Abstract
Objective
Dried blood spot (DBS) methodology offers significant advantages over venipuncture in vulnerable populations or large-scale studies, including reduced participant burden and higher response rates. Uncertainty about validity of cardiovascular risk biomarkers remains a barrier to wide-scale use. We determined the validity of DBS-derived biomarkers of CVD risk versus gold-standard assessments, and study-specific, serum-equivalency values for clinical relevance of DBS-derived values.
Methods
Concurrent venipuncture serum and DBS samples (n=150 adults) were assayed in CLIA-certified and DBS laboratories, respectively. Time controls of DBS standard samples were assayed single-blind along with test samples. Linear regression analyses evaluated DBS-to-serum equivalency values; agreement and bias were assessed via Bland-Altman plots.
Results
Linear regressions of venipuncture values on DBS-to-serum equivalencies provided R2 values for TC, HDL-C, CRP of 0.484, 0.118, 0.666, respectively. Bland-Altman plots revealed minimal systematic bias between DBS-to-serum and venipuncture values; precision worsened at higher mean values of CRP. Time controls reveal little degradation or change in analyte values for HDL-C and CRP over 30 weeks.
Conclusions
DBS-assessed biomarkers represent a valid alternative to venipuncture assessments. Large studies using DBS should include study-specific serum-equivalency determinations to optimize individual-level sensitivity, viability of detecting intervention effects, and generalizability in community-level, primary prevention interventions.
Keywords: biomarkers, dried blood spots, cardiovascular disease risk, cholesterol, CRP
Introduction
Biomarkers are critical to cardiovascular and cardiometabolic disease prevention and stratification of risk. Important biomarker analytes for cardiovascular disease (CVD) include C-reactive protein (CRP), high-density lipoprotein (HDL) cholesterol, and total cholesterol (TC). Numerous studies have demonstrated that cardiovascular risk is directly proportional to serum total cholesterol and inversely proportional to HDL cholesterol after accounting for other possible risk factors (Kannel et al. 1971, Gordon et al. 1977). CRP is a significant cardiovascular risk predictor, even among individuals with normal low-density lipoprotein (LDL) cholesterol levels (Ridker 2003), and is associated with other chronic diseases, such as type 2 diabetes (Ridker 2003, Pai et al. 2004, Boekholdt et al. 2006).
Traditionally, clinical measurement of these CVD biomarkers and interpretation of their prognostic value are based on serum blood samples derived from venipuncture (Grundy et al. 2004), a distinct disadvantage in community-based research on health determinants. Venipunctures require specialized training and protocols that increase collection and processing costs and hamper collection opportunities in non-clinical settings. Moreover, venipuncture can be a significant barrier to participation in vulnerable populations such as children, the elderly, and individuals with existing comorbidities. Dried blood spot (DBS) collection, in which capillary blood samples are placed on filter paper following a simple finger prick, presents a viable alternative. Collection and storage protocols are simpler and less costly, and finger pricks are less of a barrier to participation (McDade, Williams, and Snodgrass 2007). These advantages of the DBS method increase the feasibility of conducting large-scale, community-based studies of biomarkers for CVD and other chronic diseases in vulnerable populations (Buxton et al. 2013).
Although the clinical level of accuracy of the DBS method compared to gold-standard venipuncture is clear for neonatal screening (De Jesus et al. 2009, Therrell et al. 1996, Chamoles et al. 2004), the use of DBS for measurement of cardiovascular risk biomarkers in adult population studies is in nascent stages. For DBS methods to attain the clinical acceptance and implementation in adult populations, it is important to repeatedly and reliably test whether DBS biomarker values significantly differ from standard clinical venipuncture values and, if so, the degree to which the discrepancy varies as a function of time and analyte.
Validation studies utilizing a stepped approach are necessary in order to verify critical assumptions that ensure accuracy and precision (McDade 2013). To date, the validation findings have been mixed. While strong correlations have been found between DBS and gold-standard venipuncture for some analytes, including CRP (Crimmins et al. 2014, Lacher et al. 2013), other studies have found poor correlations for analytes such as HDL-C and total cholesterol (Lacher et al. 2013). DBS samples of certain analytes, such as CRP, appear to degrade with time and environmental exposure (Brindle et al. 2010). Specific laboratory procedures, such as time controls, may be used as quality measures of precision, substantiating the reproducibility of data and quantifying systematic error. Finally, establishing DBS-to-serum equivalence relative to whole blood methods is critical to ascertain both the quality and veracity of DBS-assessed biomarker analytes and to improve interpretability of these values.
The present study was designed to address limitations to use and interpretability of DBS-derived biomarkers of cardiovascular risk. We compared DBS- and serum venipuncture-assessed biomarker analytes including CRP, high-density lipoprotein cholesterol (HDL), and total cholesterol (TC) in order to establish the validity of DBS-derived markers of CVD risk. A second goal was to quantify study-specific serum-equivalency values in order to establish the clinical relevance of DBS-derived values compared to venipuncture values from Certified Laboratory Improvement Amendment (CLIA) laboratories.
