyses were performed with the statistical software SPSS 13.0 (SPSS Inc., Chicago, IL). A p value of less than 0.05 was accepted as statistically significant. The data are reported as the mean ± standard error of the mean (SEM).
Results
Expression of TrkA in murine Müller cells
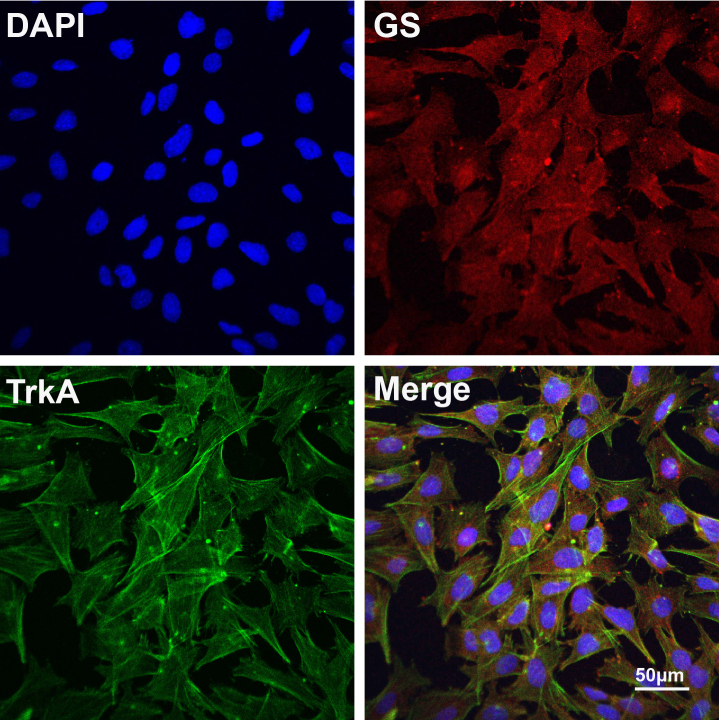
To demonstrate whether Müller cells express TrkA, we first isolated and cultured primary Müller cells from C57BL/6J mice and identified the cells by using antibodies against vimentin and GS, a specific marker of the Müller cells in the retina [33-35]. The immunostaining results revealed that most of the primary cells were GS- and vimentin-positive Müller cells (Appendix 1). TrkA, the high affinity receptor for NGF, was abundantly expressed in these primary Müller cells (Figure 1).
Figure 1.
Expression of TrkA in Müller cells. Primary mouse Müller cells were identified with immunofluorescence staining of glutamine synthetase (GS; red), a specific marker of Müller cells. TrkA (green) was expressed abundantly in the mouse Müller cells. Scale bar=50 μm.
NGF improved VEGF expression in Müller cells
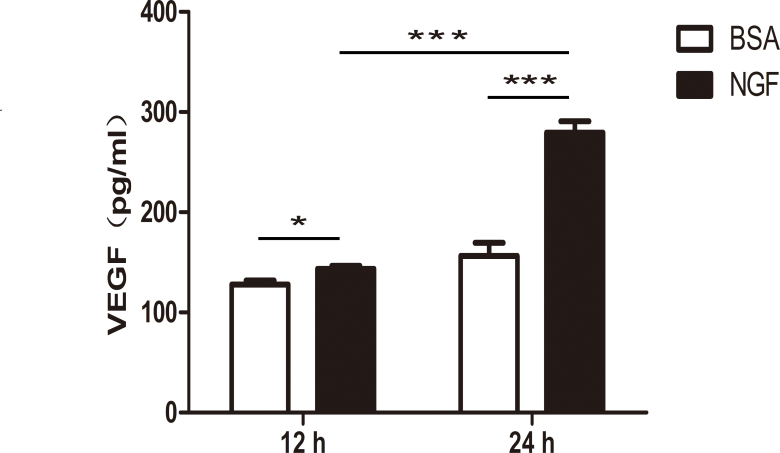
Müller cell-derived VEGF is a significant contributor to retinal neovascularization as reported. To verify whether VEGF secretion by the Müller cells was affected by NGF treatment, we treated cells with 100 ng/ml of NGF for 12 or 24 h before the supernatants were collected for ELISA. Compared to the BSA control, an increase of 12.12% and 78.64% of secreted VEGF was observed in the Müller cells in the 12 and 24 h NGF treatment groups, respectively. In addition, the VEGF increase after 24 h was significantly higher than the increase after 12 h, whereas there was no statistical significance between the 12 and 24 h samples in the BSA treatment group (Figure 2; n=6, *p<0.05, ***p<0.001).
