Abstract
Eribulin mesylate (eribulin), an analog of the marine natural product halichondrin B, is a microtubule-depolymerizing drug that has utility in the treatment of patients with breast cancer. Clinical trial results have demonstrated that eribulin treatment provides a survival advantage to patients with metastatic or locally advanced breast cancer previously treated with an anthracycline and a taxane. Furthermore, a pooled analysis of two pivotal phase III trials has demonstrated that eribulin also improves overall survival in several patient subgroups, including in women with human epidermal growth factor receptor 2 (HER2)-negative disease and triple-negative breast cancer. This review covers the preclinical research that led to the clinical testing and approval of eribulin, as well as subsequent research that was prompted by distinct and unexpected effects of eribulin in the clinic. Initial studies with halichondrin B, and then eribulin, demonstrated unique effects on tubulin binding that resulted in distinct microtubule-dependent events and antitumor actions. Consistent with the actions of the natural product, eribulin has potent microtubule-depolymerizing activities and properties that distinguish it from other microtubule targeting agents. Here, we review new results that further differentiate the effects of eribulin from other agents on peripheral nerves, angiogenesis, vascular remodeling and epithelial-to-mesenchymal transition. Together, these data highlight the distinct properties of eribulin and begin to delineate the mechanisms behind the increased survival benefit provided by eribulin for patients.
Keywords: eribulin, halichondrin B, metastatic breast cancer, microtubules, mechanism of action
Introduction
Eribulin mesylate (eribulin) is a novel microtubule targeting agent (MTA) that is used in the treatment of metastatic breast cancer. The EMBRACE clinical trial showed a survival advantage for pretreated patients with locally recurrent or metastatic breast cancer who were treated with eribulin when compared with treatment of physician’s choice (1). These positive results contributed to the approval of eribulin (Halaven®, Eisai Inc., Woodcliff Lake, NJ, USA) in the USA in 2010, and in Europe and Japan in 2011. A pooled analysis of the EMBRACE and Study 301 phase III trials has also shown that eribulin improves overall survival in several patient subgroups with advanced/metastatic breast cancer who have previously received an anthracycline and a taxane. Among those who may benefit from eribulin are women with HER2 negative and triple-negative breast cancer (2).
This review describes the initial interest in the halichondrins as potential anticancer drugs and the preclinical development of eribulin, including initial establishment of the biochemical and cellular mechanisms of its microtubule destabilizing activity. In addition, we describe new in vitro and in vivo studies triggered by unexpected clinical findings that establish a distinct biological profile of eribulin’s effects in both tumor tissue and supporting stroma. Ultimately, this review seeks to highlight the differences in the biological effects of eribulin in comparison with other MTAs that might contribute to its clinical activities.
From Halichondrin B to Eribulin
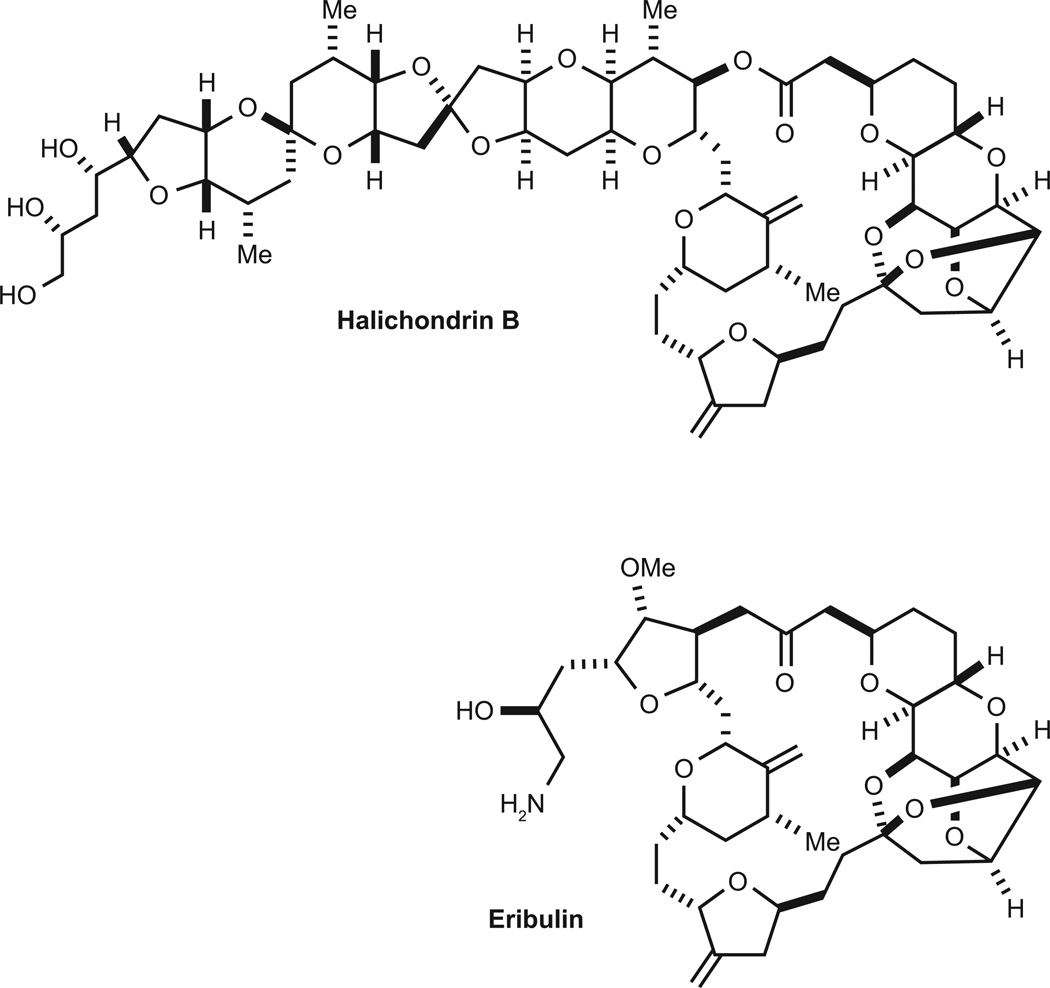
Halichondrin B (Fig. 1), was isolated in 1986 from the sponge Halichondria okadai based on its cytotoxicity (3). Halichondrin B was found to have extraordinary cytotoxic potency in vitro and antitumor activity against murine models of solid tumors and leukemia in vivo; however, only low yields of the compound could be obtained (4). Evaluation of halichondrin B in the NCI-60 cell line panel using the COMPARE algorithm revealed a pattern similar to that of other tubulin binding agents, suggesting a microtubule-dependent mechanism of action (4). Detailed mechanistic studies showed that halichondrin B inhibited tubulin polymerization and tubulin-dependent guanosine triphosphate (GTP) hydrolysis in a manner distinct from that of other microtubule destabilizers (4).