Methods
Data were obtained from research participants in two studies at different research institutions: The Be Well Work Well (BWWW; n=102) study at a Boston, Massachusetts-based hospital (Buxton et al. 2012, Sorensen et al. 2011), where data were collected from 08/2011 through 10/2012, and the Sleep and Cardiovascular Disease Risk (Sleep CVD-R; n=48) study at the University of Pittsburgh Medical Center (HL104607, M Hall, PI), where data were collected from 11/2011 through 08/2012. Institutional Review Boards at each study site provided approval for study procedures. Samples were processed within the flow of a larger workplace cohort study, the Work, Family and Health Study (WFHS)(Bray et al. 2013, King et al. 2013).
Venipuncture serum samples were collected from participants at both sites following identical procedures to ensure a uniform protocol for collection, processing, storage, and shipment. Trained study staff cleaned the DBS puncture site (middle or ring finger on the non-dominant hand) using alcohol swabs prior to pricking the side of the participant’s finger using a disposable, single-use micro-lancet (Unistik 2 Extra, Owen Mumford). Study staff wore sterile gloves and gently massaged the participant’s hand to obtain up to five free-flowing blood spots of at least 3 mm diameter. Spots were collected on a pre-printed filter paper designed for the collection of capillary whole blood (Whatman no. 903, GE Healthcare, Piscataway, NJ). Per standard procedures (Ostler, Porter, and Buxton 2014), collection cards were allowed to air-dry for at least 15 minutes, stored in a bio-hazard specimen bag with desiccant, and placed in a storage container at room temperature for weekly shipment to Brigham and Women’s Hospital (BWH) Division of Sleep Medicine, Boston, MA. Samples were rated for quality, including proper packaging, presence of moisture, and sample adequacy, as determined by visual comparison of the size of each blood spot to a pre-scaled circle on a transparent rubric. Samples were stored at −80°C prior to assay (Harborview Medical Center, University of Washington, Seattle, WA). Samples were shipped in the flow of DBS samples from the Work, Family, and Health Study (WFHS; (Bray et al. 2013, King et al. 2013)), and all collection, processing, and storage procedures were identical to those in the WFHS. The lab was blinded to the control samples identical to study samples shipped mixed among study samples.
Longitudinal Quality Assurance Measures
Blinded DBS control samples
While previous papers have tested the validity of DBS analytes compared to gold-standard venipuncture at a single time point, little is known about DBS assay degradation over time. In order to quantify variation in assay results over time, DBS control samples collected at the same time from three different individuals with known biomarker quantities were assayed periodically. These samples functioned as blinded time controls and were obtained from the venipuncture samples of three individuals, immediately applied to DBS paper, and stored at −80°C until assay. As described above, the time controls were also processed, stored, and shipped in the flow of the main study DBS samples.
Plasma control samples
As part of the DBS analysis laboratory’s standard practice of error handling, selected assay runs included analyte controls from both undiluted pooled human plasma (high control) and pooled human plasma diluted with negligible analyte plasma (low control).
Serum-Equivalent DBS Values
Because translation of DBS-obtained biomarker quantities to serum quantities facilitates clinical interpretability of these values, generation of serum equivalents represented one major focus of the Work, Family, and Health Study (Bray et al. 2013). Serum samples from non-clinically selected volunteers were assayed by local, independent, CLIA-certified laboratories (BWWW: LabCorp, Boston, MA (Buxton et al. 2012, Sorensen et al. 2011); Sleep CVD-R: University of Pittsburgh Medical Center Clinical Laboratory, Pittsburgh, PA). These serum samples were used along with their paired fingerstick DBS results to generate study-specific serum-equivalent DBS values.
Assays
Dried blood spot samples were assayed at the University of Washington (Department of Laboratory Medicine, Seattle, WA; UW Lab Med). CRP assays employed a sandwich ELISA that was an adaptation of a previously published DBS method (McDade, Burhop, and Dohnal 2004). The UW laboratory performed HDL-C and TC fluorimetric assays with previously established reliability and validity (Crimmins et al. 2014). Concurrent serum venipuncture samples were sent to independent CLIA-certified laboratories (LabCorp, Boston, MA; University of Pittsburgh Medical Center Clinical Laboratory, Pittsburgh, PA) for analysis. Using standard laboratory techniques, CRP was assayed using a particle enhanced turbidimetric assay (Roche Molecular Systems Inc.), HDL-C was assayed using a homogeneous enzymatic colorimetric test assay (Roche Molecular Systems Inc.), and TC was assayed with an enzymatic colorimetric test (Roche Molecular Systems Inc.).
Statistical Methods
To calculate DBS-to-serum equivalency values, venipuncture serum values were first regressed on DBS raw sample values to construct regression equations that predicted venipuncture serum values at each value of raw DBS. Serum-equivalent DBS values were then calculated using these equations for each raw DBS value, thereby transforming the raw DBS values into clinically-significant serum equivalencies. Simple linear regression models were then constructed for each analyte to estimate the variance in venipuncture serum concentrations explained by DBS serum-equivalent values, and R-square values were obtained. In addition, bivariate correlations were run to assess the strength of the relationship between DBS serum-equivalent and venipuncture serum values.