Figure 2.
NGF increased the expression of VEGF in Müller cells. Müller cells were treated with 100 ng/ml of nerve growth factor (NGF) compared with an equal amount of bovine serum albumin (BSA) control. After 12 or 24 h, vascular endothelial growth factor (VEGF) protein expression in the supernatants was significantly increased in the NGF-treated group according to the enzyme-linked immunosorbent assay (ELISA). Importantly, the VEGF increase after 24 h was significantly higher than that after the 12 h treatments. There was no statistically significant difference between 12 and 24 h in the BSA treatment group with a p value equal to 0.0644. n=6, *p<0.05, *** p<0.001.
NGF activates the ERK1/2 and AKT pathway after TrkA recognition
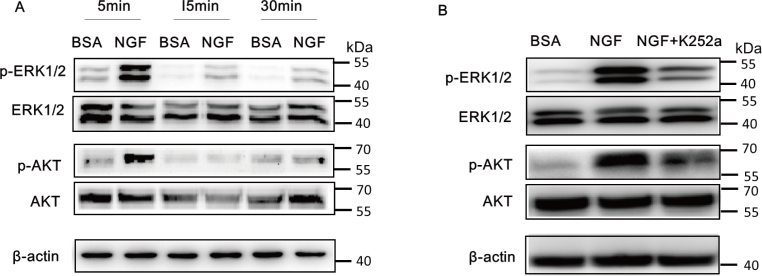
The ERK1/2 and PI3K/AKT pathways are activated after extracellular growth factors bind to their respective transmembrane receptor tyrosine kinases [36-38]. To evaluate the involvement of ERK1/2 and AKT signaling in NGF-induced VEGF generation, we used western blotting to test the phosphorylation levels after NGF treatment for 5, 15, or 30 min. The results showed that NGF treatment promoted the phosphorylation of ERK1/2 in a time-dependent manner with maximum phosphorylation occurring at 5 min. Similarly, AKT phosphorylation increased in a time-dependent manner compared with the BSA-treated controls (Figure 3A). In addition, K252a, a TrkA inhibitor, significantly reduced the phosphorylation of ERK1/2 and AKT. Taken together, these results suggested that NGF activated ERK1/2 and AKT signaling after the recognizing the TrkA receptor (Figure 3B).
Figure 3.
NGF activates the ERK1/2 and AKT pathways in Müller cells. A: Western blotting was performed to assess the phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) and AKT signaling in Müller cells after treatment with 100 ng/ml of nerve growth factor (NGF) for 5, 15, or 30 min. An equal concentration of bovine serum albumin (BSA) was used as the control. NGF promotes the phosphorylation of ERK1/2 at all time points with peak activation after 5 min of treatment. Similarly, NGF enhances the phosphorylation of AKT significantly at 5 min. B: Müller cells pretreated with 20 nM K252a for 30 min and then treated with NGF for 5 min. The western blot shows the phosphorylation of ERK1/2 and AKT in response to NGF combined with the K252a treatment was markedly decreased compared to NGF alone.
The ERK1/2 and AKT pathways were required for NGF-induced VEGF expression in Müller cells
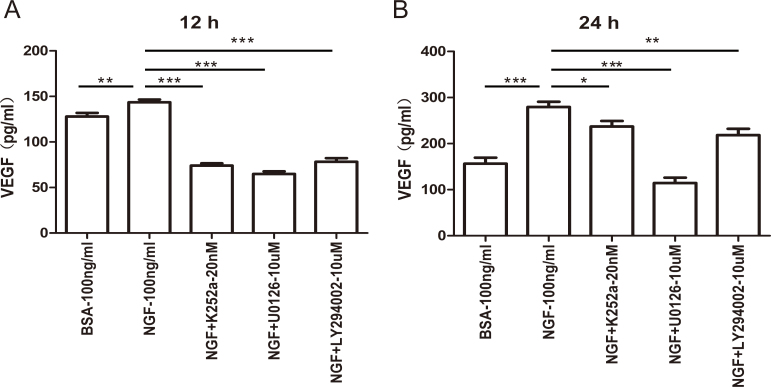
To detect whether ERK1/2 and PI3K/AKT signaling is involved in VEGF elevation by NGF, the Müller cells were treated with NGF in the presence or absence of the TrkA, ERK1/2, or PI3K/AKT inhibitors. After 12 or 24 h of stimulation, the supernatants were collected and detected with ELISA. As shown in Figure 4, NGF increased the production of VEGF in Müller cells, which was more prominent at 12 h than at 24 h. However, the K252a (TrkA inhibitor), U0126 (ERK1/2 pathway inhibitor), or LY294002 (PI3K/AKT inhibitor) combined treatment markedly decreased the increased VEGF expression induced by NGF (Figure 4; n=6, *p<0.05, **p<0.01, ***p<0.001).