Figure 1.
Chemical structures of halichondrin B and eribulin.
Halichondrin B was found to bind within a region of tubulin designated the ‘vinca domain’, at which drugs such as dolastatin 10 bind and non-competitively inhibit the binding of vinca alkaloids (4, 5). The binding properties of halichondrin B were similar to those of dolastatin 10; however, there were notable differences between the compounds, in that halichondrin B caused distinct conformational effects on tubulin (6).
Halichondrin B was therefore mechanistically interesting, with promising antitumor effects, but its complex structure and the low yield from natural sources severely limited its potential for clinical development. A breakthrough occurred in 1992, when the Kishi laboratory succeeded in the total synthesis of halichondrin B (7). This allowed the design, synthesis and evaluation of many analogs of the compound, one of which, eribulin (ER-086526, E7389, NSC-707389), is the subject of this review. The story of how the daunting 63-step chemical synthesis of eribulin was developed and made economically feasible was recently described (8).
Preclinical Studies on Eribulin
Identification of eribulin as a microtubule targeting agent
The promising biological effects of halichondrin B led to the synthesis of over 180 halichondrin B analogs (9, 10). Optimal preclinical activities were observed for two macrocyclic ketone analogs, and after extensive preclinical testing, the C35 primary amine-substituted compound (eribulin; Fig. 1) was selected for clinical development.
Towle et al. showed that eribulin had potent antiproliferative effects across a panel of eight human cancer cells lines, with an average half maximal inhibitory concentration (IC50) value of 1.8 nM (11). The cytotoxic effects of eribulin were selective for proliferating cells, because no cytotoxicity was observed against fully quiescent immortalized human fibroblasts at concentrations up to 1 µM (11). Similar to other MTAs, eribulin caused cells to accumulate in mitosis with aberrant mitotic spindles leading to apoptosis, suggesting that eribulin, like its parent halichondrin B, was a MTA (11–13). In contrast to most other MTAs, the mitotic blockade induced by eribulin was shown to be irreversible; this may be an important feature that enables even transient drug exposure to result in long-term loss of cell viability (13). The initial direct interaction of eribulin with tubulin was also demonstrated (11).
Based on a combination of experimental and modeling data, it has been proposed that eribulin binds within a pocket underneath the H3 and H11 loops of β-tubulin in a distinct manner from that of other MTAs (14). Studies with radiolabeled eribulin and tubulin heterodimers demonstrated relatively low affinity (46 µM) and 1:1 binding (15); however, eribulin binds with high affinity (3.5 µM) to polymerized microtubules in vitro, in a concentration-dependent manner reaching an extrapolated maximum of 14.7 molecules per microtubule: this is consistent with the hypothesis that eribulin acts as a microtubule end poison by binding with high affinity to each of the 13 β-tubulin subunits at the plus end of each microtubule protofilament.
Effects of eribulin on microtubule dynamics
Using live traces of single microtubules made from purified bovine tubulin, Jordan et al. discovered that eribulin promoted pausing of microtubule growth, and strongly inhibited the growth rate of microtubules (16). However, unlike vinblastine, eribulin had little to no effect on the microtubule-shortening rate. Similar results were observed in live MCF7 interphase cells expressing green fluorescent protein (GFP)-tubulin, in which 1 nM eribulin suppressed microtubule growth with little effect on disassembly, and decreased microtubule dynamicity overall (16). Eribulin also induced mitotic accumulation with aberrant mitotic spindle dynamics at 1 nM, demonstrating an ability to impact overall microtubule function at this concentration. Together, these results showed that eribulin disrupted microtubule dynamics in a manner somewhat distinct from that of vinblastine (17). The effects of eribulin on centromere dynamics during mitosis were also evaluated (18). Live U2OS cells expressing GFP-labeled centromere protein B, a component of the centromere, were used to measure functional microtubule dynamicity during mitosis. While control cells exhibited centromere dynamics of 0.84 µm/min, 60 nM eribulin decreased centromere dynamics to 0.55 µm/min. In contrast to other MTAs, eribulin did not affect the mean centromere separation distance, consistent with biochemical results indicating that eribulin disrupts microtubules differently.
