Abstract
Locally advanced esophageal cancer has been treated by a multimodality regimen encompassing combined radiochemotherapy (RCT). The tumor response to neoadjuvant RCT is a major determinant of further therapeutic strategies, whether surgery or a continuation of RCT, and therefore, also of the patient's overall prognosis. The present study included patients with histologically proven squamous cell esophageal carcinoma. The C-reactive protein (CRP) level was measured prior to and following the completion of neoadjuvant RCT. Only CRP measurements taken within 2 weeks of the start of RCT were analyzed. Further measurements were then taken at 6, 12, 18, 24, 30, 36 and 40 weeks following RCT. CRP levels were high prior to treatment; however, eventually decreased and normalized following the therapy. In univariate analysis, pre-therapeutic CRP levels had a significant influence on the response rate (P=0.033), whilst post-therapeutic CRP levels had no significant influence (P=0.383). Pre-therapeutic CRP levels, however, not post-therapeutic CRP levels were significantly correlated with the response rate (P=0.045 and P=0.444, respectively), and no association was observed between CRP levels and survival. This preliminary data indicated that the pre-therapeutic serum CRP level is a possible indicator of treatment response to RCT.
Keywords: esophageal cancer, inflammation and cancer, biomarker
Introduction
Despite improvements in the clinical outcome of patients with esophageal cancer, their prognosis remains generally very poor. Surgery alone is the standard treatment for early-stage disease, however, patients with locally advanced disease undergo a multimodality regimen encompassing combined radiochemotherapy (RCT) and surgery (1,2). RCT is administered prior to a planned operation or is used as a definitive procedure if surgery is not possible due to co-morbidity, or because complete resection is not feasible for technical reasons. Whether or not surgery is possible is always decided by an interdisciplinary team.
The tumor response to neoadjuvant RCT is a major determinant of further therapeutic strategies, whether surgery or a continuation of RCT is used, and therefore, also the overall prognosis of the patient. A significant response (complete or subtotal tumor regression) has a major bearing on the prognosis. Therefore, one option is to measure response by imaging with computed tomography (CT) or positron emission tomography/CT (PET/CT) following the administration of a sufficient radiation dose (e.g., 45 Gy), and then decide how to proceed (3).
From a clinician's perspective, the evaluation of treatment response using CT imaging alone may be difficult in numerous cases owing to its relatively low sensitivity (4,5) and the irritating co-variants of PET/CT (6–8). This is a challenge to clinicians an, therefore, the identification of biomarkers that predict the response to RCT may assist with optimizing treatment for advanced esophageal carcinoma.
The association between inflammation and cancer is widely accepted as a reliable concept (9–11). However, the complex interaction between inflammatory cascades and cancer progression is not well understood and is currently being investigated (12–14). Chronic inflammatory processes affect all stages of tumor development, as well as therapy. Cancer-associated inflammation (15) or, as it has also been referred to, inflammation-induced cancer (11), encompasses a wide array of factors that coordinate the tumor-promoting and tumor-antagonizing effects of inflammation and enable crosstalk between cancer progression and inflammatory processes.
C-reactive protein (CRP) is a representative biomarker of an acute immunogenic inflammatory response to infection or tissue damage. In addition to its well-established pathophysiological functions, CRP has been shown to mediate the complex interactions between a tumor and its inflammatory microenvironment, which in turn can promote tumor growth (16–18). Furthermore, CRP expression is upregulated by inflammatory lipid sphingosine-1-phosphate, a bioactive sphingolipid metabolite involved in cancer-associated inflammation. Increased CRP levels can then activate extracellular signal-regulated kinase, which in turn leads to the transcriptional activation of matrix metaloproetinase-9, promoting tumor invasion and metastasis (14).
A large body of evidence has emerged over the last few years for a pivotal role of CRP in cancer progression and treatment response (19–27), and there is also level I evidence for CRP having prognostic value in a number of different cancer types (28–34).
CRP dynamics in the serum have been shown to correlate with progression and poor prognosis in patients with esophageal carcinoma (35–38), and the level of preoperative CRP is an independent prognostic factor in patients with potentially resectable esophageal carcinoma (39). However, the clinical significance of CRP levels in patients with unresectable tumors and those requiring induction or even definitive CRT has not been fully investigated with respect to treatment response and prognosis (40,41), and little data are available regarding the association between CRP and the response to RCT (42,43).