Figure 4.
NGF increases VEGF expression via the TrkA receptor and is mediated by the activation of ERK1/2 and AKT signaling. Müller cells were treated with 100 ng/ml of nerve growth factor (NGF) with or without 20 nM K252a (TrkA inhibitor), 10 µM U0126 (extracellular signal-regulated kinases 1/2 (ERK1/2) pathway inhibitor), or 10 µM LY294002 (phosphatidylinositol 3-kinase (PI3K)/AKT inhibitor) for 12 or 24 h. The vascular endothelial growth factor (VEGF) protein level in the supernatants was detected with enzyme-linked immunosorbent assay (ELISA). The TrkA, ERK1/2, and PI3K/AKT inhibitors decreased the ability of NGF to promote VEGF expression to some extent. n=6, * p<0.05, ** p<0.01, *** p<0.001.
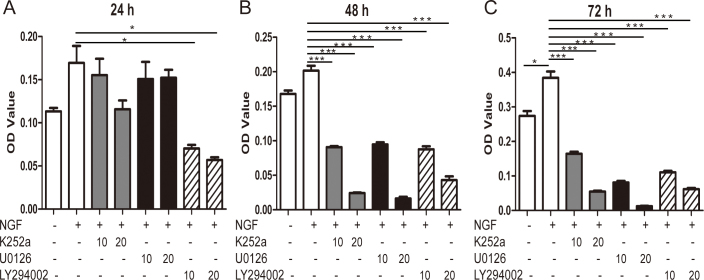
NGF improves Müller cell proliferation via ERK1/2 and AKT signaling
The ERK1/2 signaling pathway was closely related to the proliferation and differentiation of the cells, and an AKT-regulated cell cycle has been widely reported. Thus, we evaluated the role of NGF in Müller cell proliferation and used signaling inhibitors to detect the underlying mechanism. We performed a morphological assay using trypan blue staining and live cell counting and found that NGF could promote cell proliferation and ERK1/2 or PI3K/AKT was involved. We further used the MTT assay according to previous studies. As shown in Figure 5, we found NGF increased the proliferation of the Müller cells, which could be inhibited by ERK1/2 or PI3K/AKT inhibitors at different doses and time points, at 24, 48, and 72 h (n=6, *p<0.05, **p<0.01, ***p<0.001).
Figure 5.
NGF facilitates the proliferation of murine Müller cells via ERK1/2 and AKT signaling. Proliferation of Müller cells increased following nerve growth factor (NGF) treatment as analyzed with 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. In addition, the TrkA receptor, extracellular signal-regulated kinases 1/2 (ERK1/2), or phosphatidylinositol 3-kinase (PI3K)/AKT inhibitor abolished the promotion of proliferation by NGF at the 24 h (A), 48 h (B), and 72 h (C) time points. n=6, * p<0.05, ** p<0.01, *** p<0.001.
Discussion
In the retina, NGF supports the survival of retinal ganglion cells (RGCs) and photoreceptors against degeneration directly or induces other neurotrophic factor expression from Müller cells to indirectly promote photoreceptor survival [1,3]. In this study, we showed that NGF significantly increases the secretion of VEGF from Müller cells in a time-dependent manner. This effect is mediated by TrkA recognition and then the activation of the ERK1/2 and PI3K/AKT signaling pathways. Furthermore, NGF promoted Müller cell proliferation via the ERK1/2 and PI3K/AKT pathways. These data suggested that NGF has the potential to induce VEGF generation and increase Müller proliferation, which may lead to gliosis, the critical pathological changes that occur in many retinal angiogenic and proliferative diseases.