To assess the agreement between the serum-equivalent DBS and serum venipuncture measures, we calculated the mean difference of the DBS serum-equivalents and venipuncture serum analyte measurements and corresponding SDs. Bland-Altman plots were created to assess agreement for the biomarkers TC, HDL-C, and CRP by plotting the means of the DBS serum-equivalent and gold-standard venipuncture serum values against difference scores between the two measures (Bland and Altman 1999). A positive or negative mean difference represents a serum-equivalent DBS overestimation or underestimation of venipuncture-assessed biomarker concentrations, respectively, and the degree of scatter in difference values on the Y-axis indicates the amount of variability between methods.
Study Schematics
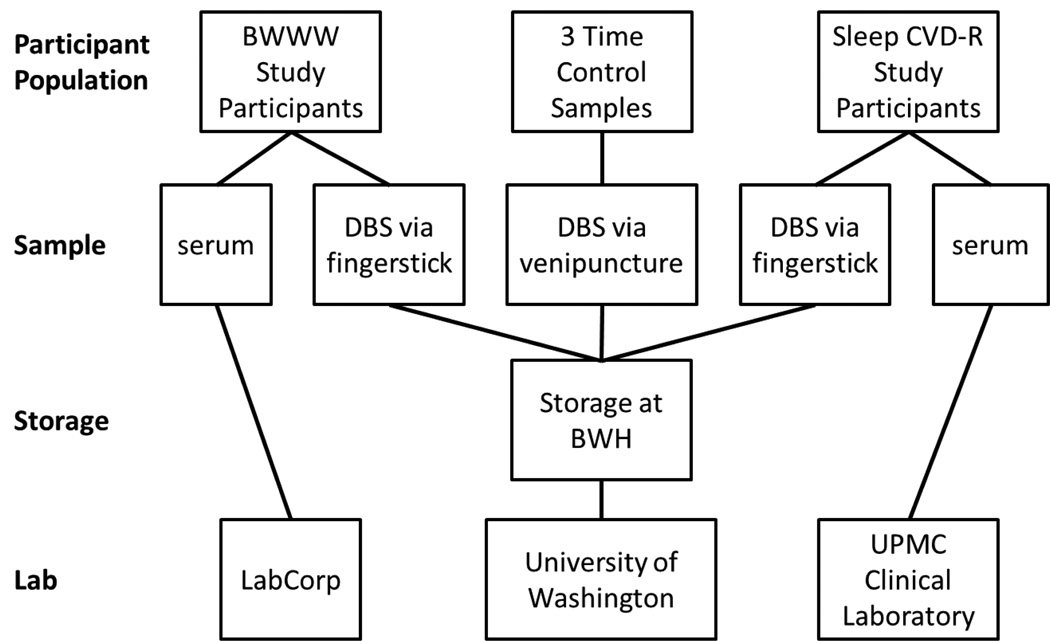
Figure 1 represents a visual depiction of the conceptual framework, providing an overview of the operationalization of precision and accuracy within the sub-study.
Figure 1. Schematic of the Dried Blood Spot Biomarker Serum Equivalency and Quality Control Sub-Study.
This schematic depicts blood sample types for the Be Well Work Well (BWWW) Study, Sleep and Cardiovascular Disease Risk (Sleep CVD-R) Study, and blinded time control sample. All three of these control samples were processed and shipped to the DBS lab using identical procedures as used for the main study samples including use of main study blood spot cards, and mixed among the main study sample shipments. The University of Washington lab was thus maintained blind to the use of any control samples as all samples appeared identical. For the paired control samples, the serum venipuncture collection occurred within minutes of the DBS fingerstick collection. Serum samples were assayed at independent CLIA-certified labs.
