Abstract
Recent advances in genomics, proteomics, cell biology and biochemistry of tumors have revealed new pathways that are aberrantly activated in numerous cancer types. However, the enormous amount of data available in this field may mislead scientists in focused research. As cancer cell growth and progression is often dependent upon the phosphoinositide 3-kinase (PI3K)/AKT pathway, there has been extensive research into the proteins implicated in the PI3K pathway. Using data available in the Human Protein Atlas database, the current study investigated the expression of 25 key proteins that are known to be involved with PI3K pathway activation in a distinct group of 20 cancer types. These proteins are AKTIP, ARP1, BAD, GSK3A, GSK3B, MERTK-1, PIK3CA, PRR5, PSTPIP2, PTEN, FOX1, RHEB, RPS6KB1, TSC1, TP53, BCL2, CCND1, WFIKKN2, CREBBP, caspase-9, PTK2, EGFR, FAS, CDKN1A and XIAP. The analysis revealed pronounced expression of specific proteins in distinct cancer tissues, which may have the potential to serve as targets for treatments and provide insights into the molecular basis of cancer.
Keywords: cancer, tumorigenesis, Human Protein Atlas, PI3K, AKT, TSC1, EGFR, PTEN
Introduction
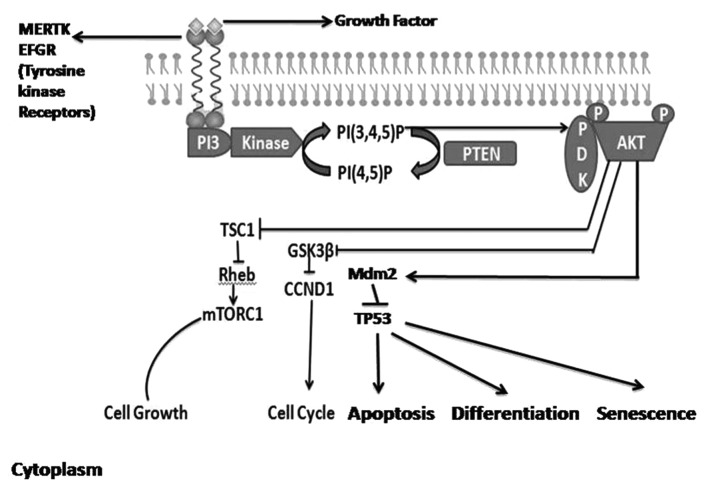
Phosphoinositide 3-kinases (PI3Ks) are an evolutionarily conserved family of lipid kinases that promote various cellular functions, including cell growth, metabolism and survival (1,2). The lipid second messengers that are generated in this reaction interact with specialized lipid-binding domains that are present in a wide variety of signaling molecules. PI3Ks may be classified into one of three classes, each of which possesses different structures and characteristics (3). The PI3K pathway may be activated by upstream receptor tyrosine kinases, leading to the generation of phosphatidylinositol-3,4,5-trisphosphate (PIP3) via the phosphorylation of phosphatidylinositol-4,5-bisphosphate. The phosphatase and tensin homolog (PTEN) may dephosphorylate PIP3, which terminates PI3K signaling. The accumulation of PIP3 activates a signaling cascade, commencing with the phosphorylation (activation) of the protein serine-threonine kinase AKT (also known as protein kinase B) at threonine 308 by phosphoinositide-dependent kinase 1. Activation of AKT serves a crucial role in essential cellular functions, including cell proliferation and survival, via the phosphorylation of a variety of substrates (Fig. 1) (4).
Figure 1.
PI3K/AKT signaling pathway. Binding of the ligand to membrane receptor tyrosine kinases activates PI3K, which phosphorylates PIP2 to produce PIP3. PIP3 recruits PDK1 to the plasma membrane. PDK1 phosphorylates and activates AKT, which regulates various cellular processes. The lipid phosphate activity of cytoplasmic PTEN dephosphorylates PIP3, thereby decreasing PIP3 levels and increasing levels of PIP2, resulting in a concomitant decrease in AKT activity. PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; PIP2 [PI(4,5)P], phosphatidylinositol 4,5-bisphosphate; PIP3 [PI(3,4,5)P], phosphatidylinositol (3,4,5)-trisphosphate; PDK1, phosphoinositide-dependent kinase 1; PTEN, phosphatase and tensin homolog.
Although the PI3K/AKT pathway has been extensively investigated in detail in distinct in vitro and in vivo systems (5), its role in molecular targeted therapy for cancer required further study. Molecular targeted therapies (e.g. inhibitors of target molecules with critical roles in tumor growth and progression) have been investigated in various cancer models, particularly hematological malignancies, such as leukemia, lymphoma and myeloma, due to the ease in obtaining samples for examination (6). The PI3K/AKT pathway has been reported to be activated in numerous types of malignancy (7), and inhibitors associated with this pathway have been shown to induce apoptosis in targeted tumor cells (8).
