Abstract
Pneumonia is an infectious disease of the lung causing mortality. Mycoplasma pneumonia (MP) is an atypical bacterial pneumonia that damages several organs. Lung computed tomography (CT) has been utilized in its identification. The aim of the present study was to examine the value of computed tomography diagnosis for pediatric MP. The present study prospectively analyzed the clinical and imaging data of 1,280 cases of pediatric MP in the out- and inpatient departments from March, 2010 to March, 2014; analyzed the morphology and distribution of the pneumonic lesion in the lungs; and summarized the value of CT diagnosis for pediatric MP. In the included children, there were 688 cases of lesions in the unilateral lobe, 592 cases of lesions in the bilateral lobes, 1,101 cases of extensive patchy opacity, 496 cases of mottled opacity, 432 cases of increased lung marking, 256 cases of streak opacity, 192 cases of ground-glass opacity, 992 cases of thickened bronchial wall in the lesions, 128 cases of lymphadenopathy in the hilar lymph nodes and mediastinal lymph nodes, and the lung CT showed 32 cases of pulmonary cavity and 144 cases of pleural effusion. In conclusion, the CT signals of pediatric MP had several types with some children exhibiting complicated changes. The child's clinical manifestation and symptoms should thus be considered in the diagnosis to improve the diagnostic rate.
Keywords: computed tomography diagnosis, pediatric pneumonia, mycoplasma pneumonia, clinical value
Introduction
Pneumonia is a lung infection caused by a variety of germs, including viruses, bacteria, fungi and parasites (1). Symptoms of pneumonia vary depending on child's age and the cause of the pneumonia. The main symptoms include sore throat, headache, cough and fever (1,2). Pneumonia is a major infectious disease responsible for significant morbidity and mortality worldwide.
Pneumonia caused by mycoplasma bacteria is a less serious form of infection (3). The child's secretion sputum is contagious and spreads easily in children. Mycoplasma pneumonia (MP) is a form of atypical bacterial pneumonia that damages several organs and systems through local infection of the respiratory tract. It was extensively spread in children with an increasing morbidity and is rapidly developing into a prevalent disease (4–6).
A chest X-ray is the optimal option for diagnosing pneumonia. However, this test may not be ideal to identify the cause of pneumonia. Clinical tests are not considered significant, therefore a lung computed tomography (CT) scan was necessary for the early diagnosis of the disease (7).
The diagnosis of pediatric pneumonia should be performed based on the major clinical manifestations and corresponding CT examination of the children. The present study prospectively analyzed the clinical data of 1,280 children to assess the diagnostic value of CT examination in pediatric MP. Gaining understanding of the children's characteristic imaging data was beneficial to the clinical diagnosis and treatment of pediatric MP.
Materials and methods
General information
The current study included 1,280 children (680 males and 600 females) with pediatric MP in the out- and inpatient departments from March, 2010 to March, 2014. The age range of the patients was 3 months to 10 years, with a mean age of 4.3±1.8 years, while the range of disease course was 3–15 days, with a mean course of 8.9±2.1 days. The clinical manifestations of all the patients were paroxysmal cough and irritating cough. Most children were complicated with fever, expectoration, chest stuffiness, and pharyngolaryngitis to varying degrees. All the children met the diagnostic criteria of pediatric MP in practical pediatrics (8). The study was approved by the Ethics Committee of Xuzhou Children's Hospital (Jiangsu, China).
Inclusion criteria
Inclusion criteria for the study were: i) Age range 3 months to 12 years (6); ii) major clinical manifestations meeting the clinical diagnostic criteria, including cough, fever, loss of appetite, and moist rales in the lung; iii) disease course of 4–15 days; iv) having signed informed consent with the agreement of guardians and v) exclusion of children with bronchitis, asthma, and respiratory infection due to cold (9).