Effects of eribulin in murine xenograft models
Eribulin demonstrated a wide spectrum of antitumor activity in human xenograft models of breast cancer, colon cancer, fibrosarcoma, glioblastoma, head and neck cancer, leiomyosarcoma, ovarian cancer, melanoma, pancreatic cancer, non-small-cell lung cancer and small-cell lung cancer (11, 19). In addition, eribulin also had activity in cells from a range of pediatric solid tumors and acute lymphocytic leukemia in vitro and in xenograft models (20). Collectively, these preclinical studies established eribulin as a promising compound, that retained the unique properties of halichondrin B and had excellent preclinical in vivo activity. Importantly, eribulin also had an acceptable toxicity profile and therapeutic window in mice across several dosing schedules.
Clinical Observations and Resulting Studies
The EMBRACE trial compared eribulin with treatment of physician’s choice, and showed an overall survival advantage in patients treated with eribulin (1), kindling new research to identify which properties of the drug might contribute to this survival advantage. In addition, while the overall side effect profile was similar between eribulin and the physician’s choice arm (which included other tubulin-targeting agents such as vinorelbine and several non-MTA therapies), there were also some differences. This led to further preclinical research, as described below.
Peripheral neuropathy
Peripheral neuropathy is a well-documented toxicity of MTAs that can lead to treatment discontinuation or dose reduction. Observations during the clinical evaluation of eribulin indicated that the incidence and severity of peripheral neuropathy might differ between eribulin and other MTAs (21). These initial observations prompted comparative in vitro studies on the effects of eribulin, ixabepilone, and paclitaxel in mice to determine differences in the effects of these agents on peripheral nerves.
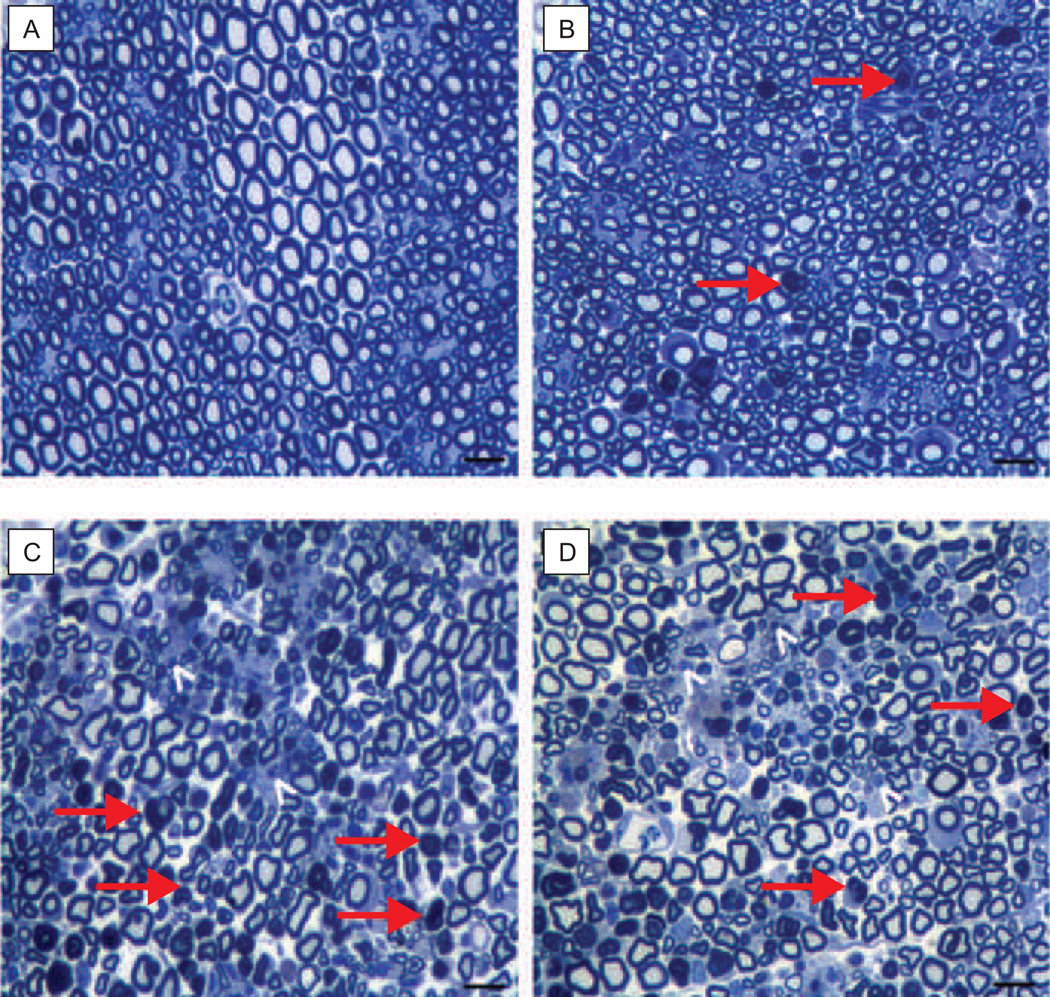
MTA-induced peripheral neuropathy in mice has been measured using behavioral assays and causes altered sensitivity to painful stimuli (22). These behavioral changes, indicative of peripheral neuropathy, are associated with diminished peripheral nerve conduction velocity, amplitude and morphology. After defining the individual maximum tolerated doses (MTDs) in mice, MTAs were tested for their effects on parameters associated with peripheral neuropathy in caudal and digital nerves at fractional doses (0.25, 0.5, 0.75 and 1) of the MTD on a 2-week schedule (23). Eribulin did not change the nerve conduction velocity of either nerve type, but did increase the caudal nerve amplitude at the MTD and 0.75 MTD. In contrast, at the MTD and 0.75 MTD, ixabepilone and paclitaxel significantly decreased both nerve conduction velocity and amplitude. All three drugs caused dose-dependent pathologies to L4 and L5 dorsal root ganglia and sciatic nerves, including axonal degeneration, cytoplasmic vacuolation and dark inclusions (Fig. 2). Pathological changes occurred with each drug, but paclitaxel and ixabepilone initiated more severe changes in sciatic nerves than did eribulin. Tissue histology of the sciatic nerve showed that eribulin-induced pathologies were less frequent and less severe than those observed with the other agents. These investigators next studied whether eribulin exacerbated pre-existing peripheral neuropathy in mice (24). Peripheral neuropathy was induced with a 2-week cycle of 0.75 MTD paclitaxel. After a 2-week recovery, the mice were switched to either 0.5 MTD eribulin or 0.5 MTD paclitaxel for another 2 weeks. Nerve conduction velocity and amplitude measurements demonstrated that eribulin did not significantly worsen the existing nerve function; however, the second treatment with paclitaxel did exacerbate the pre-existing neuropathy. Nevertheless, both eribulin and paclitaxel treatments caused an increase in the number of degenerated axons in the sciatic nerve as compared with pre-existing neuropathy, suggesting that both drugs caused some degree of damage.