NGF, the first discovered member of the neurotrophin family, has been extensively researched for its protective role in neurons. The expression of NGF and its high affinity receptor TrkA is increased compensatively in many pathological conditions to protect tissue function, such as ocular hypertension, neurogenic inflammation, oxygen-induced retinopathy, etc. [21,39,40]. However, limited research has shown the role of NGF in Müller glia cells. Gliosis plays an important role in neurodegeneration, diabetic retinopathy, retinal detachment as well as retinal injury, and even in the physiologic aging of the retina [25,26,41,42]. In this study, we revealed that NGF promoted proliferation of Müller cells, indicating that an excess dose of NGF might induce gliosis, which might lead to secondary injury.
Müller cells, as an important bridge connecting nerves and blood vessels in the retina, are an important source of growth factors and cytokines. Among them, VEGF is the most potent proangiogenic factor and contributes to retinal neovascularization under different conditions. Many studies reported that VEGF is mostly derived from Müller cells in pathological conditions [11,23,43,44]. As a pool of VEGF, Müller cells produce and secrete VEGF in response to many stimuli. Our previous study showed that retinal glia activation is involved in inflammation-induced angiogenesis in the retina and that an intravitreal NGF injection enhances retinal neovascularization [21,45]. Our findings in this study extend that NGF has the potential to promote angiogenesis via VEGF elevation in Müller cells and the activation and proliferation of Müller cells. We found that NGF significantly increases VEGF expression in Müller cells. In addition, because NGF utilizes a receptor-coupling event, we also found that TrkA blockade, the receptor for NGF, abolished the VEGF increase, indicating that NGF induced VEGF expression via TrkA binding on Müller cells. Further research on the proangiogenic effect of NGF dependent on or independent of VEGF is warranted. In addition, increasing evidence suggests the importance of NGF-TrkA signaling in Müller cells. NGF/TrkA plays an important role in the neural retina under normal or pathological conditions. Garcia et al. showed that TrkA was widely distributed in Müller cells after retinal ischemia–reperfusion in vivo [46]. Q. Jian et al. found that cultured rat bone marrow mesenchymal stem cells (rBMSCs) secrete nerve growth factor in vitro and that rBMSCs transplantation induced TrkA expression, cell proliferation, and dedifferentiation of Müller cells in vivo [10]. Consistent with previous reports, our results showed that NGF promotes VEGF production and cellular proliferation of Müller cells in vitro, which indicates that NGF has the potential to be involved in the retinal pathological condition by targeting Müller cells.
In this study, NGF significantly upregulated VEGF expression in Müller cells in vitro, which is mediated by the binding of the TrkA receptor. Mechanistically, we found that NGF induced VEGF expression in Müller cells via PI3K/AKT and ERK1/2 signaling, which are the canonical kinase cascades. NGF activated ERK1/2 and AKT signaling 5 min after stimulation; the VEGF increase occurred at 12 and 24 h, and was more obvious after the 24 h stimulation. A possible reason may be that protein synthesis takes some time due to the complicated and time-consuming processes, which include DNA transcription, mRNA translation, and post-translational modification protein trafficking and secretion. Several pathways are shown to be involved in the angiogenesis process induced by the angiogenic growth factors. The PI3K/AKT pathway is activated upon the binding of extracellular growth factors to their respective transmembrane receptor tyrosine kinases. It is an important signaling molecule that is associated with endothelial cell survival and migration [47]. Consistent with other research, we found that the PI3K/AKT pathway mediated the survival and proliferation of Müller cells as well as VEGF generation, which are all involved in angiogenesis. In addition, the ERK1/2 pathway is activated by VEGF and FGF has also been implicated in the regulation of endothelial cell motility and survival. In this study, an ERK1/2 inhibitor blocked the activation of ERK1/2 and suppressed VEGF secretion and Müller cell proliferation, indicating that ERK1/2 activation is also required in gliosis. Therefore, the western blot and MTT assay results revealed that the inhibitory effects of the TrkA and PI3K/AKT inhibitors seem to be quite potent, while both drugs have only moderate effects on blocking NGF-induced VEGF production. The suppressive role of the ERK1/2 inhibitor in NGF-induced VEGF production appeared more potent than that of the PI3K/AKT inhibitor, indicating that the ERK1/2 signaling pathway played a more important role in VEGF secretion than the AKT signaling pathway. Taken together, these data suggested that NGF exerted various roles dominantly mediated by different signaling pathways. In addition, a TrkA inhibitor also suppressed the phosphorylation of AKT and ERK1/2 signaling, confirming that the TrkA receptor is involved in signal transduction to AKT and ERK1/2 signaling.