Examination methods
Whole lung scanning was performed from apex pulmonis to basis pulmonis with a Philips Brilliance 16-slice spiral CT (Philips Healthcare, DA Best, The Netherlands). The slice width and spacing were 5 mm, the pitch was 1.20, and the scanning time was 1.0 sec. The voltage was maintained at 120 kV, the constant at 80–160 mA, the mediastinal window was W450:L45, and the lung window was W1,000:L2,500. The vertical and coronal planes of the lesion were reconstructed spontaneously. CT scan with contrast was performed after the plain scan and the scan parameters were the same as with those of the plain scan. The contrast agent was iohexol, which was injected by a high-pressure injector at a rate of 1.5–3 ml/sec. The mean CT value of the margin and interior of the lesion was calculated. Children with poor compliance were orally administered with 3% chloral hydrate (1.5 ml/kg). Children requiring special care completed the examinations with the accompaniment of his/her parents'/guardians (10).
CT images
The location, size, shape, density, distribution, internal structure, margin of the lesion, pleural effusion, hilar lymph nodes and mediastinal lymph nodes from the lung and mediastinal window were observed. The imaging data of the lesion, lesion site, its adjacent tissue and organ lesions obtained from all the CT examinations were analyzed.
Observational measurements
The lung CT imaging data of all the children were observed. The number of lobe lesions, including the lesions in the unilateral and bilateral lobes, the number of lesions in the left and right lobes and the main imaging features of the lesion lobes were recorded (11).
Results
Analysis of the lung CT imaging data of the children
As shown in Table I, of all the children, there were 688 cases of unilateral lobe lesions, 592 cases of bilateral lobe lesions, including 338 cases of left upper lobe lesions, 416 cases of left lower lobe lesions, 370 cases of right upper lobe lesions, 240 cases of left middle lobe lesions, and 435 cases of right lower lobe lesions. The lesions were predominantly extensive patchy opacity, mottled opacity, increased lung marking, streak opacity and ground-glass opacity. In addition, there were 992 cases of thickened bronchial wall in the lesions, 128 cases of lymphadenopathy in hilar lymph nodes and mediastinal lymph nodes, and lung CT showed 32 cases of pulmonary cavity and 144 cases of pleural effusion.
Table I.
The analysis of the lung CT imaging data of the children (no., %)
| Characteristics | No. (%) |
|---|---|
| Lesion distribution | |
| Lesion in unilateral lobe | 688 (53.8) |
| Right upper lobe | 370 (28.9) |
| Right middle lobe | 240 (18.8) |
| Right lower lobe | 435 (34.0) |
| Left upper lobe | 338 (26.4) |
| Left lower lobe | 416 (32.5) |
| Lesions in bilateral lobes | 592 (46.3) |
| Features of the CT imaging of the lesions | |
| Extensive patchy opacity | 1,101 (86.0) |
| Mottled opacity | 496 (38.8) |
| Increased lung marking | 432 (33.8) |
| Streak opacity | 256 (20.0) |
| Ground-glass opacity | 192 (15.0) |
| Other lung manifestations | |
| Thickened bronchial wall | 992 (77.5) |
| Hilar and mediastinal lymphadenopathy | 128 (10.0) |
| Pulmonary cavity | 32 (2.5) |
| Pleural effusion | 144 (11.3) |
CT, computed tomography.