The present study investigated the possible association between CRP and the response to RCT, and that between CRP and prognosis, by measuring CRP dynamics in serum the during the induction of RCT for patients with clinical T3 or T4 esophageal squamous cell carcinoma, all of whom underwent subsequent esophagectomy. The present study assessed the potential of serum CRP as a biomarker for the prediction of response to RCT.
Materials and methods
Patient selection and data acquisition
The present study included patients with histologically proven squamous cell esophageal carcinoma, predominantly located in middle or upper third of the esophagus, and staged II or III, according to the UICC classification (w). Patients with distant metastases were excluded.
The present study was approved by the responsible ethics committees and was performed in accordance with the Helsinki agreement. Each individual case was discussed at a multidisciplinary team meeting involving radiation oncologists, dedicated surgeons, medical oncologists, pathologists, and radiologists. Pre-therapeutic CRP levels were measured. The data were prospectively collected and stored in databases, from which the demographics, co-morbidity, tumor pathology, morbidity and mortality were subsequently retrieved. Case records were subsequently searched for any missing data. Survival data were obtained either from Cancer Registries or by direct contact with physicians.
Staging, neoadjuvant treatment and surgery
Pre-therapeutic staging included upper endoscopy with biopsy and endoscopic ultrasound, abdominal and thoracic CT and, in all cases, a PET/CT scan. Neoadjuvant RCT consisted of 5 cycles of carboplatin (AUC 2) and paclitaxel (45 mg/m2) in combination with 45–50 Gy of radiotherapy using the volumetric modulated arc therapy technique. The treatment was delivered as an in-patient regimen. Surgery was performed 6 weeks following the completion of RCT.
RCT was delivered by the same radiation oncologists and the delineation of target volumes was consistently supervised by two experienced radiation oncologists, as well as the preparation of chemotherapy and lab work-up. Surgery was performed by the same three experienced surgeons, using an abdominal and transthoracic approach. The stomach was used as the conduit for reconstruction in all cases. The standard UICC classification (44) was used to evaluate pathology.
CRP measurement
The CRP level was measured prior to and following the completion of neoadjuvant RCT. Only CRP measurements taken within 2 weeks of the start of RCT were analyzed. Further measurements were then taken at 6, 12, 18, 24, 30, 36 and 40 weeks after RCT.
CRP measurements were performed in serum samples with an automated immunoturbidimetric analyzer (Cobas® Integra 800; Roche Diagnostics, Basel, Switzerland). Normal reference values of <10 and <8 mg/l were provided by the manufacturers, respectively.
Statistical analysis
Statistical analysis was performed using SPSS® (version 22) for Windows (IBM SPSS, Chigago, IL, USA). The data are expressed as either the mean ± standard deviation or the median with their 95% confidence intervals (CI). A comparison of data between the two patient groups was performed using χ2 tests for categorical data and Mann-Whitney U tests for continuous data. Correlations between variables were tested for using the Pearson test, survival was calculated using the Kaplan-Meier method and differences between groups were evaluated using the log rank test. In order to determine the influence of different variables on the outcome, Cox regression analyses were performed. P<0.05 was considered to indicate a statistically significant difference.
Results
Response to RCT
All patient characteristics and tumor variables are summarized in Table I. All patients received RCT, which was delivered as a neoadjuvant therapy in 34 cases (73.91%) and as a definitive treatment in 12 cases (26.08%). A response was observed in 34 cases (73.91%), of which, 6 (17.6%) were a complete response and 28 (82.3%) were a partial remission. No response was reported in 12 cases (26%).
Table I.