Aberrant activation of the PI3K pathway may promote carcinogenesis and tumor angiogenesis (9,10). For example, a previous study reported that ~30% of breast cancer cases demonstrated activating missense mutations of phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α (PIK3CA), the gene encoding the catalytic p110α subunit of class I PI3K (2); this mutated gene provides cells with a growth advantage and promotes tumorigenesis (11). In addition, dysregulated PI3K pathway signaling has been implicated in conferring resistance to conventional therapies, including biologics, hormonal therapy, tyrosine kinase inhibitors, radiation and cytotoxic drugs in breast cancer, glioblastoma and non-small cell lung cancer (12).
Wet laboratory research has revealed enormous data in the field of cancer research, and expression levels of certain proteins can be found at the Human Protein Atlas (www.proteinatlas.org). However, these proteins are not classified according to a specific disease or disorder. The aim of the present study was to utilize data deposited in the Human Protein Atlas to investigate the protein expression level of 25 proteins that are known to be implicated in the PI3K pathway in various cancer tissues. The proteins investigated were as follows: AKTIP, ARP1, BAD, GSK3A, GSK3B, MERTK-1, PIK3CA, PRR5, PSTPIP2, PTEN, FOX1, RHEB, RPS6KB1, TSC1, TP53, BCL2, CCND1, WFIKKN2, CREBBP, capase-9, PTK2, EGFR, FAS, CDKN1A and XIAP. The analysis reveals a pronounced expression of specific proteins in distinct cancer tissues, which may be potential targets for cancer treatment and provide insights into the molecular basis of cancer.
Materials and methods
Data were collected from the Human Protein Atlas database (www.proteinatlas.org) via manual searches of the desired gene names. The expression levels of 25 specific proteins that are known to be involved in the PI3K pathway were investigated in 20 different cancer tissues types: Carcinoid, glioma, liver cancer, lymphoma, melanoma, ovarian cancer, pancreatic cancer, skin cancer, testis, urothelial, lung cancer, breast cancer, cervical cancer, colorectal cancer, head and neck, renal, thyroid, prostate, endometrial and stomach cancer.
The expression of the 25 proteins in the different cancer tissues were reported as high, medium or low (excluding no expression, which was considered as a separate category) relative to normal tissues as shown in the database. Thereafter, the percentage of high, medium and low expression in each tissue type was calculated by dividing the number of patients exhibiting high expression, for example, over the total number of patients in the sample for each tissue type. The number of patients per sample ranged from 8–18. Furthermore, high and medium percentages were combined as the biological impact of high and medium expression was believed to be similar. Graphs were created using Microsoft Excel 12.0 (Microsoft Corporation, Redmond, WA, USA) to represent the percentage of each level of protein expression as it was expressed in these patients.
Results and Discussion
In this study, the expression levels of 25 proteins in tissues from 20 cancer types were analyzed utilizing the Human Protein Atlas (www.proteinatlas.org). The following proteins examined: AKTIP, ARP1, BAD, GSK3A, GSK3B, MERTK-1, PIK3CA, PRR5, PSTPIP2, FOX1, RHEB, TSC1, TP53, BCL2, CCND1, WFIKKN2, CREBBP, RPS6KB1, caspase-9, EGFR, PIK2, FAS, CDKN1A, XIAP and PTEN. The physiological activity and full name of these proteins, as well as their role in cancer initiation and control, is summarized in Table I (13–43).
Table I.
Summary of the physiological function and associated signaling pathways of the 25 proteins studied.