The analysis of imaging features
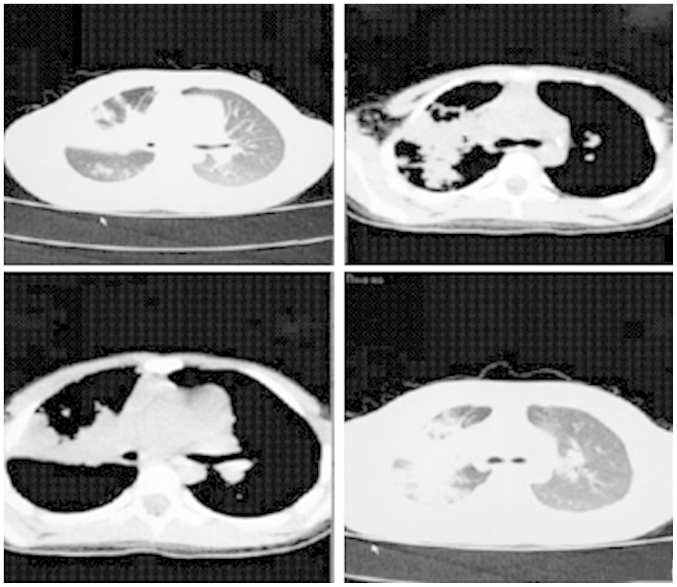
In a case involving a female child ages 5-years and 11 months old, who was hospitalized due to fever and cough for 4 days, the diagnosis was bilateral pneumonia and predominantly pneumonia in the upper lobe of the right lung. As shown in Fig. 1, the major imaging features included extensive high-density opacity in the upper lobe of the right lung, with the sign of bronchial inflation, less uniform lesion density, blurry boundary and slight thickening of the adjacent pleura, flocculent opacity and small patchy opacity in the lower lobe of the right lung. Increased vascular bundles in the bilateral bronchi were observed albeit they were blurry. Trachea and bilateral primary bronchi were unobstructed. No obvious density was identified in the mediastinum, a thymus shadow was observed in the anterior superior mediastinum, no obvious abnormal shape and size of the heart shadow was identified and no clear free liquid was evident in the bilateral thoracic cavities. No obvious abnormality in the bilateral thoracic spine and ribs was evident, and the structure of the soft tissue in the chest wall was clear.
Figure 1.
The imaging of bilateral inflammation in the children.
Discussion
Pediatric MP is an infective respiratory disease common in children due to MP (12–14), accounting for approximately 50% of cases. However, the morbidity of this disease has been on the increase (15). This disease can damage various organs and affect the child's normal growth and development. The disease has a short incubation period with the main symptoms of manifesting at the early phase of this disease head pain, fever, cough and pharyngalgia. Pediatric MP is seasonal, occurring primarily in spring and autumn. The child's secreta such as body fluid and sputum become contagious. Extensive infection occurs when the mycoplasma is mutated, and even death may occur when the disease is severe (16–18).
The X-ray imaging changes were identified as more significant than those of clinical manifestation (19,20). X-ray examination showed that at the early phase, the clinical manifestations of most children were interstitial pneumonia, increased lung marking, reticular opacity, and uniform blurry opacity or patchy opacity when the condition was worsened with most lesions being close to the hilus and predominantly in the lower lung. Extensive infiltration and solid changes were rare, with some children having hilar lymphadenopathy, while a few children had pleural effusion (21). In comparison with X-ray, CT examination determined bronchitis, mixed pathological changes, and interstitial pneumonia. Therefore, CT was usually used for the diagnosis of children. Increasing reports have focused on the use of CT in the diagnosis of pneumonia. Xinbo (22) analyzed the CT examinations of children with pediatric pneumonia and the results indicated that the lung lesions were predominantly unilateral, the proportion of solid infiltration lesions was high, and the lung bronchial wall was thickened while some children had pleural effusion and rare hilar lymphadenopathy (23,24). In the current study, analysis of the data indicated that: i) the CT imaging of pediatric MP was a mainly unilateral lesion (53.8%), and secondly bilateral lesion (46.3%). Thus, bilateral lesions were more prevalent with the lesion in the lower lobe being mostly prevalent in all parts of the lung (the incidence of bilateral lesions in the lower lobes was >30.0%.) ii) The CT imaging of pediatric MP indicated various types of lesions in the lung tissues, including extensive patchy opacity, mottled opacity, increased lung marking, streak opacity and ground-glass opacity, with extensive patchy opacity being the main lesion, accounting for 86.0% of cases. iii) Analysis of the CT imaging data of 1,280 children with pediatric MP indicated that the children had simple pathological lobe lesions as well as complicated lesions in adjacent intrathoracic organs, predominantly thickened bronchial wall (77.5%). Other signs were not significant and only identified in some children, including hilar and mediastinal lymphadenopathy, pulmonary cavity and pleural effusion. Therefore, a comprehensive diagnosis should be performed based on the major clinical manifestations and corresponding CT examinations of the children with pediatric MP. A differential diagnosis should be performed with other types of pneumonia to determine the clinical treatment option, achieve early treatment, improve the diagnostic and cure rates, and promote early recovery (25,26).