Characteristics of the 46 patients undergoing radiochemotherapy followed by surgery.
| Variable | No. of patients |
|---|---|
| Mean age in years (range) | 65.3 (51–81) |
| Gender | |
| Female | 11 (23.9%) |
| Male | 35 (76%) |
| Tumor location | |
| Upper | 6 (13%) |
| Middle | 38 (82.6%) |
| Lower | 2 (4.3%) |
| Tumor depth | |
| T1 | 0 (0%) |
| T2 | 8 (17.3%) |
| T3 | 30 (65.2%) |
| T4 | 8 (17.3 %) |
| Nodal status | |
| N1 | 25 (54.3%) |
| N2 | 18 (39.1%) |
| N3 | 3 (17.3%) |
| Tumor stage | |
| IB | 1 (2.1%) |
| II–III | 45 (97.8%) |
| IV | 0 (0%) |
CRP levels during and following RCT, and the association with response to RCT
Table II shows pre- and post-RCT measurements of CRP. CRP levels were high prior to treatment, however, eventually decreased and normalized following therapy. In univariate analysis, pre-therapeutic CRP levels had a significant influence on the response rate (P=0.033), whilst post-therapeutic CRP levels had no significant influence (P=0.383).
Table II.
CRP measurement prior to and following radiochemotherapy (n=42 patients).
| Measurement | Normal | High | Low |
|---|---|---|---|
| Pre-therapy | |||
| CRP | 22 (47.9%) | 24 (52.2%) | 0 |
| Post-therapy | |||
| CRP | 26 (56.5%) | 20 (43.5%) | 0 |
Normal concentrations of CRP = <0.5 mg/dl. CRP, C-reactive protein.
CRP levels during and after RCT, and correlation with response to RCT
Pre-therapeutic CRP levels, however, not post-therapeutic CRP levels were significantly correlated with the response rate (P=0.045 and P=0.444, respectively), and no association was observed between CRP levels and survival. Fig. 1 summarizes the association between the levels of CRP and response to RCT.
Figure 1.
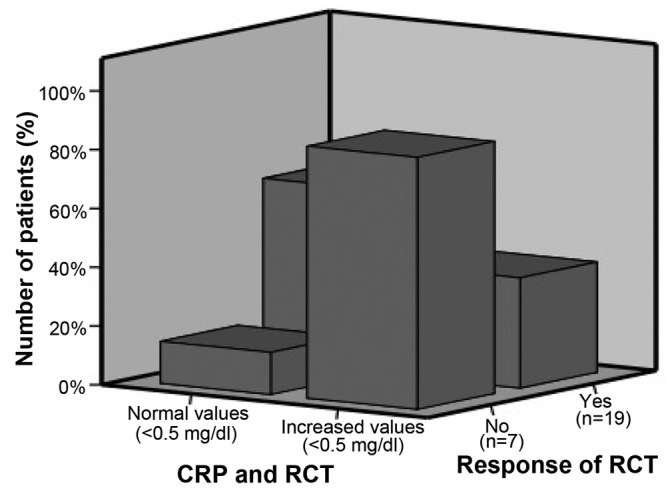
Association between CRP and the response to RCT. CRP, C-reactive protein; RCT, radiochemotherapy.
Discussion
The present study an association between the response of squamous cell esophageal carcinoma to neoadjuvant RCT and the pre-therapeutic levels of CRP. A significant response to treatment, either complete or subtotal tumor regression, is a key indicator of prognosis. However, it is difficult to evaluate response by CT imaging alone due to its relatively low sensitivity (4,5), and clinical evaluation remains challenging. An inflammatory biomarker such as CRP, which is easy to measure and reflects the response to treatment, can help guide clinical decision making.
Guillem and Triboulet (35) revealed that patients with CRP levels >6 mg/l more frequently failed to respond to RCT (P=0.035), tended to have a shorter overall survival (P=0.061), and had a significantly shorter disease-free survival (P=0.016). The authors concluded that the pretreatment measurement of serum CRP levels in esophageal cancer patients can be used in routine practice as an indicator of prognosis. This was the first attempt to associated CRP serum levels to neoadjuvant RCT response. A subsequent study demonstrated that, amongst patients with cancer of the gastroesophageal junction, the pre-therapeutic CRP level was a prognostic indicator, in agreement with the results of our pilot study. A multivariate analysis revealed that only the positive to total lymph node ratio [hazard ratio (HR), 2.02; 95% CI, 1.44–2.84; P<0.001] and preoperative CRP concentration (HR, 3.53; 95% CI, 1.88–6.64; P<0.001) were independent predictors of cancer-specific survival. Patients with no evidence of a preoperative systemic inflammatory response (CRP ≤10 mg/l) had a median survival of 79 months compared with 19 months for those with an elevated systemic inflammatory response (P<0.001). Together with these findings, the present study indicated that CRP is a potentially predictive cancer biomarker (36). The same research team subsequently reported a possible association between CRP and Albumin levels, and the response of cancer to treatment. Another attempt to associated inflammation with cancer was the development of the inflammation-based prognostic score (37), however, the usefulness of this score has not been confirmed, and was not supported by the present data.