| Protein | Description/full name | Main function | References |
|---|---|---|---|
| TSC1 | Tuberous sclerosis 1 | Tumor suppressor; stimulates specific GTPases | (13) |
| EGFR | Epidermal growth factor receptor | Activates PI3K, which activates Akt (promotes cell survival and proliferation) | (14) |
| MERTK | MER proto oncogene tyrosine kinase | Tyrosine kinase receptor with oncogenic properties that is often overexpressed or activated in various types of malignancy; member of the MER/AXL/TYRO3 receptor kinase family; regulates melanoma cell migration and survival | (15,16) |
| RHEB | Ras homolog enriched in brain | Regulates growth and cell cycle progression via the insulin/TOR/S6K signaling pathway | (17) |
| RPS6KB1 | Ribosomal protein S6 β1 | mTOR downstream protein; promotes protein synthesis, cell growth, and cell proliferation | (18) |
| CCND1 | Cyclin D1 | Functions as a regulatory subunit of cyclin-dependent kinase (CDK)4 or CDK6, whose activity is required for cell cycle G1/S transition | (19) |
| TP53 | Tumor protein p53 | Tumor suppressor; induces cell cycle arrest, apoptosis, senescence, DNA repair or changes in metabolism | (20) |
| PTEN | Phosphatase and tensin homolog | Tumor suppressor; mostly mutated in a large number of cancers | (21,22) |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α | Belongs to the PI3K family; has roles in various signaling pathways; involved in proliferation, oncogenic transformation, cell survival and migration, and intracellular protein trafficking | (23,24) |
| GSK3A | Glycogen synthase kinase 3α | Control of several regulatory proteins including glycogen synthase and transcription factors | (25) |
| BAD | BCL2-associated agonist of cell death | Regulator of programmed cell death; proapoptotic activity of this protein is regulated through its phosphorylation; AKT and mitogen-activated protein kinase are involved in the regulation of this protein | (26,27) |
| AKTIP | AKT interacting protein | Mediates insulin-induced translocation of glucose transporter-4 to the cell surface | (28) |
| GSK3B | Glycogen synthase kinase 3β | Involved in energy metabolism, neuronal cell development and body pattern formation | (29) |
| ARP1 | Actin-related protein 1 | Transcriptional regulator involved in basal and hormone-regulated activity of prolactin; regulates the cell-specific trafficking of a receptor protein involved in apoptosis | (30,31) |
| PRR5 | Proline rich 5 (renal) | Regulates platelet-derived growth factor receptor | (32) |
| FOX1 | Fox-1 homolog. Also known as: ataxin 2-binding protein 1 (A2BP1); or hexaribonucleotide-binding | Possesses RNA binding motif; associated with spinocerebellar ataxia due to its binding to ataxin-2; regulator of tissue-specific alternative splicing in mammals protein 1 (HRNBP1) | (33) |
| BCL2 | B-cell lymphoma 2 | Oncogene; blocks apoptosis | (34,35) |
| WFIKKN2 | WAP, follistatin/kazal, immunoglobulin, kunitz | Protease-inhibitor that contains distinct protease inhibitor domains and netrin domain containing 2 | (36) |
| CREBBP | CREB binding protein | Regulates embryonic development, growth control, and homeostasis by coupling chromatin remodeling to transcription factor recognition | (37) |
| Caspase-9 | Apoptosis-related cysteine peptidase | Plays a central role in the execution-phase of cell apoptosis | (38) |
| PTK2 | Protein tyrosine kinase 2. Also known as: focal adhesion kinase (FAK) | Focal adhesion-associated protein involved in cellular adhesion and spreading processes; when it is blocked, cancer cells become less metastatic due to decreased mobility | (38,39) |
| FAS | Cell surface death receptor. Also known as: apoptosis antigen 1 (APO-1 or APT) | Plays a central role in the physiological regulation of programmed cell death | (40) |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A. Also known as: P21; CIP1; SDI1; WAF1; CAP20; CDKN1; MDA-6; or p21CIP1 | Inhibits the activity of cyclin-CDK2 or -CDK4 complexes, and thus functions as a regulator of cell cycle progression at G1 phase | (41) |
| PSTPIP2 | Proline-serine-threonine phosphatase-interacting protein 2 | Substrate for a number of important signaling proteins | (42) |
| XIAP | X-linked inhibitor of apoptosis. Also known as: IAP3 and BIRC | Inhibits apoptosis | (43) |
The results revealed that 9 of the 25 proteins tested exhibited high expression levels in various cancer tissues. These proteins were PIK3CA, RPS6KB1, MERTK, RHEB, EGFR, TSC1, CCND1, TP53 and PTEN. The other 16 proteins exhibited low or no expression in tumor tissues (data not shown).
The expression level for each protein tested was categorized as either high/medium or low. The protein TSC1 exhibited high/medium expression in all types of cancer tissue tested. TSC1 exhibited ~100% high/medium expression in breast, cervical, colorectal, head and neck, lymphoma, ovarian, pancreatic, prostate, skin, stomach, testis and urothelial cancer tissues. It expression was ~90% high/medium in endometrial, glioma, liver and lung cancers (Fig. 2).
Figure 2.

Expression levels of tuberous sclerosis 1 (TSC1) protein in different cancer tissues based on Human Protein Atlas.
EGFR protein had high/medium expression level in >50% of carcinoid, head and neck, glioma, renal and urothelial cancer tissues (Fig. 3). For MERTK protein the high/medium expression rate was >50% in liver and thyroid cancer tissues, and 100% in renal cancer tissues. It was not detected in carcinoid, glioma, or head and neck cancer tissues (Fig. 4).