In conclusion, the diagnosis of pediatric MP should be performed based on the major clinical manifestations and CT imaging data of the children with pediatric MP to accomplish early diagnosis and treatment. Understanding of the children's characteristic imaging data was beneficial to clinical diagnosis and treatment.
References
- 1.Rambaud-Althaus C, Althaus F, Genton B, D'Acremont V. Clinical features for diagnosis of pneumonia in children younger than 5 years: A systematic review and meta-analysis. Lancet Infect Dis. 2015;15:439–450. doi: 10.1016/S1473-3099(15)70017-4. [DOI] [PubMed] [Google Scholar]
- 2.José RJ, Periselneris JN, Brown JS. Community-acquired pneumonia. Curr Opin Pulm Med. 2015;21:212–218. doi: 10.1097/MCP.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 3.Edelstein I, Rachina S, Touati A, Kozlov R, Henin N, Bébéar C, Pereyre S. Mycoplasma pneumoniae Monoclonal P1 Type 2c Outbreak, Russia, 2013. Emerg Infect Dis. 2016;22:348–350. doi: 10.3201/eid2202.151349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aydogan P, Kahyaoglu S, Saygan S, Kaymak O, Mollamahmutoglu L, Danisman N. Does cervical ureaplasma/mycoplasma colonization increase the lower uterine segment bleeding risk during cesarean section among patients with placenta previa? A cross-sectional study. Eur Rev Med Pharmacol Sci. 2014;18:2243–2247. [PubMed] [Google Scholar]
- 5.Zhifeng M. The difference between the clinical manifestations of X-ray and CT of pediatric mycoplasma pneumonia. Chin Healthc Nutr. 2014;24:1153–1154. [Google Scholar]
- 6.Wu SH, Chen XQ, Kong X, Yin PL, Dong L, Liao PY, Wu JM. Characteristics of respiratory syncytial virus-induced bronchiolitis co-infection with Mycoplasma pneumoniae and add-on therapy with montelukast. World J Pediatr. 2015 Apr 6; doi: 10.1007/s12519-015-0024-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Kazama I, Tamada T, Nakajima T. Macroscopic haemoglobinuria associated with Mycoplasma pneumoniae infection successfully treated by clarithromycin. Infez Med. 2015;23:74–78. [PubMed] [Google Scholar]
- 8.Fukang Z, Shiting F, Jingdi C, et al. The represents of pulmonary tuberculoma and inammatory pseudotumor at helical dynamic enhanced CT. Chinese Journal of CT and MRI. 2011;9:32–34. [Google Scholar]
- 9.Feng S, Huiquan Y, Hong C, et al. The analysis of the clinical features and extrapulmonary manifestations of 60 children with pediatric Mycoplasma pneumonia. Chin Integr Pediatr. 2012;4:261–263. [Google Scholar]
- 10.Xiangying H, Zhaoding H, Kaimei W, et al. The analysis of the clinical features and imaging features of 260 children with pediatric Mycoplasma pneumonia. Chin J Misdiag. 2011;11:5929–5930. [Google Scholar]
- 11.Chung HL, Shin JY, Ju M, Kim WT, Kim SG. Decreased interleukin-18 response in asthmatic children with severe Mycoplasma pneumoniae pneumonia. Cytokine. 2011;54:218–221. doi: 10.1016/j.cyto.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanjun D, Ruimeng Y, Yunhai H, et al. The exploration of 320 cases of first-pass perfusion imaging CT in the differentiation of benign and malignant tumor. Chin J CT and MRI. 2014;4:16–17. 44. [Google Scholar]