Figure 2.
Effect of MTDs of eribulin mesylate, paclitaxel and ixabepilone on sciatic nerve morphology. Tissue from mice treated with A, vehicle, B, eribulin, C, paclitaxel or D, ixabepilone. Severe pathological changes consistent with axonal degeneration of both large and small fibers are highlighted with red arrows and white arrowheads, respectively. No regeneration (e.g. thin myelinated fibers) was evident with paclitaxel or ixabepilone. Scale bar, 20 µm. MTD, maximum tolerated dose (23).
(Reprinted from Wozniak K M, Nomoto K, Lapidus R G et al. Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer Research 2011;71(11):3952–62. Reproduced with permission from AACR.).
It has been noted that the peripheral neuropathy induced by MTAs initially affects the longest nerves of the body, those innervating the feet and hands (25). The susceptibility of these long neurons to MTAs suggests disruption of essential microtubule-dependent transport. The effects of eribulin and other MTAs on microtubule-dependent trafficking, anterograde and retrograde transport in squid axoplasm were evaluated (26). Ixabepilone and vincristine both caused a 27% decrease in anterograde trafficking and a ~20% decrease in retrograde trafficking at 1 µM. In contrast, eribulin and paclitaxel caused less than a 20% decrease in anterograde trafficking at concentrations of up to 10 µM, with no significant difference in retrograde transport. Importantly, there were no gross changes in axoplasm microtubule structures under any of these conditions. Consistent with the axoplasm data, experiments with purified biochemical components demonstrated a direct inhibition of kinesin function with 10 µM ixabepilone or vincristine, but not with eribulin or paclitaxel. These mechanistic studies, using a variety of models, support the idea that eribulin has distinct effects on peripheral nerves and are consistent with clinical results demonstrating that eribulin has a low frequency of treatment discontinuation due to peripheral neuropathy (1, 27, 28).
Angiogenesis and vascular effects
Angiogenesis is necessary for tumor growth and metastasis, and is an important drug target. Angiogenesis is complex, involving not only endothelial cells, but also stromal pericytes that support and stabilize endothelial cells (29, 30).
The effects of eribulin on endothelial cells or pericytes alone or in co-culture were compared with the effects of paclitaxel (31). The proliferation of human umbilical endothelial cells was potently inhibited by both eribulin and paclitaxel, with similar IC50 values of 0.54 nM and 0.41 nM, respectively. Interestingly, human brain vascular pericytes (HBVPs) were less sensitive to both drugs, with IC50 values of 1.19 nM for eribulin and 2.19 nM for paclitaxel. Gene expression analyses revealed that eribulin and paclitaxel altered similar genes in endothelial cells (59% overlap; most with decreased expression levels), but had distinct profiles in pericytes. In the HBVPs, only 12% of gene expression changes overlapped between eribulin and paclitaxel treatment. Eribulin caused a general decrease in transcription, while paclitaxel caused an increase. Of the genes affected by both drugs, 22 were down-regulated by eribulin but up-regulated by paclitaxel. These data indicate that, compared with endothelial cells, HBVPs respond differently to MTA treatment, and that eribulin and paclitaxel cause divergent effects on pericyte gene expression.