Motoyama et al (38) revealed evidence for an association between CRP polymorphisms and CRP levels following esophagectomy for thoracic esophageal cancer. The authors demonstrated that, 12 h following surgery, CRP levels were significantly higher in patients harboring the CRP 1,059 G/C genotype (0.0266), and that this difference remained for 36 h after surgery (217±63 vs. 140±51 mg/l; P=0.0020). Logistic regression models revealed that patients harboring the CRP 1,059 G/G genotype had a significantly higher likelihood of a post-surgery increase in serum CRP (38).
Zingg et al (43) analyzed data for 70 patients exhibiting normal CRP levels, and 20 patients with raised CRP. The groups revealed no difference with respect to in descriptive, co-morbidities, white cell counts, pathological data or morbidity. In-hospital mortality was more frequent in the raised CRP group (3 vs. 1 patient; P=0.048), and Kaplan-Meier survival analysis revealed a significant survival advantage for patients with a normal CRP levels compared with those with raised CRP levels (median survival, 65.4 vs. 18.7 months; log rank test, P=0.027). The Cox regression analysis identified three independent prognostic factors for survival: UICC stage (IIB/III vs. I/IIA; HR, 3.48; P=0.007), extent of resection (HR, 6.33; P=0.002) and CRP levels (raised vs. normal; HR, 5.07; P=0.001). The authors concluded that pre-therapeutic CRP levels are an independent prognostic marker for survival following neoadjuvant treatment in patients with esophageal cancer and may be of value in the re-staging process following neoadjuvant treatment (43). This is in agreement with the findings reported in the present study.
In conclusion, the present preliminary data indicated that the pre-therapeutic serum CRP level is a possible indicator of treatment response, however, this finding requires confirmation in a prospective trial with a larger patient number.
Acknowledgements
The authors would like to thank staff members, nurses and technologists.
References
- 1.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Australasian Gastro-Intestinal Trials Group: Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 2.Blum MA, Taketa T, Sudo K, Wadhwa R, Skinner HD, Ajani JA. Chemoradiation for esophageal cancer. Thorac Surg Clin. 2013;23:551–558. doi: 10.1016/j.thorsurg.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Blom RL, Steenbakkers IR, Lammering G, Vliegen RF, Belgers EJ, de Jonge C, Schreurs WM, Nap M, Sosef MN. PET/CT-based metabolic tumour volume for response prediction of neoadjuvant chemoradiotherapy in oesophageal carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:1500–1506. doi: 10.1007/s00259-013-2468-x. [DOI] [PubMed] [Google Scholar]
- 4.Hulshoff JB, Smit JK, van der Jagt EJ, Plukker JT. Evaluation of progression prior to surgery after neoadjuvant chemoradiotherapy with computed tomography in esophageal cancer patients. Am J Surg. 2014;280:73–79. doi: 10.1016/j.amjsurg.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 5.Krasna MJ. Radiographic and endosonographic staging in esophageal cancer. Thorac Surg Clin. 2013;23:453–460. doi: 10.1016/j.thorsurg.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Lutz MP, Zalcberg JR, Ducreux M, Ajani JA, Allum W, Aust D, Bang YJ, Cascinu S, Hölscher A, Jankowski J, et al. Highlights of the EORTC St. Gallen International Expert Consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer-differential treatment strategies for subtypes of early gastroesophageal cancer. Eur J Cancer. 2012;48:2941–2953. doi: 10.1016/j.ejca.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Shi W, Wang W, Wang J, Cheng H, Huo X. Meta-analysis of 18FDG PET-CT for nodal staging in patients with esophageal cancer. Surg Oncol. 2013;22:112–116. doi: 10.1016/j.suronc.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Marzola MC, De Manzoni G, Grassetto G, Cordiano C, Al-Nahhas A, Alavi A, Rubello D. Extended staging of oesophageal cancer using FDG-PET - a critical appraisal. Eur J Radiol. 