Figure 3.
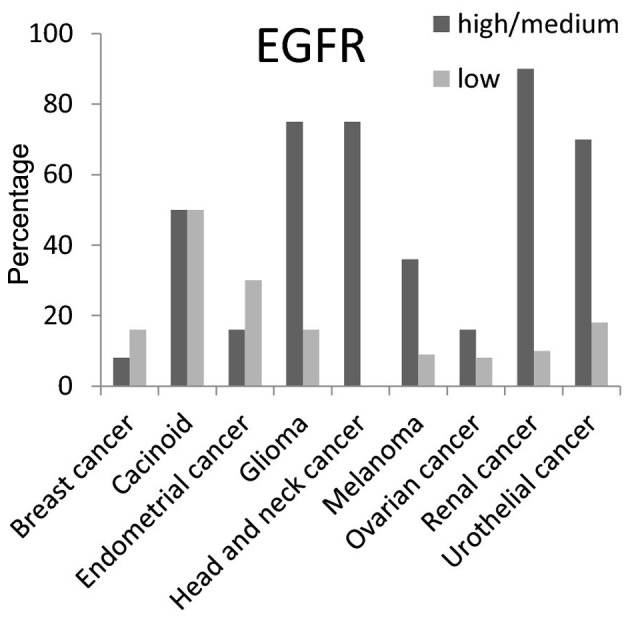
Expression percentages of epidermal growth factor receptor (EGFR) protein in 10 different cancer tissues based on Human Protein Atlas.
Figure 4.
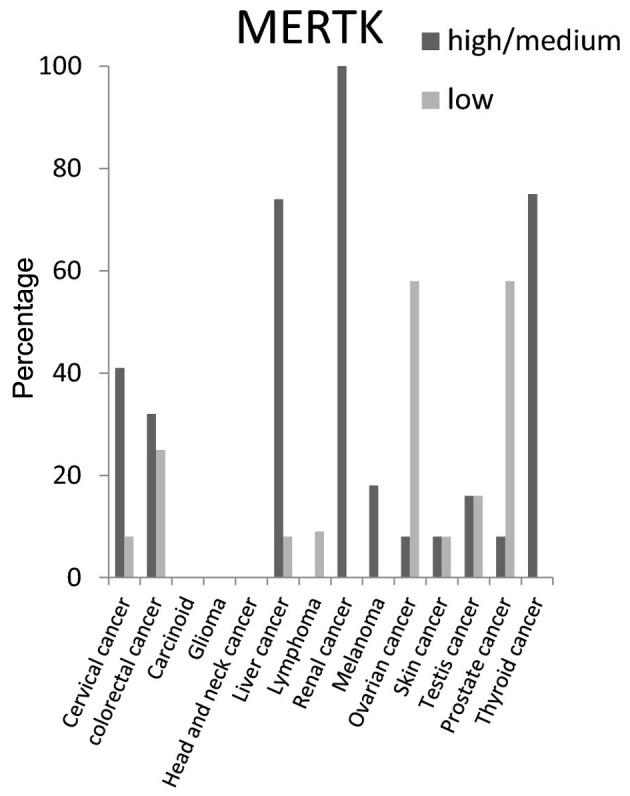
Expression percentages of MER proto oncogene tyrosine kinase (MERTK) protein in different cancer tissues based on Human Protein Atlas.
For RHEB protein, the highest expression level was present in >50% of breast, endometrial, ovarian, pancreatic and stomach cancer tissues, but was not detected in glioma and lymphoma cancer tissues (Fig. 5).
Figure 5.
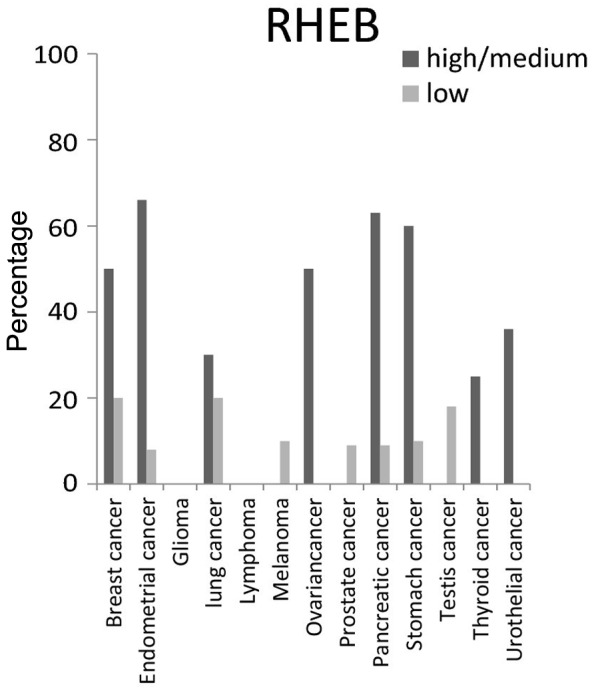
Expression percentages of Ras homolog enriched in brain (RHEB)protein in different cancer tissues based on Human Protein Atlas.