Results
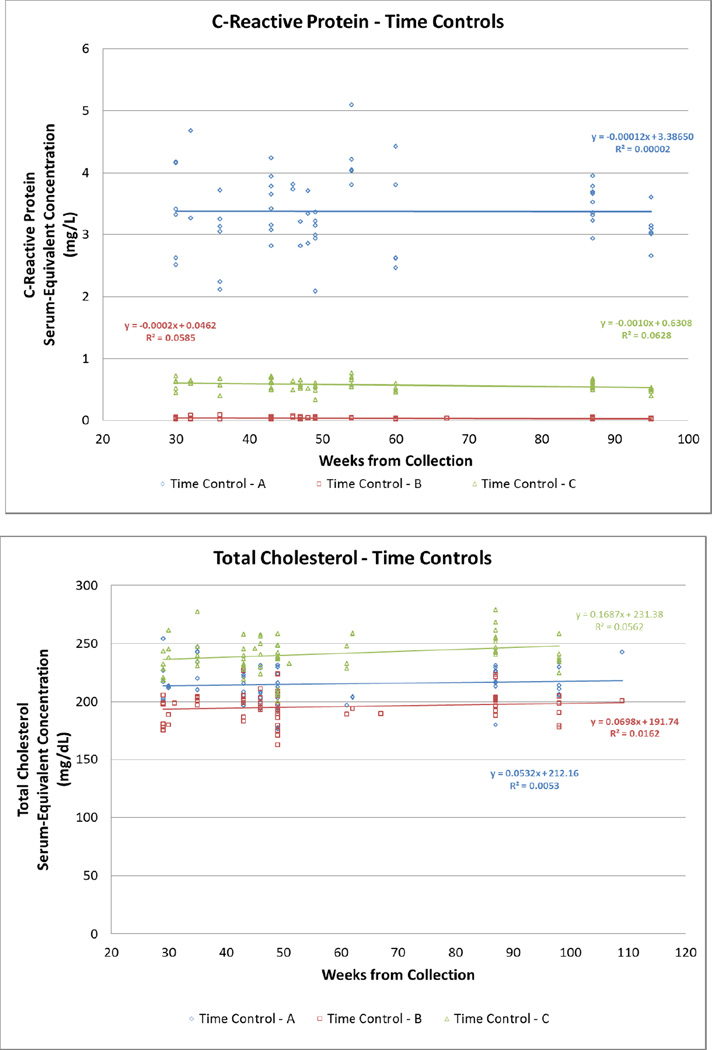
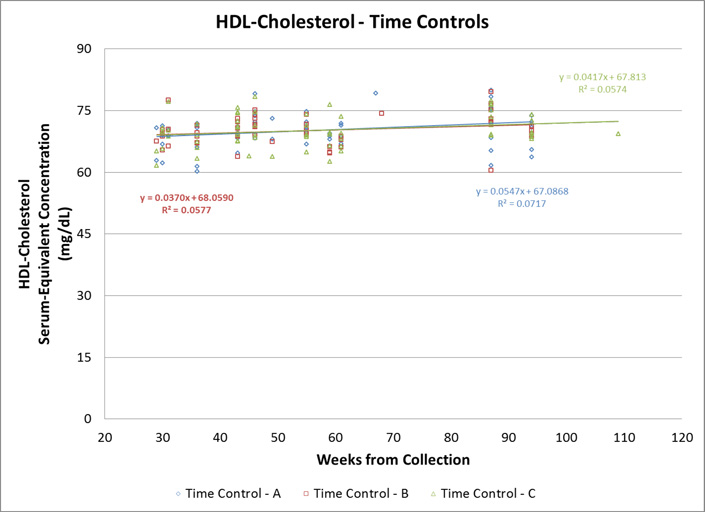
The sample included 150 participants of varying ethnicities, BMI values, and ages (Table 1). The mean, standard deviation, and minimum and maximum values for raw DBS, DBS-to-serum equivalents, and serum values for TC, HDL-C, and CRP are shown in Table 2. Figure 2 displays the laboratory-assessed serum-equivalent values for the blinded high, medium, and low controls over a period of 30 weeks. Since each set of points represents the same control, evidence of a slope or non-zero R2 value signifies a variation in the values recorded from the same source at different times. CRP and HDL-C had slopes and regression coefficients close to zero, while TC had rather large slopes such as −2.32 and −1.09 for two of its controls.
Table 1.
Sample Characteristics.
| Total n=150 |
|
|---|---|
| Mean ± SD (range) | |
| Age (years) | 46.60 ± 13.83 (21–74) |
| BMI (kg/m2) | 27.19 ± 6.44 (18–68) |
| Number (percent) | |
| Female | 134 (89.3%) |
| Race | |
| Hispanic or Latino | 3 (2.0%) |
| African-American | 8 (5.3%) |
| Asian | 2 (1.3%) |
| White | 135 (90.0%) |
| Unknown | 2 (1.3%) |
Table 2.
Distributions of raw dried blood spots (DBS) compared to DBS-to-serum equivalents and serum blood.
| Analyte (unit) |
Specimen | Mean | SD | Minimum Value |
Maximum Value |
|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | Raw DBS | 308.85 | 61.98 | 162.97 | 555.59 |
| DBS-to-serum | 193.03 | 28.38 | 126.22 | 306.04 | |
| Serum | 192.81 | 40.68 | 126.00 | 444.00 | |
| HDL cholesterol (mg/dL) | Raw DBS | 86.70 | 21.67 | 49.83 | 150.42 |
| DBS-to-serum | 61.66 | 5.89 | 51.63 | 78.99 | |
| Serum | 62.25 | 17.52 | 26.00 | 119.00 | |
| C-reactive protein (mg/L) | Raw DBS | 3.31 | 4.73 | 0.14 | 31.19 |
| DBS-to-serum | 1.40 | 2.12 | −0.02 | 13.89 | |
| Serum | 1.41 | 2.59 | 0.02 | 17.55 |
Figure 2. Serum Equivalent concentrations for C-reactive Protein, Total Cholesterol and High-Density Cholesterol levels in three different standards compared to time of collection.