- 13.Yimenicioğlu S, Yakut A, Ekici A, Carman Bora K, Cagrı Dinleyici E. Mycoplasma pneumoniae infection with neurologic complications. Iran J Pediatr. 2014;24:647–651. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuo Z, Li F, Chen X, Jin P, Guo Q, Wang H. Mycoplasma pneumonia combined with pulmonary infarction in a child. Int J Clin Exp Med. 2015;8:1482–1486. [PMC free article] [PubMed] [Google Scholar]
- 15.Horiba K, Gotoh K, Hattori F, Takeuchi S, Nishimura N, Ozaki T, Shiraki K. Analysis of 23S rRNA of Mycoplasma pneumoniae detected from pediatric inpatients with community-acquired pneumonia in a regional hospital in Japan. Kansenshogaku Zasshi. 2014;88:715–716. doi: 10.11150/kansenshogakuzasshi.88.715. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 16.Fuchuan W, Peigang N, Tao G, et al. The multi-slice spiral CT examination of pneumococcal pneumonia. Chin J CT and MRI. 2014;11:17–19. [Google Scholar]
- 17.Dumke R, Stolz S, Jacobs E, Juretzek T. Molecular characterization of macrolide resistance of a Mycoplasma pneumoniae strain that developed during therapy of a patient with pneumonia. Int J Infect Dis. 2014;29:197–199. doi: 10.1016/j.ijid.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Prindaville B, Newell BD, Nopper AJ, Horii KA. Mycoplasma pneumonia-associated mucocutaneous disease in children: dilemmas in classification. Pediatr Dermatol. 2014;31:670–675. doi: 10.1111/pde.12482. [DOI] [PubMed] [Google Scholar]
- 19.Gu L, Chen X, Li H, Qu J, Miao M, Zhou F, Zhu Y, Wang X, Wang C, Liu Y, et al. A case of lethal hemolytic anemia associated with severe pneumonia caused by Mycoplasma pneumoniae. Chin Med J (Engl) 2014;127:3839. [PubMed] [Google Scholar]
- 20.Cheng HH, Tang TT, He Q, Huang LJ, Lin XL, Chen M, Yang C, Geng DF, Jiang SP. Beneficial effects of statins on outcomes in pneumonia: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2014;18:2294–2305. [PubMed] [Google Scholar]
- 21.Zhou FJ, Zhou CY, Tian YJ, Xiao AJ, Li PL, Wang YH, Jia JW. Diagnostic value of analysis of H-FABP, NT-proBNP, and cTnI in heart function in children with congenital heart disease and pneumonia. Eur Rev Med Pharmacol Sci. 2014;18:1513–1516. [PubMed] [Google Scholar]
- 22.Xinbo Z. The characteristics of the CT imaging of pediatric mycoplasma pneumonia and the treatments. Chin Commun Phys Med. 2012;14:233–233. [Google Scholar]
- 23.Sen V, Kelekci S, Sen Selimoglu H, Yolbas I, Günes A, Abakay O, Gurkan Fuat M. An evaluation of cases of pneumonia that occurred secondary to hydrocarbon exposure in children. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 1):9–12. [PubMed] [Google Scholar]
- 24.Ucgun I, Dagli CE, Kiremitci A, Yildirim H, Ak G, Aslan S. Effects of isolation rooms on the prevalence of hospital acquired pneumonia in a respiratory ICU. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 1):2–8. [PubMed] [Google Scholar]
- 25.Kim YH, Kim JE, Hyun MC. Cytokine response in pediatric patients with pandemic influenza H1N1 2009 virus infection and pneumonia: comparison with pediatric pneumonia without H1N1 2009 infection. Pediatr Pulmonol. 2011;46:1233–1239. doi: 10.1002/ppul.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno P, Ricci A, Pezzuto A, Martone L, Gencarelli G, Mariotta S. Severe pneumonia caused by Nocardia farcinica and complicated by Staphylococcus haemoliticus superinfection. Eur Rev Med Pharmacol Sci. 2011;15:401–405. [PubMed] [Google Scholar]