The RPS6KB1 protein expression level had ~100% high/medium in 9 cancer tissue tested: Carcinoid, colorectal, glioma, head and neck, ovarian, prostate, renal, skin and testis cancer tissues (Fig. 6).
Figure 6.
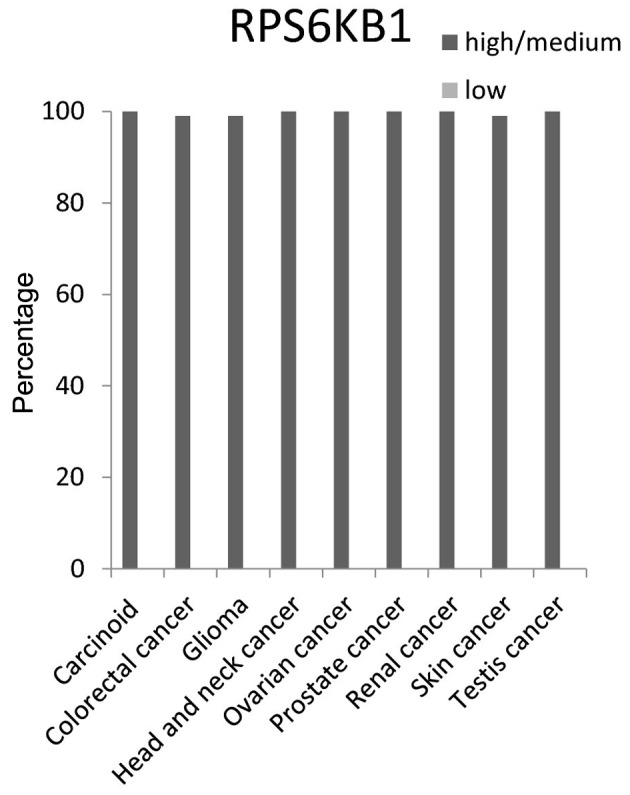
Expression percentages of ribosomal protein S6 β1 (RPS6KB1) protein in different cancer tissues based on Human Protein Atlas.
CCND1 protein high/medium expression level was present in ~50% of head and neck cancer and melanoma tissues (Fig. 7). The high/medium expression percentage of TP53 protein was ≥50% in colorectal, head and neck, ovarian, pancreatic and urothelial tissues, but was not detected at all in carcinoid, prostate and thyroid cancer tissues (Fig. 8).
Figure 7.

Expression percentages of Cyclin D1 (CCND1) protein in different cancer tissues based on Human Protein Atlas.
Figure 8.
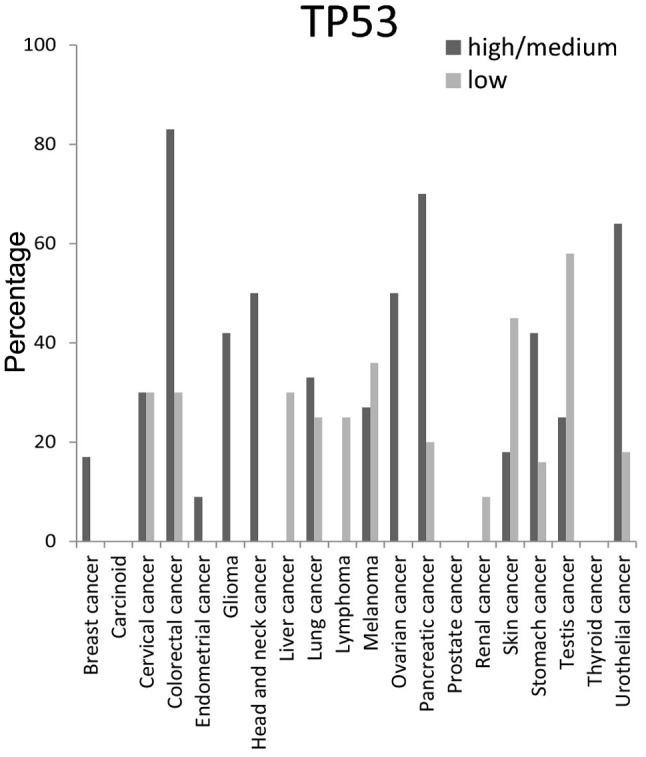
Expression percentages of tumor protein p53 (TP53) in different cancer tissues based on Human Protein Atlas.