Each set of colored points represent the serum equivalent concentration from the same source, collected at one time, tested over a period of 65 weeks. DBS samples were created at one time using venipuncture blood and were stored frozen at −80°C until assay between 29–109 weeks following collection. A distribution of the same colored points along the y-axis signifies different values recorded for serum equivalent concentrations from the same source.
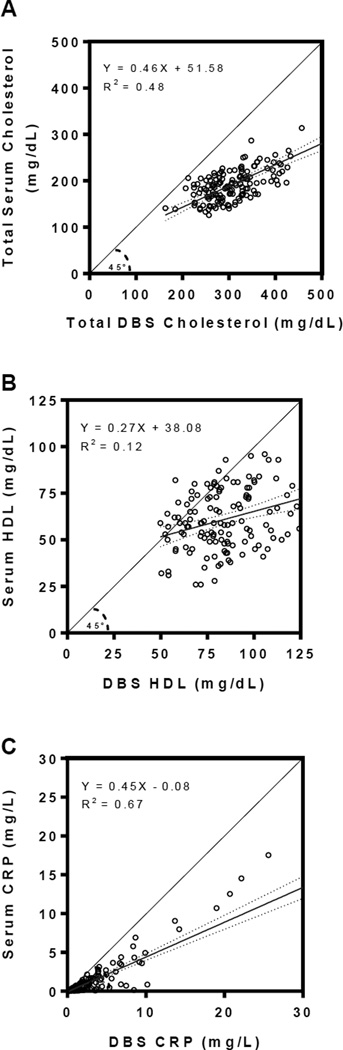
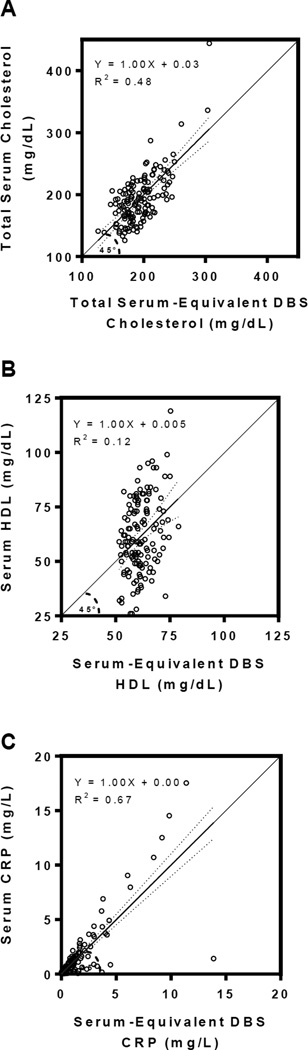
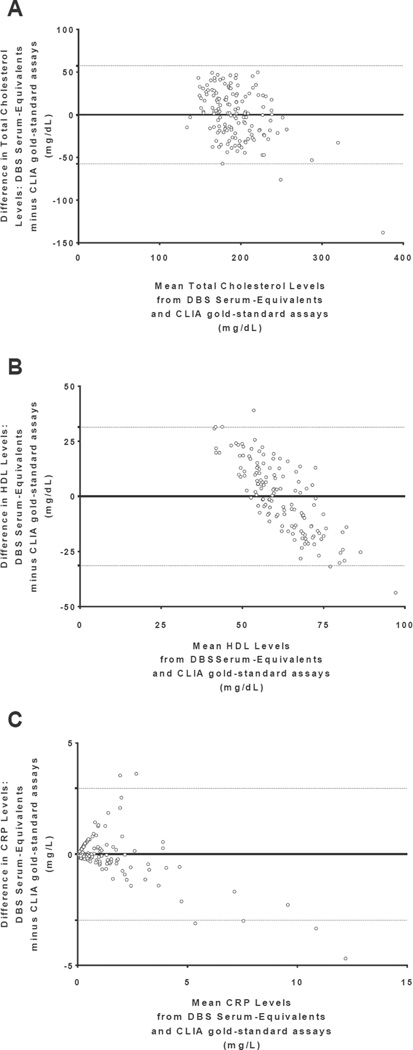
For each analyte, linear regression analyses obtained the following DBS-to-serum equivalency equations [Figure 3]: YTC serum = 0.458 * TC DBS raw + 51.581; YHDL-C serum = 0.272 * HDL-C DBS raw + 38.075; YCRP serum = 0.448 * CRP DBS raw – 0.084 (all regression coefficients p<0.001). Using these relationships, each raw DBS value was then transformed into a serum-equivalent DBS value to provide clinically-relevant serum equivalencies. The scatterplots for each analyte in Figure 4 compared the serum-equivalent DBS values from the designated laboratory to the paired venipuncture serum samples for each participant. The R2 values and standard errors of the estimate (SEE) for the analytes TC, HDL-C, and CRP were 0.484 (SEETC = 29.389), 0.118 (SEEHDL = 16.075), and 0.666 (SEECRP = 1.518), respectively. Figure 5 displays the Bland-Altman plots of mean serum-equivalent DBS and venipuncture serum values against difference values between these two measures for each biomarker. Visual inspection of the HDL-C plot indicates no overall systematic bias, although DBS serum-equivalent values overestimate the gold standard at lower mean HDL-C levels and underestimate gold standard values at higher mean HDL-C levels (overall bias = 0.004 mg/dL, 95 percent limits of agreement = −31.38–31.40). Visual inspection of the TC plot suggests that serum-equivalent DBS values were evenly clustered around venipuncture serum values across all mean TC levels (overall bias = 0.67 mg/dL, 95 percent limits of agreement = −57.34–57.47), indicating minimal bias. While the CRP plot is also suggestive of an overall minimal bias (overall bias = −.001 mg/L, 95 percent limits of agreement = −3.00–2.96), visual inspection reveals greater variability between the methods across the distribution of mean CRP levels, such that there is increasing overestimation and underestimation of venipuncture serum values at higher mean levels. For all three of the studied biomarkers, the bivariate correlations between serum-equivalent DBS and venipuncture serum values were significant at the p<0.01 level (rTC=0.696, rHDL=0.344, rCRP=0.816).