2012;81:21–30. doi: 10.1016/j.ejrad.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Marchesi F, Portal C, Allavena P, Sica A. Linking inflammation reactions to cancer: Novel targets for therapeutic strategies. Adv Exp Med Biol. 2008;610:112–127. doi: 10.1007/978-0-387-73898-7_9. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 11.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 12.Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ham M, Moon A. Inflammatory and microenvironmental factors involved in breast cancer progression. Arch Pharm Res. 2013;36:1419–1431. doi: 10.1007/s12272-013-0271-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim ES, Cha Y, Ham M, Jung J, Kim SG, Hwang S, Kleemann R, Moon A. Inflammatory lipid sphingosine-1-phosphate upregulates C-reactive protein via C/EBPβ and potentiates breast cancer progression. Oncogene. 2014;33:3583–3593. doi: 10.1038/onc.2013.319. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A, Pierotti MA. Cancer and inflammation: A complex relationship. Cancer Lett. 2008;267:180–181. doi: 10.1016/j.canlet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Morrison WB. Inflammation and cancer: A comparative view. J Vet Intern Med. 2012;26:18–31. doi: 10.1111/j.1939-1676.2011.00836.x. [DOI] [PubMed] [Google Scholar]
- 18.Lukaszewicz-Zając M, Mroczko B, Kozłowski M, Nikliński J, Laudański J, Siewko M, Szmitkowski M. Comparative evaluation of serum C-reactive protein (CRP) levels in the different histological subtypes of esophageal cancer (squamous cell carcinoma and adenocarcinoma of esophagus) J Clin Lab Anal. 2012;26:73–81. doi: 10.1002/jcla.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe T, Shibata M, Nishiyama H, Soeda S, Furukawa S, Gonda K, Takenoshita S, Fujimori K. Serum levels of rapid turnover proteins are decreased and related to systemic inflammation in patients with ovarian cancer. Oncol Lett. 2014;7:373–377. doi: 10.3892/ol.2013.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swede H, Hajduk AM, Sharma J, Rawal S, Rasool H, Vella AT, Tobet RE, Stevens RG. Baseline serum C-reactive protein and death from colorectal cancer in the NHANES III cohort. Int J Cancer. 2014;134:1862–1870. doi: 10.1002/ijc.28504. [DOI] [PubMed] [Google Scholar]
- 21.Xu M, Zhu M, Du Y, Yan B, Wang Q, Wang C, Zhao J. Serum C-reactive protein and risk of lung cancer: A case-control study. Med Oncol. 2013;30:319. doi: 10.1007/s12032-012-0319-4. [DOI] [PubMed] [Google Scholar]
- 22.Tibau A, Ennis M, Goodwin PJ. Post-surgical highly sensitive C-reactive protein and prognosis in early-stage breast cancer. Breast Cancer Res Treat. 2013;141:485–493. doi: 10.1007/s10549-013-2694-8. [DOI] [PubMed] [Google Scholar]
- 23.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Laird BJ, Kaasa S, McMillan DC, Fallon MT, Hjermstad MJ, Fayers P, Klepstad P. Prognostic factors in patients with advanced cancer: A comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res. 2013;19:5456–5464. doi: 10.1158/1078-0432.CCR-13-1066. [DOI] [PubMed] [Google Scholar]
- 25.Kersten C, Louhimo J, Algars A, Lahdesmaki A, Cvancerova M, Stenstedt K, Haglund C, Gunnarsson U. Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol. 2013;52:1691–1698. doi: 10.3109/0284186X.2013.835494. [DOI] [PubMed] [Google Scholar]
- 26.Mohri Y, Tanaka K, Ohi M, Toiyama Y, Yasuda H, Inoue Y, Uchida K, Kusunoki M. Inflammation-based prognostic score as a predictor of postoperative gastric cancer recurrence. Anticancer Res. 2012;32:4581–4584. [PubMed] [Google Scholar]
- 27.Jiang X, Hiki N, Nunobe S, Kumagai K, Kubota T, Aikou S, Sano T, Yamaguchi T. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. Br J Cancer. 2012;107:275–279. doi: 10.1038/bjc.2012.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZQ, Chu L, Fang JM, Zhang X, Zhao HX, Chen YJ, Xu Q. Prognostic role of C-reactive protein in prostate cancer: A systematic review and meta-analysis. Asian J Androl. 2014;16:467–471. doi: 10.4103/1008-682X.123686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Q, Yu XF, Zhang SD, Wang HH, Wang HY, Teng LS. Prognostic role of C-reactive protein in gastric cancer: A meta-analysis. Asian Pac J Cancer Prev. 2013;14:5735–5740. doi: 10.7314/APJCP.2013.14.10.5735. [DOI] [PubMed] [Google Scholar]