To study the effects of eribulin and paclitaxel on capillaries, endothelial cells and pericytes were co-cultured. Resultant capillary networks were insensitive to high concentrations (1 µM) of eribulin or paclitaxel over 2 days, but after 3 days of treatment eribulin initiated a loss of the capillary networks with an IC50 of 3.6 nM. These networks remained resistant to paclitaxel until day 5, when the IC50 for capillary loss was 13 nM. In the absence of pericytes, endothelial cell tubules (formed in a collagen gel) were equally sensitive to inhibition by eribulin or paclitaxel after 4 days, demonstrating the critical role played by pericytes in establishing differential sensitivity between MTAs. These results highlight differences between eribulin and paclitaxel with regard to their overall antiangiogenic and antivascular activities, suggesting that they might affect these processes differently in vivo, and that the basis for such differences may reside in differential sensitivities of pericytes to the two agents.
Effects of eribulin on vascular remodeling
Tumor vasculature is very different from normal vasculature, with tortuous, abnormal vessels that are targets for anticancer therapies. The abnormal tumor vasculature, together with tumor and stromal proliferation, results in a high interstitial tumor pressure, further impeding tumor perfusion and contributing to the hypoxic tumor environment (32). Tumor hypoxia and the accompanying acidity are implicated in tumor progression, metastasis and drug resistance. Several microtubule depolymerizing agents cause rapid vascular changes associated with destruction of intratumoral vasculature, leading to a rapid shutdown of tumor perfusion, and tumor necrosis (33–35).
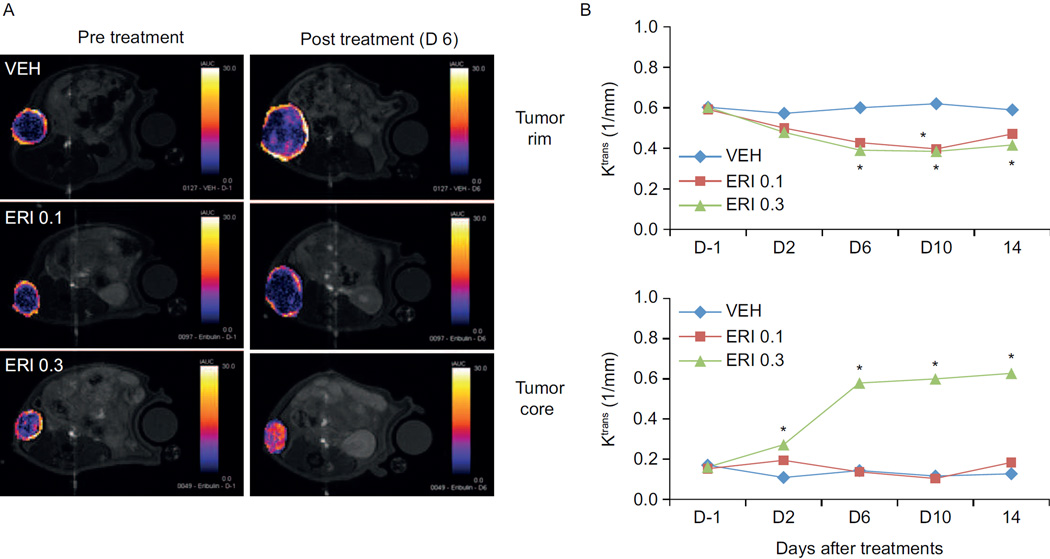
Considering the antivascular effects observed with microtubule depolymerizing agents, it was important to evaluate the effects of eribulin on tumor perfusion. Dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) experiments in the nude rat MX-1 and MDA-MB-231 human xenograft models of breast cancer showed that 0.3 mg/kg doses of eribulin caused an increase in the tumor perfusion transfer coefficient (Ktrans), indicative of increased perfusion in the tumor core. Representative DCE-MRI images of the MX-1 tumors before and after eribulin treatment are shown in Figure 3, together with the average Ktrans measurements of the tumor rim and core (36). Similar results were obtained in a murine xenograft model, using Hoechst 33342 dye to measure perfusion (31). Tumors also showed an increase in the number of microvessels after eribulin treatment, consistent with increased perfusion. These data collectively demonstrate that eribulin causes tumor vascular remodeling, leading to increased perfusion.
Figure 3.
Eribulin increases tumor vascular perfusion in an MX-1 rat xenograft model of human breast cancer. A. Representative DCE-MRI images of MX-1 tumors showing initial area under the curve (iAUC) maps prior to or on day 6 following eribulin treatment (0.1 or 0.3 mg/kg, days 0 and 4). A difference in perfusion between the tumor rim and tumor core is seen prior to treatment as indicated by lighter colors on the tumor rim and darker colors in the core. Changes in tumor perfusion after treatment are indicated by the change in color of the tumor core. B. Average volume transfer constant values (Ktrans) in tumor rim or tumor core regions as determined by DC-MRI. *P < 0.05 versus vehicle. VEH, vehicle; ERI, eribulin; 0.1, 0.3, dose in mg/kg, every fourth day beginning in day 0; iAUC, initial area under the curve (36).
(Reprinted from Funahashi Y, Okamoto K, Adachi Y et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Science 2014;105(10):1334–42. Copyright: The Authors. Reproduced with permission from John Wiley and Sons).
Mechanistic studies showed that eribulin decreased the expression of genes associated with angiogenesis. This included genes involved in the vascular endothelial growth factor (VEGF), Wnt, Notch and ephrin signaling pathways, and in the epithelial-to-mesenchymal transition (EMT), a process known to be driven in part by hypoxia (37). At the protein level, eribulin decreased the expression of both VEGF and CA9, markers of hypoxia.