Figure 3. Analyte levels measured by dried blood spots compared to gold standard venipuncture serum measurements.
A. Levels of Total cholesterol (TC), B. High-density lipoprotein (HDL) cholesterol, and C. C-reactive protein (CRP). Individual data points represent the DBS levels (X-axis) compared to corresponding venipuncture serum measurements from the same individual and assayed by a CLIA-certified laboratory (Y-axis) for each analyte.
Figure 4. DBS-to-serum equivalent analyte levels compared to gold standard venipuncture serum measurements.
A. Total cholesterol (TC), B. High-density lipoprotein (HDL) cholesterol, and C. C-reactive protein (CRP). Raw DBS values were transformed into serum-equivalent DBS values using empirically-derived calibrations between these measures (see results) to provide clinically-relevant serum equivalencies for each analyte.
Figure 5. Bland-Altman Plots of analyte levels measured by dried blood spots compared to gold standard venipuncture serum measurement.
A. Total cholesterol (TC), B. HDL cholesterol, and C. C-reactive protein (CRP). This plot depicts the difference between serum-equivalent DBS and venipuncture serum measurements compared to the corresponding means of the values derived from both measures. The horizontal line at zero represents zero bias and the additional reference lines represent the upper and lower 95% confidence limits for the difference scores. Equal point distribution on either side of the zero bias and within the standard deviation limits of difference indicates minimal difference between values derived from the two methods.
Discussion
One of the primary aims of this study was to translate DBS-assessed biomarker values into a clinically-relevant scale (per CLIA laboratory gold-standard determinations) in order to improve interpretability with other studies. DBS sample values are based on whole blood, and the concentration of analyte is reduced in comparison with serum due to the presence of a large volume of red blood cells, which may dilute the sample (McDade, Williams, and Snodgrass 2007). The DBS-to-serum equivalency is, therefore, necessary to ensure clinically interpretable results from DBS values when serum values represent the clinical standard, although this translation may not result in reduced variability within the samples all of the time.
Furthermore, our use of blinded controls to create a study-specific DBS-to-serum equivalent relationship allowed for a study-specific representation of serum-equivalent values perhaps more useful than laboratory controls, which may use less directly comparable samples. This study-specific approach can increase the power of DBS data to detect individual-level differences in longitudinal studies, rather than just large group- or population-level differences, particularly important in the presence of an individual-level intervention. To ensure DBS quality control and increase reliability in future studies, we recommend that approximately 10 percent of additional, blinded study samples be collected independently and be subjected to the same pre- and post-collection procedure as the main study samples in order to calculate serum equivalents. Blinded samples should be reinserted into the main study assay flow. While samples for quality control might be considered burdensome, we argue that the benefits of having study-specific serum-equivalent values from DBS assay results outweigh this cost for some study designs. Our confidence in the overall process and procedures for the larger study was strengthened by the minimal variability in control (blinded) samples assessed over time.
Dried Blood Spot Method Considerations
Although the DBS methodology offers advantages over venipuncture, certain aspects of DBS should be considered before its implementation within a study. Due to their higher variability as compared to venipuncture, some DBS assays are more appropriate in population-level, public health research, while venipuncture sampling remains the gold standard for blood-based, diagnostic testing of individuals. As new biomarkers become available for clinical standard measurement, they will first need to be adapted to DBS assays before they can be reliably measured by DBS. However, this opportunity to expand biomarker DBS serum-equivalents illustrates the flexibility of this new methodology. In addition, it should be noted that some labs currently lack a DBS sample protocol or the capacity to analyze DBS samples while maintaining clinical applicability through validation and verification (McDade, Williams, and Snodgrass 2007). There are costs associated with the DBS methodology, including the purchase of laboratory grade freezers for storage and DBS protocol training for non-medically trained staff (McDade, Williams, and Snodgrass 2007). Nonetheless, the total costs of DBS are generally lower than those of serum venipuncture methods.