In summary, we showed that Müller cells express the high-affinity NGF receptor TrkA. The NGF/TrkA interaction induced VEGF secretion via the PI3K/AKT and ERK1/2 signaling pathway in Müller cells. In addition, NGF promoted the proliferation of Müller cells that was also mediated by PI3K/AKT and ERK1/2 signaling, indicating the potent gliosis role of NGF. Thus, NGF has a potential role in Müller cell–induced angiogenesis and gliosis, in addition to its role in neuroprotection, providing directions for future research on neural–vascular coupling and communication in the retina.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81470646 and 81271010). The authors declare no competing financial interests.
Appendix 1. Identification of the cultured retinal Müller cells.
The cultured retinal Müller cells were identified by immunocytochemistry using antibodies against the Müller cell markers GS and vimentin, which is a cytoskeletal intermediate filament protein in Müller cells. The nuclei were stained with DAPI. Almost all cells were positive for GS and vimentin (A). The negative controls are shown in B. To access the data, click or select the words “Appendix 1.”
References
- 1.Carmignoto G, Maffei L, Candeo P, Canella R, Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci. 1989;9:1263–72. doi: 10.1523/JNEUROSCI.09-04-01263.1989. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2467970&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambiase A, Aloe L. Nerve growth factor delays retinal degeneration in C3H mice. Graefes Arch Clin Exp Ophthalmol. 1996;234(Suppl 1):S96–100. doi: 10.1007/BF02343055. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8871157&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Lenzi L, Coassin M, Lambiase A, Bonini S, Amendola T, Aloe L. Effect of exogenous administration of nerve growth factor in the retina of rats with inherited retinitis pigmentosa. Vision Res. 2005;45:1491–500. doi: 10.1016/j.visres.2004.12.020. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15781068&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 4.Siliprandi R, Canella R, Carmignoto G. Nerve growth factor promotes functional recovery of retinal ganglion cells after ischemia. Invest Ophthalmol Vis Sci. 1993;34:3232–45. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8225858&dopt=Abstract [PubMed] [Google Scholar]
- 5.Falsini B, Chiaretti A, Barone G, Piccardi M, Pierri F, Colosimo C, Lazzareschi I, Ruggiero A, Parisi V, Fadda A, Balestrazzi E, Riccardi R. Topical nerve growth factor as a visual rescue strategy in pediatric optic gliomas: a pilot study including electrophysiology. Neurorehabil Neural Repair. 2011;25:512–20. doi: 10.1177/1545968310397201. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21444653&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Tuszynski MH, Thal L, Pay M, Salmon DP. U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–5. doi: 10.1038/nm1239. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15852017&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 7.Carmignoto G, Comelli MC, Candeo P, Cavicchioli L, Yan Q, Merighi A, Maffei L. Expression of NGF receptor and NGF receptor mRNA in the developing and adult rat retina. Exp Neurol. 1991;111:302–11. doi: 10.1016/0014-4886(91)90097-v. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1847878&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti S, Sima AA, Lee J, Brachet P, Dicou E. Nerve growth factor (NGF), proNGF and NGF receptor-like immunoreactivity in BB rat retina. Brain Res. 1990;523:11–5. doi: 10.1016/0006-8993(90)91630-y. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2169962&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 9.Micera A, Lambiase A, Aloe L, Bonini S, Levi-Schaffer F. Nerve growth factor involvement in the visual system: implications in allergic and neurodegenerative diseases. Cytokine Growth Factor Rev. 2004;15:411–7. doi: 10.1016/j.cytogfr.2004.09.003. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15561599&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 10.Jian Q, Li Y, Yin ZQ. Rat BMSCs initiate retinal endogenous repair through NGF/TrkA signaling. Exp Eye Res. 2015;132:34–47. doi: 10.1016/j.exer.2015.01.008. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25584870&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, Le YZ. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219:446–54. doi: 10.1002/path.2611. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19768732&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 12.Gallina D, Zelinka CP, Cebulla C, Fischer AJ. Activation of glucocorticoid receptors in Muller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol. 2015;273:114–25. doi: 10.1016/j.expneurol.2015.08.007. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26272753&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–36. doi: 10.1523/JNEUROSCI.22-21-09228.2002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12417648&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iandiev I, Biedermann B, Bringmann A, Reichel MB, Reichenbach A, Pannicke T. Atypical gliosis in Muller cells of the slowly degenerating rds mutant mouse retina. Exp Eye Res. 2006;82:449–57. doi: 10.1016/j.exer.2005.07.018. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16154566&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 15.Distler C, Dreher Z. Glia cells of the monkey retina–II. Muller cells. Vision Res. 1996;36:2381–94. doi: 10.1016/0042-6989(96)00005-3. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8917802&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 16.Barnett NL, Pow DV, Bull ND. Differential perturbation of neuronal and glial glutamate transport systems in retinal ischaemia. Neurochem Int. 2001;39:291–9. doi: 10.1016/s0197-0186(01)00033-x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11551669&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Nakazawa T, Matsubara A, Noda K, Hisatomi T, She H, Skondra D, Miyahara S, Sobrin L, Thomas KL, Chen DF, Grosskreutz CL, Hafezi-Moghadam A, Miller JW. Characterization of cytokine responses to retinal detachment in rats. Mol Vis. 2006;12:867–78. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16917487&dopt=Abstract [PubMed] [Google Scholar]
- 18.Romon R, Adriaenssens E, Lagadec C, Germain E, Hondermarck H, Le Bourhis X. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol Cancer. 2010;9:157. doi: 10.1186/1476-4598-9-157. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20569463&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazarovici P, Marcinkiewicz C, Lelkes PI. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr Pharm Des. 2006;12:2609–22. doi: 10.2174/138161206777698738. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16842161&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 20.Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappala G, Pafumi C, Bernardini R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–9. doi: 10.1096/fj.01-1000fje. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12154004&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Wang D, Liu Y, Luo Y, Ma W, Xiao W, Yu Q. Neuronal-driven angiogenesis: role of NGF in retinal neovascularization in an oxygen-induced retinopathy model. Invest Ophthalmol Vis Sci. 2010;51:3749–57. doi: 10.1167/iovs.09-4226. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20207957&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu M, Le YZ, Ge J, Johnson RS, Ma JX. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Muller cells. Diabetologia. 2011;54:1554–66. doi: 10.1007/s00125-011-2081-0. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21360191&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Xu XL, Elliott MH, Zhu ML, Le YZ. Muller Cell-Derived VEGF Is Essential for Diabetes-Induced Retinal Inflammation and Vascular Leakage. Diabetes. 2010;59:2297–305. doi: 10.2337/db09-1420. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20530741&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bringmann A, Reichenbach A. Role of Muller cells in retinal degenerations. Front Biosci. 2001;6:E72–92. doi: 10.2741/bringman. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11578954&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 25.Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003;230:263–90. doi: 10.1016/s0074-7696(03)30005-1. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14692684&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 26.Okada M, Matsumura M, Ogino N, Honda Y. Muller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefes Arch Clin Exp Ophthalmol. 1990;228:467–74. doi: 10.1007/BF00927264. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2227494&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 27.Guidry C. The role of Muller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24:75–86. doi: 10.1016/j.preteyeres.2004.07.001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15555527&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 28.Iandiev I, Uhlmann S, Pietsch UC, Biedermann B, Reichenbach A, Wiedemann P, Bringmann A. Endothelin receptors in the detached retina of the pig. Neurosci Lett. 2005;384:72–5. doi: 10.1016/j.neulet.2005.04.056. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15885900&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 29.Rattner A, Nathans J. The genomic response to retinal disease and injury: Evidence for endothelin signaling from photoreceptors to glia. J Neurosci. 2005;25:4540–9. doi: 10.1523/JNEUROSCI.0492-05.2005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15872101&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geller SF, Lewis GP, Fisher SK. FGFR1, signaling, and AP-1 expression after retinal detachment: Reactive Muller and RPE cells. Invest Ophthalmol Vis Sci. 2001;42:1363–9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11328752&dopt=Abstract [PubMed] [Google Scholar]