An increase in tumor perfusion would be expected to alleviate the hypoxic environment of the tumor. This could improve subsequent systemic chemotherapy, both by reducing hypoxia-driven chemoresistance and by enhancing intratumoral delivery of drugs. This hypothesis was tested using capecitabine with and without eribulin pretreatment. Strikingly, eribulin-pretreated tumors were significantly more sensitive to subsequent capecitabine treatment than non-pretreated tumors, consistent with the hypothesis that improved perfusion due to eribulin treatment could increase the cytotoxic efficacy of a subsequent treatment. Thus, eribulin induces tumor vasculature remodeling, leading to increased perfusion of the tumor, which can result in diminished hypoxia and enhanced antitumor efficacy of subsequent therapies. It is interesting to speculate that such vascular changes, at this time only observed in preclinical models, might contribute to the clinical efficacy and survival advantage observed in the EMBRACE trial.
Epithelial-to-mesenchymal transition
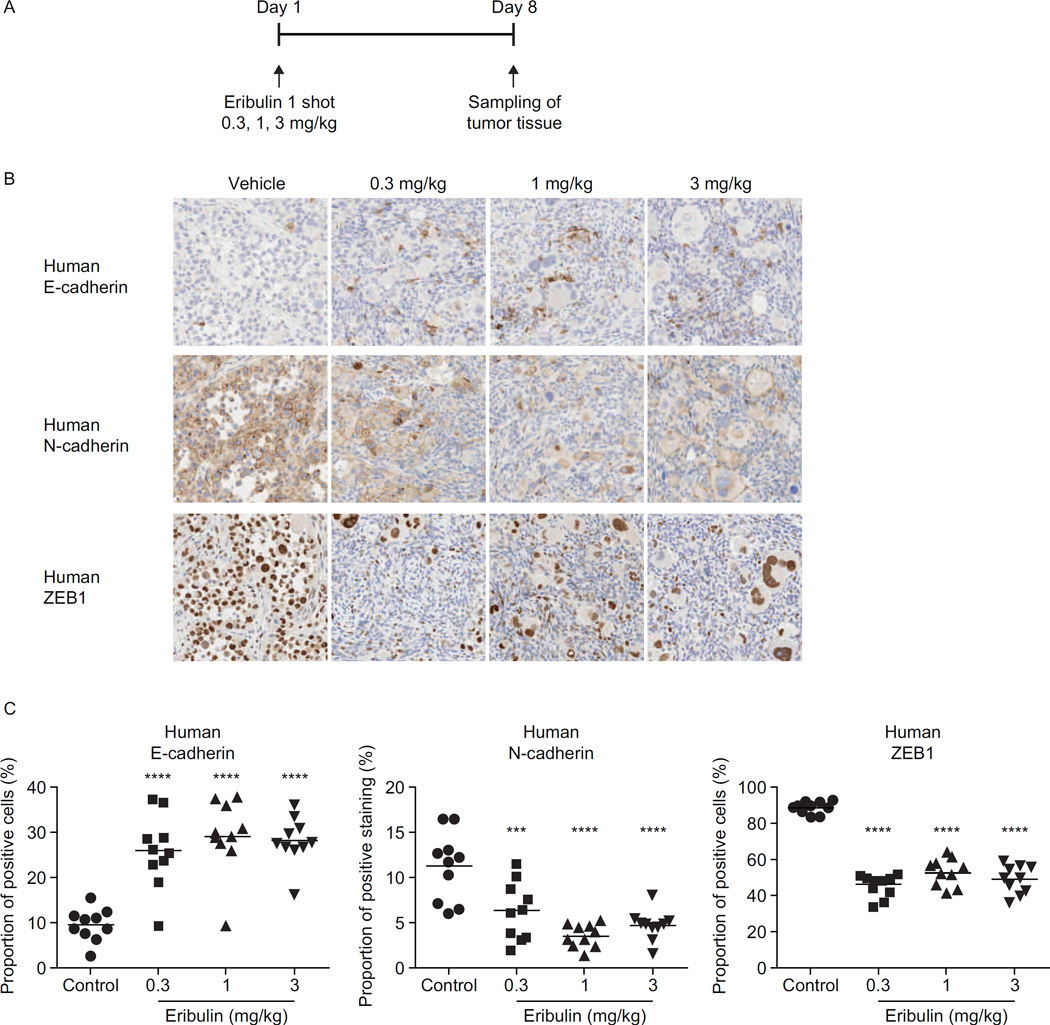
Similar to angiogenesis, EMT is considered a crucial process in tumor progression and metastasis. During EMT, the gene signature and phenotype of epithelial cells change in such a way that they adopt mesenchymal characteristics that have been implicated in increased drug resistance, enhanced invasion and metastasis, and a shift toward stem cell phenotypes (38). Drugs with the ability to inhibit or to reverse EMT are highly desired. Due to the survival advantage that eribulin demonstrated clinically, it was hypothesized that eribulin may affect EMT. Notably, treatment with 1 nM eribulin for 1 week in three mesenchymal-like triple-negative breast cancer cell lines triggered reversal of EMT in surviving cells as suggested by increased epithelial-like morphology and significant changes in gene profiles and protein marker expression (39). The surviving cells showed increased messenger RNA (mRNA) expression of the epithelial markers E-cadherin and keratin 18, as well as decreased expression of multiple mesenchymal markers including N-cadherin, vimentin, TWIST and ZEB-1.