The expression level of PTEN protein (a tumor suppressor gene) was low in various cancer tissues as was expected. A high/medium PTEN expression level was present in <50% of breast, cervical, endometrial, glioma, head and neck, liver, pancreatic and skin cancer tissues; however, high/medium expression was present at a rate of ~75% in melanoma (Fig. 9).
Figure 9.
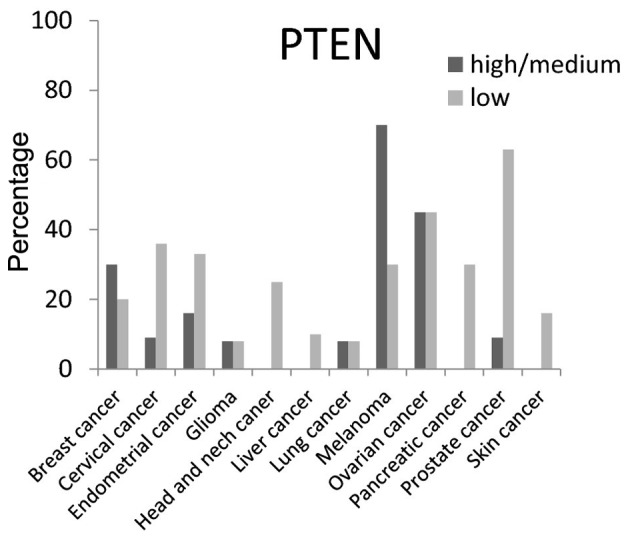
Expression percentages of phosphatase and tensin homolog (PTEN) protein (tumor suppressor gene) based on Human Protein Atlas.
PIK3CA protein expression level was high/medium in around 100% of lymphoma, ovarian and pancreatic cancer tissues, 90% of liver cancer tissues, 85% of melanoma and prostate cancer tissues, 70% of carcinoid and stomach cancer tissues and 65% of cervical cancer tissues (Fig. 10).
Figure 10.
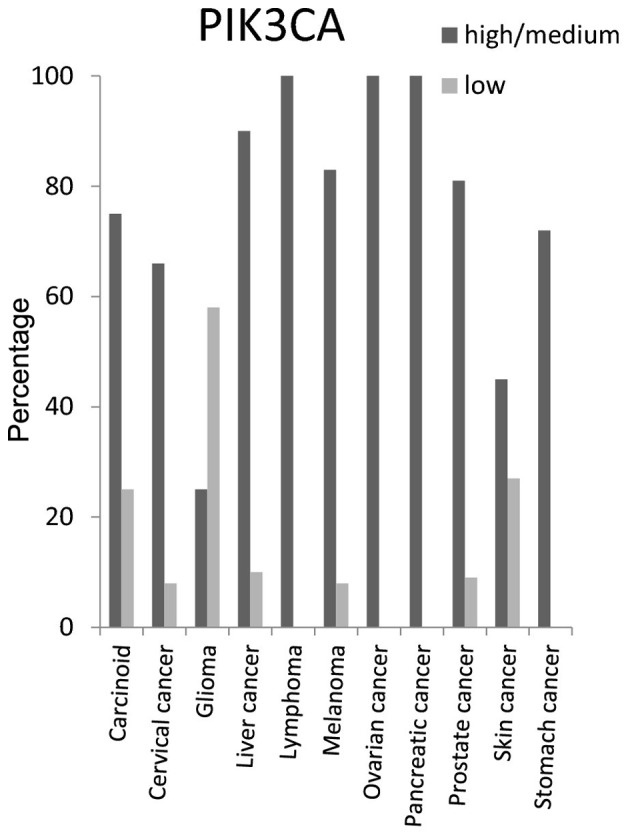
Expression percentage of phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α (PIK3CA) protein based on Human Protein Atlas.
Taking this data together, the current analysis reveals a pronounced expression of specific proteins in distinct cancer tissues. These proteins may be potential candidates to serve as targets for cancer treatments and provide insights into the molecular basis of cancer. PI3Ks initiate signaling through a network of downstream effector pathways. Due to the direct implication of the pathway in numerous cancer types, this pathway has become the target for novel cancer therapies. This bird's-eye view study highlights 9 proteins that are involved in the PI3K pathway and which may be potential targets for cancer treatment. These proteins are highly expressed in several cancer tissues as indicated. Designing new drugs that modulate the activity of these proteins may decrease cancer growth, migration and metastasis.
Acknowledgements
This study was supported by Alqasemi Research Fund and the Association of Arab Universities Research Fund.