- 31.Zhang F, Tang ZS, Hou X, Lennartsson J, Li Y, Koch AW, Scotney P, Lee C, Arjunan P, Dong LJ, Kumar A, Rissanen TT, Wang B, Nagai N, Fons P, Fariss R, Zhang YQ, Wawrousek E, Tansey G, Raber J, Fong GH, Ding H, Greenberg DA, Becker KG, Herbert JM, Nash A, Yla-Herttuala S, Cao YH, Watts RJ, Li XR. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009;106:6152–7. doi: 10.1073/pnas.0813061106. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19369214&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P, Lennartsson J, Zhu C, Qu Y, Fang C, Hua J, Matsuo O, Fong GH, Ding H, Cao Y, Becker KG, Nash A, Heldin CH, Li X. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118:913–23. doi: 10.1172/JCI33673. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18259607&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards MM, McLeod DS, Bhutto IA, Villalonga MB, Seddon JM, Lutty GA. Idiopathic preretinal glia in aging and age-related macular degeneration. Exp Eye Res. 2015 doi: 10.1016/j.exer.2015.07.016. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26220834&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen F, Chen B, Danias J, Lee KC, Lee H, Su Y, Podos SM, Mittag TW. Glutamate-induced glutamine synthetase expression in retinal Muller cells after short-term ocular hypertension in the rat. Invest Ophthalmol Vis Sci. 2004;45:3107–12. doi: 10.1167/iovs.03-0948. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15326127&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 35.Valamanesh F, Monnin J, Morand-Villeneuve N, Michel G, Zaher M, Miloudi S, Chemouni D, Jeanny JC, Versaux-Botteri C. Nestin expression in the retina of rats with inherited retinal degeneration. Exp Eye Res. 2013;110:26–34. doi: 10.1016/j.exer.2013.01.013. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23399867&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 36.Elshaer SL, Abdelsaid MA, Al-Azayzih A, Kumar P, Matragoon S, Nussbaum JJ, El-Remessy AB. Pronerve Growth Factor Induces Angiogenesis via Activation of TrkA: Possible Role in Proliferative Diabetic Retinopathy. J Diabetes Res. 2013 doi: 10.1155/2013/432659. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23998130&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jadhao CS, Bhatwadekar AD, Jiang Y, Boulton ME, Steinle JJ, Grant MB. Nerve growth factor promotes endothelial progenitor cell-mediated angiogenic responses. Invest Ophthalmol Vis Sci. 2012;53:2030–7. doi: 10.1167/iovs.11-8430. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22410557&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinle JJ, Granger HJ. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton Neurosci. 2003;108:57–62. doi: 10.1016/S1566-0702(03)00151-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14614965&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 39.Rudzinski M, Wong TP, Saragovi HU. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J Neurobiol. 2004;58:341–54. doi: 10.1002/neu.10293. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14750147&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 40.Bronzetti E, Artico M, Kovacs I, Felici LM, Magliulo G, Vignone D, D’Ambrosio A, Forte F, Di Liddo R, Feher J. Expression of neurotransmitters and neurotrophins in neurogenic inflammation of the rat retina. European journal of histochemistry. EJH. 2007;51:251–60. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18162454&dopt=Abstract [PubMed] [Google Scholar]
- 41.Illes P, Verkhratsky A. Purinergic neurone-glia signalling in cognitive-related pathologies. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.08.005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26256423&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 42.Reichenbach A, Bringmann A. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.05.005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25998275&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 43.Wang JJ, Zhu M, Le YZ. Functions of Muller cell-derived vascular endothelial growth factor in diabetic retinopathy. World J Diabetes. 2015;6:726–33. doi: 10.4239/wjd.v6.i5.726. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26069721&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–9. doi: 10.1073/pnas.92.3.905. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7846076&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He C, Sun YY, Ren XR, Lin Q, Hu X, Huang X, Su SB, Liu YZ, Liu XL. Angiogenesis Mediated by Toll-Like Receptor 4 in Ischemic Neural Tissue. Arterioscler Thromb Vasc Biol. 2013;33:330. doi: 10.1161/ATVBAHA.112.300679. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23241411&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 46.Garcia TB, Pannicke T, Vogler S, Berk BA, Grosche A, Wiedemann P, Seeger J, Reichenbach A, Herculano AM, Bringmann A. Nerve growth factor inhibits osmotic swelling of rat retinal glial (Muller) and bipolar cells by inducing glial cytokine release. J Neurochem. 2014;131:303–13. doi: 10.1111/jnc.12822. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25041175&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 47.Lv WW, Qin SN, Chen CQ, Zhang JJ, Ren TS, Xu YN, Zhao QC. Isoindolone derivative QSN-10c induces leukemic cell apoptosis and suppresses angiogenesis via PI3K/AKT signaling pathway inhibition. Acta Pharmacol Sin. 2014;35:625–35. doi: 10.1038/aps.2013.194. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24786233&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]