Changes in the protein levels of E-cadherin, N-cadherin and vimentin in surviving eribulin-treated cells mirrored the changes in mRNA levels (39). Consistent with these effects, fully viable, surviving MX-1 cells showed significantly diminished capacities for in vitro migration and invasion. In addition, eribulin also caused similar EMT reversal in MX-1 tumor xenografts in vivo (39). Consistent with the direct effects on cells in vitro, eribulin caused a shift of residual tumors to a more epithelial phenotype, with increased E-cadherin and lower tumor expression of N-cadherin and ZEB-1 (Fig. 4). To evaluate the metastatic propensity of drug-treated cells, MX-1 cells were treated in vitro with vehicle, eribulin or 5-fluorouracil (5-FU), and equal numbers of surviving cells were injected into the tail veins of mice. Treatment with eribulin, but not with 5-FU, strongly inhibited lung nodule formation 15 days after injection of the cells, and 60% of the mice survived for 80 days following the injection. In contrast, all of the mice injected with vehicle or 5-FU-pretreated cells died within 21 days following injection. While these results are exciting, further studies are necessary to determine if this contributes to the survival advantage of eribulin.
Figure 4.
Eribulin reverses EMT in MX-1 human breast cancer xenografts in vivo. A, Schematic representation of treatment scheme. B, Representative immunohistochemistry images of human (i.e. tumor, not host) E-cadherin (upper), N-cadherin (middle) and ZEB-1 (lower) in tumor specimens from animals treated with eribulin 0.3, 1 and 3 mg/kg. Images taken at 100 × magnification. C, Quantification of immunohistochemistry staining of the markers shown in B. Data for individual tumors are presented as points, with mean ± standard error of the mean of the group shown by horizontal lines and error bars (n = 10). ***P < 0.001, ****P < 0.0001 versus control group (Dunnett-type multiple comparison test). EMT, epithelial-to-mesenchymal transition; IHC, immunohistochemistry; WB, Western blotting; ZEB-1, zinc finger E-box-binding homeobox 1 (39).
(Reprinted from Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. British journal of cancer. 2014;110:1497-505. Reproduced with permission from Nature Publishing Group.
Concluding Remarks
Many different assay systems measuring diverse parameters collectively show that eribulin has many characteristics that make it distinct from other MTAs. Although still speculative, the specific biochemical effects of eribulin may underlie its unique effects on peripheral nerves, angiogenesis, vascular remodeling and EMT. Studies continue to demonstrate that while different MTAs have many shared mechanisms of action, they also clearly have non-overlapping effects. Recent evidence by several researchers has highlighted the importance of microtubules in normal interphase events, suggesting that the clinical distinctions between MTAs could be due, at least in part, to their differential effects on interphase microtubules (40). Additionally, there is evidence that eribulin has profound effects on the tumor microenvironment that differ from those of paclitaxel and possibly other MTAs. These mechanistic studies have the potential to improve and guide the use of MTAs in specific tumor types and in mechanistically driven drug combinations. It is likely that ongoing research into these features of MTAs will reveal many of the underlying reasons for the efficacy of these agents in cancer therapy, and show how they can best be used to improve patient survival.
Acknowledgments
The authors extend sincere thanks to Dr. Bruce A. Littlefield (Eisai Inc., Andover, MA, USA) for his insights and contributions to this review. Editorial support was provided by Oxford PharmaGenesis, Oxford, UK, and was funded by Eisai Inc., Woodcliff Lake, NJ, USA.
Financial support: Editorial support for this article was funded by Eisai Inc.
Potential conflicts of interest: S.L. Mooberry currently has a sponsored research agreement with Eisai Inc. and serves on an eribulin preclinical advisory board for Eisai.
References
- 1.Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 2.Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148:553–561. doi: 10.1007/s10549-014-3144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirata Y, Uemura D. Halichondrins-antitumor polyether macrolides from a marine sponge. Pure Appl Chem. 1986;58:701–710. [Google Scholar]
- 4.Bai RL, Paull KD, Herald CL, Malspeis L, Pettit GR, Hamel E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J Biol Chem. 1991;266:15882–15889. [PubMed] [Google Scholar]
- 5.Bai RL, Pettit GR, Hamel E. Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J Biol Chem. 1990;265:17141–17149. [PubMed] [Google Scholar]
- 6.Luduena RF, Roach MC, Prasad V, Pettit GR. Interaction of halichondrin B and homohalichondrin B with bovine brain tubulin. Biochem Pharmacol. 1993;45:421–427. doi: 10.1016/0006-2952(93)90079-c. [DOI] [PubMed] [Google Scholar]
- 7.Aicher TD, Buszek KR, Fang FG, Forsyth CJ, Jung SH, Kishi Y, et al. Total synthesis of halichondrin B and norhalichondrin B. J Am Chem Soc. 1992;114:3162–3164. [Google Scholar]
- 8.Yu MJ, Zheng W, Seletsky BM. From micrograms to grams: scale-up synthesis of eribulin mesylate. Nat Prod Rep. 2013;30:1158–1164. doi: 10.1039/c3np70051h. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Habgood GJ, Christ WJ, Kishi Y, Littlefield BA, Yu MJ. Structure-activity relationships of halichondrin B analogues: modifications at C.30-C.38. Bioorganic & medicinal chemistry letters. 2000;10:1029–1032. doi: 10.1016/s0960-894x(00)00150-5. [DOI] [PubMed] [Google Scholar]