References
- 1.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3.Akinleye A, Avvaru P, Furqan M, Song Y, Liu D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6:88. doi: 10.1186/1756-8722-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ocana A, Vera-Badillo F, Al-Mubarak M, Templeton AJ, Corrales-Sanchez V, Diez-Gonzalez L, Cuenca-Lopez MD, Seruga B, Pandiella A, Amir E. Activation of the PI3K/mTOR/AKT pathway and survival in solid tumors: Systematic review and meta-analysis. PLoS One. 2014;9:e95219. doi: 10.1371/journal.pone.0095219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott PA, Adams S. Small-molecule protein kinase inhibitors and their effects on the immune system: Implications for cancer treatment. Immunotherapy. 2011;3:213–227. doi: 10.2217/imt.10.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, Ji J, Monks A, Low JA, Chen A, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–2711. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vara Fresno JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JM, Kunnimalaiyaan S, Kunnimalaiyaan M, Gamblin TC. Inhibition of the AKT pathway in cholangiocarcinoma by MK2206 reduces cellular viability via induction of apoptosis. Cancer Cell Int. 2015;15:13. doi: 10.1186/s12935-015-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 10.Patel S. Exploring novel therapeutic targets in GIST: Focus on the PI3K/Akt/mTOR pathway. Curr Oncol Rep. 2013;15:386–395. doi: 10.1007/s11912-013-0316-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 13.Demetriades C, Doumpas N, Teleman AA. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell. 2014;156:786–799. doi: 10.1016/j.cell.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yewale C, Baradia D, Vhora I, Patil S, Misra A. Epidermal growth factor receptor targeting in cancer: A review of trends and strategies. Biomaterials. 2013;34:8690–8707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 15.Schlegel J, Sambade MJ, Sather S, Moschos SJ, Tan AC, Winges A, DeRyckere D, Carson CC, Trembath DG, Tentler JJ, et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J Clin Invest. 2013;123:2257–2267. doi: 10.1172/JCI67816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings CT, DeRyckere D, Earp HS, Graham DK. Molecular pathways: MERTK signaling in cancer. Clin Cancer Res. 2013;19:5275–5280. doi: 10.1158/1078-0432.CCR-12-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karassek S, Berghaus C, Schwarten M, Goemans CG, Ohse N, Kock G, Jockers K, Neumann S, Gottfried S, Herrmann C, et al. Ras homolog enriched in brain (Rheb) enhances apoptotic signaling. J Biol Chem. 2010;285:33979–33991. doi: 10.1074/jbc.M109.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du W, Gerald D, Perruzzi CA, Rodriguez-Waitkus P, Enayati L, Krishnan B, Edmonds J, Hochman ML, Lev DC, Phung TL. Vascular tumors have increased p70 S6-kinase activation and are inhibited by topical rapamycin. Lab Invest. 2013;93:1115–1127. doi: 10.1038/labinvest.2013.98. [DOI] [PubMed] [Google Scholar]
- 19.Juskevicius D, Ruiz C, Dirnhofer S, Tzankov A. Clinical, morphologic, phenotypic and genetic evidence of cyclin D1-positive diffuse large B-cell lymphomas with cyclin D1 gene rearrangements. Am J Surg Pathol. 2014;38:719–727. doi: 10.1097/PAS.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 20.Hejnold M, Dyduch G, Białas M, Demczuk S, Ryś J, Chłosta P, Szopiński T, Okoń K. Selected immunohistochemical features of conventional renal cell carcinomas coexpressing P53 and MDM2. Pol J Pathol. 2014;65:113–119. doi: 10.5114/pjp.2014.43960. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Chen P, Liu XX, Zhao W, Shi L, Gu XW, Zhu CR, Zhu HH, Zong L. Prognostic impact of gastrointestinal bleeding and expression of PTEN and Ki-67 on primary gastrointestinal stromal tumors. World J Surg Oncol. 2014;12:89. doi: 10.1186/1477-7819-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/S1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 23.Qiu W, Tong GX, Turk AT, Close LG, Caruana SM, Su GH. Oncogenic PIK3CA mutation and dysregulation in human salivary duct carcinoma. Biomed Res Int. 2014;2014:810487. doi: 10.1155/2014/810487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho DC. Targeting the PI3K/Akt/mTOR pathway in malignancy: Rationale and clinical outlook. BioDrugs. 2014;28:373–381. doi: 10.1007/s40259-014-0090-5. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang N, Zhu J, Liu D, Li YL, Chen BJ, He YQ, Liu K, Mo XM, Li WM. Overexpression of Bcl-2-associated death inhibits A549 cell growth in vitro and in vivo. Cancer Biother Radiopharm. 2012;27:164–168. doi: 10.1089/cbr.2011.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooray S. The pivotal role of phosphatidyl inositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- 28.Yang F, Deng R, Qian XJ, Chang SH, Wu XQ, Qin J, Feng GK, Ding K, Zhu XF. Feedback loops blockade potentiates apoptosis induction and antitumor activity of a novel AKT inhibitor DC120 in human liver cancer. Cell Death Dis. 2014;5:e1114. doi: 10.1038/cddis.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of gycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Xin YF, Xu WJ, Liu ZM, Qiu XB, Qu XK, Xu L, Li X, Yang YQ. Prevalence and spectrum of PITX2c mutations associated with congenital heart disease. DNA Cell Biol. 2013;32:708–716. doi: 10.1089/dna.2013.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Símová S, Klíma M, Cermak L, Sourková V, Andera L. Arf and Rho GAP adapter protein ARAP1 participates in the mobilization of TRAIL-R1/DR4 to the plasma membrane. Apoptosis. 2008;13:423–436. doi: 10.1007/s10495-007-0171-8. [DOI] [PubMed] [Google Scholar]
- 32.Gan X, Wang J, Wang C, Sommer E, Kozasa T, Srinivasula S, Alessi D, Offermanns S, Simon MI, Wu D. PRR5L degradation promotes mTORC2-mediated PKC-delta phosphorylation and cell migration downstream of G α 12. Nat Cell Biol. 2012;14:686–696. doi: 10.1038/ncb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuroyanagi H. Fox-1 family of RNA-binding proteins. Cell Mol Life Sci. 2009;66:3895–3907. doi: 10.1007/s00018-009-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg EF, Lavik AR, Distelhorst CW. Bcl-2 regulation of the inositol 1,4,5-trisphosphate receptor and calcium signaling in normal and malignant lymphocytes: Potential new target for cancer treatment. Biochim Biophys Acta. 2014;1843:2205–2210. doi: 10.1016/j.bbamcr.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bose P, Rahmani M, Grant S. Coordinate PI3K pathway and Bcl-2 family disruption in AML. Oncotarget. 2012;3:1499–1500. doi: 10.18632/oncotarget.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szlama G, Trexler M, Patthy L. Latent myostatin has significant activity and this activity is controlled more efficiently by WFIKKN1 than by WFIKKN2. FEBS J. 2013;280:3822–3839. doi: 10.1111/febs.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MS, Yoo NJ, Lee SH. Expressional and mutational analysis of CREBBP gene in gastric and colorectal cancers with microsatellite instability. Pathol Oncol Res. 2014;20:221–222. doi: 10.1007/s12253-013-9668-3. [DOI] [PubMed] [Google Scholar]
- 38.Unno H, Futamura K, Morita H, Kojima R, Arae K, Nakae S, Ida H, Saito H, Matsumoto K, Matsuda A. Silica and double-stranded RNA synergistically induce bronchial epithelial apoptosis and airway inflammation. Am J Respir Cell Mol Biol. 2014;51:344–353. doi: 10.1165/rcmb.2013-0281OC. [DOI] [PubMed] [Google Scholar]
- 39.Lackie JM, Coote JG, Lloyd CW, editors. The Dictionary of Cell & Molecular Biology. 5th. Academic Press; San Diego, CA: 2013. [DOI] [Google Scholar]
- 40.Villa-Morales M, Cobos MA, González-Gugel E, Álvarez-Iglesias V, Martínez B, Piris MA, Carracedo A, Benítez J, Fernández-Piqueras J. FAS system deregulation in T-cell lymphoblastic lymphoma. Cell Death Dis. 2014;5:e1110. doi: 10.1038/cddis.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta R, Dong Y, Solomon PD, Wettersten HI, Cheng CJ, Min JN, Henson J, Dogra SK, Hwang SH, Hammock BD, et al. Synergistic tumor suppression by combined inhibition of telomerase and CDKN1A. Proc Natl Acad Sci USA. 2014;111:E3062–E3071. doi: 10.1073/pnas.1411370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thapa N, Anderson RA. PIP2 signaling, an integrator of cell polarity and vesicle trafficking in directionally migrating cells. Cell Adh Migr. 2012;6:409–412. doi: 10.4161/cam.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyamoto M, Takano M, Iwaya K, Shinomiya N, Kato M, Aoyama T, Sasaki N, Goto T, Suzuki A, Hitrata J, Furuya K. X-chromosome-linked inhibitor of apoptosis as a key factor for chemoresistance in clear cell carcinoma of the ovary. Br J Cancer. 2014;110:2881–2886. doi: 10.1038/bjc.2014.255. [DOI] [PMC free article] [PubMed] [Google Scholar]