Limitations to the Study
It is possible that increasing the number of samples set aside for quality control may have resulted in a more accurate study-specific DBS-to-serum equivalence relationship. In addition, although our current sample size (n=150) was of sufficiently good quality, additional samples used as blinded controls may have produced more strongly correlated equivalence relationships. Due to the relatively small sample size, it is possible that we did not have a full distribution of each analyte, resulting in relatively few data points at the tails of each distribution, particularly for CRP. While some DBS collection protocols recommend that the cards be allowed to dry for two hours prior to packaging, all cards in the present study followed a previously established and validated protocol, which found that 15 minutes was sufficient to fully air dry the cards (Ostler, Porter, and Buxton 2014).
Importance to Biomarker Research
Our study provides further validation for the use of DBS methodology for TC, HDL cholesterol, and CRP biomarker collection, contributing to the growing literature on DBS biomarker validation (e.g. (Brindle et al. 2010, Crimmins et al. 2014, Lacher et al. 2013)). The present study translated raw DBS values to serum equivalents, which we argue will allow for the interpretation of DBS values on a clinical scale, providing valuable diagnostic and predictive capability of cardiometabolic disease risk biomarkers. This equivalence method allows for the identification and consideration of pre-analytic sources of error in the final values, as control samples and study samples undergo identical processing. Nonetheless, DBS does exhibit greater variation and assay error for the tested analytes, suggesting that DBS may have greater utility in group or population studies and may be less useful in individual clinical decisions than standard venipuncture samples. A particular strength of the present study was the demonstration through the use of time controls that DBS samples remain fairly constant over time, particularly for the analytes CRP and HDL cholesterol. The DBS method allows for biomarker assays to be implemented in studies using larger, community-based samples and those that target more vulnerable populations, broadening the applicability and generalizability of biomarker use in CVD research.
Acknowledgments
This work was supported by a grant from the National Institute for Occupational Safety and Health (U19 OH008861) for the Harvard School of Public Health Center for Work, Health and Well-being. This study would not have been accomplished without the participation of Partners HealthCare System and leadership from Dennis Colling, Sree Chaguturu, and Kurt Westerman. The authors would like to thank Partners Occupational Health Services including Marlene Freeley for her guidance, as well as Elizabeth Taylor, Elizabeth Tucker O’Day, and Terry Orechia. We also thank individuals at each of the hospitals including Jeanette Ives Erickson and Jacqueline Somerville in Patient Care Services leadership, and Jeff Davis and Julie Celano in Human Resources. We also thank Chris Kenwood of NERI for his statistical and programming support and Deirdre McLaren for her assistance with the production of this manuscript. This study was further supported by grants from the National Institutes of Health U01-AG027669, R01-HL107240, R01-HL104607, UL1-RR024153, UL1-TR000005, and T32-HL007901; additional support was provided by a Robert Wood Johnson Foundation pilot grant and Partners Occupational Health.
Source of Funding: Ms. Samuelsson received support from grant #T32 HL-007560-29 and is currently supported by grant #R01-HL104607 from the NIH. Dr. Hall is supported by grants from the NIH (#R01-HL104607, UL1-TR000005). Dr. McLean is supported by a grant from the NIH (#T32 HL-007901). Mr. Porter is supported by grants from the NIH (#U01-AG027669, R01-HL107240). Dr. Berkman is supported by a grant from the NIH (#U01-AG027669). Dr. Marino is supported by a grant from the NIH (R01-HL-107240). Dr. Sembajwe is supported by a pilot grant from the Robert Wood Johnson Foundation. Dr. McDade is supported by a grant from the NIH (#U01-AG027669). Dr. Buxton is supported by a grant from the NIH (#R01-HL107240). Dr. Buxton serves as a consultant and expert witness for Dinsmore, LLC (plaintiff attorney) in a case unrelated to the current manuscript involving sleep, circadian rhythms, and diabetes in railroad workers. Dr. Buxton serves on the scientific advisory board of Matsutani America and as a consultant to the Wake Forest University Medical Center (NC) on an unrelated study of sleep and pediatric atopic dermatitis.
Acronyms
- DBS
dried blood spots
- CVD
cardiovascular disease
- CRP
C-reactive protein
- HDL
high-density lipoprotein
- TC
total cholesterol
- CLIA
Clinical Laboratory Improvement Amendments
Footnotes
Conflicts of Interest: For the remaining authors no conflicts of interest were declared.
Contributor Information
Laura B. Samuelsson, Department of Psychology, University of Pittsburgh, Pittsburgh, PA.
Martica H. Hall, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Shakir McLean, Warren Alpert Medical School, Brown University, Providence, RI.
James H. Porter, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; Center for Population and Development Studies, Harvard School of Public Health, Cambridge, MA.
Lisa Berkman, Department of Epidemiology, Harvard Center for Population and Development Studies, Cambridge, MA; Harvard School of Public Health, Boston, MA.
Miguel Marino, Department of Family Medicine, Oregon Health and Science University, Portland, OR.
Grace Sembajwe, CUNY School of Public Health, Hunter College, New York, NY.