- 30.Guo YZ, Pan L, Du CJ, Ren DQ, Xie XM. Association between C-reactive protein and risk of cancer: A meta-analysis of prospective cohort studies. Asian Pac J Cancer Prev. 2013;14:243–248. doi: 10.7314/APJCP.2013.14.1.243. [DOI] [PubMed] [Google Scholar]
- 31.Zhou B, Liu J, Wang ZM, Xi T. C-reactive protein, interleukin 6 and lung cancer risk: A meta-analysis. PLoS One. 2012;7:e43075. doi: 10.1371/journal.pone.0043075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qayyum T, McArdle PA, Lamb GW, Going JJ, Orange C, Seywright M, Horgan PG, Oades G, Aitchison MA, Edwards J. Prospective study of the role of inflammation in renal cancer. Urol Int. 2012;88:277–281. doi: 10.1159/000334971. [DOI] [PubMed] [Google Scholar]
- 33.Han Y, Mao F, Wu Y, Fu X, Zhu X, Zhou S, Zhang W, Sun Q, Zhao Y. Prognostic role of C-reactive protein in breast cancer: A systematic review and meta-analysis. Int J Biol Markers. 2011;26:209–215. doi: 10.5301/JBM.2011.8872. [DOI] [PubMed] [Google Scholar]
- 34.Heikkilä K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S, Lawlor DA. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: Findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 35.Guillem P, Triboulet JP. Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus. 2005;18:146–150. doi: 10.1111/j.1442-2050.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 36.Crumley AB, McMillan DC, McKernan M, Going JJ, Shearer CJ, Stuart RC. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer. 2006;94:1568–1571. doi: 10.1038/sj.bjc.6603150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crumley AB, Stuart RC, McKernan M, McDonald AC, McMillan DC. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG-ps) in patients receiving palliative chemotherapy for gastroesophageal cancer. J Gastroenterol Hepatol. 2008;23:e325–e329. doi: 10.1111/j.1440-1746.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- 38.Motoyama S, Miura M, Hinai Y, Maruyama K, Usami S, Nakatsu T, Saito H, Minamiya Y, Suzuki T, Ogawa J. C-reactive protein 1059G>C genetic polymorphism influences serum C-reactive protein levels after esophagectomy in patients with thoracic esophageal cancer. J Am Coll Surg. 2009;209:477–483. doi: 10.1016/j.jamcollsurg.2009.06.365. [DOI] [PubMed] [Google Scholar]
- 39.Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Aoki T, Sugaya M, Miyazawa Y, Hayashi H, Miyazaki S, Ochiai T. Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2003;83:248–252. doi: 10.1002/jso.10275. [DOI] [PubMed] [Google Scholar]
- 40.Wang CY, Hsieh MJ, Chiu YC, Li SH, Huang HW, Fang FM, Huang YJ. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol. 2009;92:270–275. doi: 10.1016/j.radonc.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Groblewska M, Mroczko B, Sosnowska D, Szmitkowski M. Interleukin 6 and C-reactive protein in esophageal cancer. Clin Chim Acta. 2012;413:1583–1590. doi: 10.1016/j.cca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Miyata H, Yamasaki M, Kurokawa Y, Takiguchi S, Nakajima K, Fujiwara Y, Mori M, Doki Y. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med. 2011;2:879–885. doi: 10.3892/etm.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zingg U, Forberger J, Rajcic B, Langton C, Jamieson GG. Association of C-reactive protein levels and long-term survival after neoadjuvant therapy and esophagectomy for esophageal cancer. J Gastrointest Surg. 2010;14:462–469. doi: 10.1007/s11605-009-1113-2. [DOI] [PubMed] [Google Scholar]
- 44.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM: Classification of Malignant Tumours. 7th. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]