- 10.Zheng W, Seletsky BM, Palme MH, Lydon PJ, Singer LA, Chase CE, et al. Macrocyclic ketone analogues of halichondrin B. Bioorganic & medicinal chemistry letters. 2004;14:5551–5554. doi: 10.1016/j.bmcl.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 11.Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer research. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 12.Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, et al. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer research. 2004;64:5760–5766. doi: 10.1158/0008-5472.CAN-04-1169. [DOI] [PubMed] [Google Scholar]
- 13.Towle MJ, Salvato KA, Wels BF, Aalfs KK, Zheng W, Seletsky BM, et al. Eribulin induces irreversible mitotic blockade: implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer research. 2011;71:496–505. doi: 10.1158/0008-5472.CAN-10-1874. [DOI] [PubMed] [Google Scholar]
- 14.Bai R, Nguyen TL, Burnett JC, Atasoylu O, Munro MH, Pettit GR, et al. Interactions of halichondrin B and eribulin with tubulin. Journal of chemical information and modeling. 2011;51:1393–1404. doi: 10.1021/ci200077t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49:1331–1337. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Molecular cancer therapeutics. 2005;4:1086–1095. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 17.Ngan VK, Bellman K, Panda D, Hill BT, Jordan MA, Wilson L. Novel actions of the antitumor drugs vinflunine and vinorelbine on microtubules. Cancer research. 2000;60:5045–5051. [PubMed] [Google Scholar]
- 18.Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Molecular cancer therapeutics. 2008;7:2003–2011. doi: 10.1158/1535-7163.MCT-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Towle MJ, Nomoto K, Asano M, Kishi Y, Yu MJ, Littlefield BA. Broad spectrum preclinical antitumor activity of eribulin (Halaven®): optimal effectiveness under intermittent dosing conditions. Anticancer research. 2012;32:1611–1619. [PubMed] [Google Scholar]
- 20.Kolb EA, Gorlick R, Reynolds CP, Kang MH, Carol H, Lock R, et al. Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatr Blood Cancer. 2013;60:1325–1332. doi: 10.1002/pbc.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cigler T, Vahdat LT. Eribulin mesylate for the treatment of breast cancer. Expert opinion on pharmacotherapy. 2010;11:1587–1593. doi: 10.1517/14656566.2010.486790. [DOI] [PubMed] [Google Scholar]
- 22.Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2009;6:620–629. doi: 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wozniak KM, Nomoto K, Lapidus RG, Wu Y, Carozzi V, Cavaletti G, et al. Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer research. 2011;71:3952–3962. doi: 10.1158/0008-5472.CAN-10-4184. [DOI] [PubMed] [Google Scholar]
- 24.Wozniak KM, Wu Y, Farah MH, Littlefield BA, Nomoto K, Slusher BS. Neuropathy-inducing effects of eribulin mesylate versus paclitaxel in mice with preexisting neuropathy. Neurotoxicity research. 2013;24:338–344. doi: 10.1007/s12640-013-9394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8:244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 26.LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2013;37:231–239. doi: 10.1016/j.neuro.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamucci T, Michelotti A, Pizzuti L, Mentuccia L, Landucci E, Sperduti I, et al. Eribulin mesylate in pretreated breast cancer patients: a multicenter retrospective observational study. Journal of Cancer. 2014;5:320–327. doi: 10.7150/jca.8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahdat LT, Garcia AA, Vogel C, Pellegrino C, Lindquist DL, Iannotti N, et al. Eribulin mesylate versus ixabepilone in patients with metastatic breast cancer: a randomized Phase II study comparing the incidence of peripheral neuropathy. Breast Cancer Res Treat. 2013;140:341–351. doi: 10.1007/s10549-013-2574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. The International journal of developmental biology. 2011;55:261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 30.Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. American journal of hematology. 2010;85:593–598. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 31.Agoulnik SI, Kawano S, Taylor N, Oestreicher J, Matsui J, Chow J, et al. Eribulin mesylate exerts specific gene expression changes in pericytes and shortens pericyte-driven capillary network in vitro. Vascular cell. 2014;6:3. doi: 10.1186/2045-824X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nature reviews Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 33.Kanthou C, Tozer GM. Microtubule depolymerizing vascular disrupting agents: novel therapeutic agents for oncology and other pathologies. International journal of experimental pathology. 2009;90:284–294. doi: 10.1111/j.1365-2613.2009.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill SA, Lonergan SJ, Denekamp J, Chaplin DJ. Vinca alkaloids: anti-vascular effects in a murine tumour. European journal of cancer. 1993;29A:1320–1324. doi: 10.1016/0959-8049(93)90082-q. [DOI] [PubMed] [Google Scholar]
- 35.Kruczynski A, Poli M, Dossi R, Chazottes E, Berrichon G, Ricome C, et al. Anti-angiogenic, vascular-disrupting and anti-metastatic activities of vinflunine, the latest vinca alkaloid in clinical development. European journal of cancer. 2006;42:2821–2832. doi: 10.1016/j.ejca.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer science. 2014;105:1334–1342. doi: 10.1111/cas.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung HY, Fattet L, Yang J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3173. (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinestel K, Eder S, Schrader AJ, Steinestel J. Clinical significance of epithelialmesenchymal transition. Clinical and translational medicine. 2014;3:17. doi: 10.1186/2001-1326-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. British journal of cancer. 2014;110:1497–1505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res. 2012;18:51–63. doi: 10.1158/1078-0432.CCR-11-0999. [DOI] [PubMed] [Google Scholar]