Thomas W. McDade, Department of Anthropology and Institute for Policy Research, Northwestern University, Evanston, IL.
Orfeu M. Buxton, Division of Sleep Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston MA; Department of Social and Behavioral Sciences, Harvard School of Public Health, Cambridge, MA; Department of Biobehavioral Health, Pennsylvania State University, State College, PA.
References
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Boekholdt SM, Hack CE, Sandhu MS, Luben R, Bingham SA, Wareham NJ, Peters RJ, Jukema JW, Day NE, Kastelein JJ, Khaw KT. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis. 2006;187(2):415–422. doi: 10.1016/j.atherosclerosis.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Bray JW, Kelly E, Hammer LB, Almeida DM, Dearing JW, Berkowitz King R, Buxton OM. RTI Press. Research Triangle Park, NC: 2013. An integrative, multilevel, and transdisciplinary research approach to challenges of work, family, and health. [PubMed] [Google Scholar]
- Brindle E, Fujita M, Shofer J, O'Connor KA. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods. 2010;362(1–2):112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Hopcia K, Sembajwe G, Porter JH, Dennerlein JT, Kenwood C, Stoddard AM, Hashimoto D, Sorensen G. Relationship of sleep deficiency to perceived pain and functional limitations in hospital patient care workers. Journal of Occupational and Environmental Medicine. 2012;54(7):851–858. doi: 10.1097/JOM.0b013e31824e6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Klein LC, Whinnery J, Williams S, McDade T. Biomarkers in Work and Family Research. In: Grzywacz JG, Demerouti E, editors. New Frontiers in Work and Family Research. East Sussex, UK: Psychology Press; Routledge: Taylor & Francis Group; 2013. pp. 170–190. [Google Scholar]
- Chamoles NA, Niizawa G, Blanco M, Gaggioli D, Casentini C. Glycogen storage disease type II: enzymatic screening in dried blood spots on filter paper. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2004;347(1–2):97–102. doi: 10.1016/j.cccn.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Crimmins E, Kim JK, McCreath H, Faul J, Weir D, Seeman T. Validation of blood-based assays using dried blood spots for use in large population studies. Biodemography Soc Biol. 2014;60(1):38–48. doi: 10.1080/19485565.2014.901885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus VR, Zhang XK, Keutzer J, Bodamer OA, Muhl A, Orsini JJ, Caggana M, Vogt RF, Hannon WH. Development and evaluation of quality control dried blood spot materials in newborn screening for lysosomal storage disorders. Clinical Chemistry. 2009;55(1):158–164. doi: 10.1373/clinchem.2008.111864. [DOI] [PubMed] [Google Scholar]
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. The American Journal of Medicine. 1977;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(8):e149–e161. doi: 10.1161/01.ATV.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham Study. Annals of Internal Medicine. 1971;74(1):1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- King RB, Karuntzos GT, Casper LM, Moen P, Davis KD, Berkman L, Durham M, Kossek EE. Work-family Balance Issues and Work-Leave Policies. In: Gatchel RJ, Schultz IZ, editors. Handbook of Occpational Health and Wellness. New York: Springer; 2013. pp. 323–340. [Google Scholar]
- Lacher DA, Berman LE, Chen TC, Porter KS. Comparison of dried blood spot to venous methods for hemoglobin A1c, glucose, total cholesterol, high-density lipoprotein cholesterol, and C-reactive protein. Clin Chim Acta. 2013;422:54–58. doi: 10.1016/j.cca.2013.03.032. [DOI] [PubMed] [Google Scholar]
- McDade TW. Development and validation of assay protocols for use with dried blood spot samples. American Journal of Human Biology. 2013;26(1):1–9. doi: 10.1002/ajhb.22463. [DOI] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clinical Chemistry. 2004;50(3):652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- Ostler MW, Porter JH, Buxton OM. Dried blood spot collection of health biomarkers to maximize participation in population studies. Journal of Visualized Experiments. 2014;28(83):e50973. doi: 10.3791/50973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. The New England Journal of Medicine. 2004;351(25):2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108(19):2292–2297. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- Sorensen G, Stoddard AM, Stoffel S, Buxton OM, Sembajwe G, Hashimoto D, Dennerlein JT, Hopcia K. The role of the work context in multiple wellness outcomes for hospital patient care workers. Journal of Occupational and Environmental Medicine. 2011;53(8):899–910. doi: 10.1097/JOM.0b013e318226a74a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrell BL, Hannon WH, Pass KA, Lorey F, Brokopp C, Eckman J, Glass M, Heidenreich R, Kinney S, Kling S, Landenburger G, Meaney FJ, McCabe ER, Panny S, Schwartz M, Shapira E. Guidelines for the retention, storage, and use of residual dried blood spot samples after newborn screening analysis: statement of the Council of Regional Networks for Genetic Services. Biochemical and Molecular Medicine. 1996;57(2):116–124. doi: 10.1006/bmme.1996.0017. [DOI] [PubMed] [Google Scholar]