Abstract
Background
Microtubule-targeting agents (MTAs) are a mainstay in breast cancer treatment, yet patient responses differ. The underlying mechanisms of these differences are unknown. While MTAs are mitotic inhibitors, recent evidence highlights that non-mitotic effects of these drugs can contribute to their anticancer effects. It is critical to identify the non-mitotic mechanisms that could contribute to differences among MTAs. However, it is not clear whether rapidly dividing cells in culture are optimal tools to address these mechanistic questions in interphase cells.
Materials and Methods
Detailed concentration response curves for five MTAs in a panel of diverse breast cancer cell lines were generated.
Results
Substantial differences among both drugs and cell lines, consistent with the clinical scenario, were observed. Importantly, these differences do not correlate with cell doubling time.
Conclusion
The interphase actions of MTAs are critical to the full spectrum of their effects in cancer cells, even in cell culture models.
Keywords: Microtubule, microtubule destabilizer, eribulin, paclitaxel, docetaxel, vinorelbine, ixabepilone
Microtubule-targeting agents (MTAs) are among the most effective anticancer agents. Paclitaxel, docetaxel and ixabepilone are microtubule stabilizing drugs that bind within the taxane pocket in the microtubule lumen. Drug occupancy enhances intrinsic stability of microtubules, resulting in microtubule bundles (7, 13, 24). Although they bind within the same site on tubulin, paclitaxel and docetaxel initiate the formation of microtubules with different numbers of protofilaments (1) and have different effects on MAP2- and tau-stabilized microtubules (10); in addition, a lack of cross resistance between the two drugs has been noted (27). Clinical distinctions between paclitaxel and docetaxel were described as early as 1997 (28). Thus, even closely related taxanes have subtle, but relevant differences that translate into differential clinical efficacies in some patients. The epothilone ixabepilone also binds to β-tubulin within the taxane pocket (14), and a lack of cross-resistance between taxanes and ixabepilone in breast cancer patients has also been reported (11). Microtubule de-stabilizers with demonstrated clinical utility in metastatic breast cancer include vinorelbine and eribulin. These drugs bind tubulin within the vinca domain and inhibit microtubule polymerization, leading to loss of cellular microtubules (7, 13). Vinorelbine has binding properties different from other vinca alkaloids (9) and distinct clinical utilities (2, 13, 18). Eribulin is a non-competitive inhibitor of vinca binding (5), and has unique pre-clinical and clinical activities that distinguish it from other MTAs (8). Thus, the generalization of these drugs as MTAs does not capture the significant mechanistic differences among them. Although each of these MTAs is available for treatment of metastatic breast cancer, there is currently little evidence to guide a physician’s decision to rationally select among them based on individual patients’ tumor characteristics. Efforts to understand the nature of the differences among these agents could contribute to providing such a rationale.
It has long been thought that the anti-mitotic effects of MTAs are the primary mechanism of their anti-tumor actions. However, recent observations in laboratory and clinical settings demonstrated that mitosis is likely not the sole mechanism by which MTAs exert their anticancer effects (4, 12, 15, 16, 19, 20). Median doubling time of tumors in patients is exponentially longer than that of cancer cells in tissue culture or murine xenografts (15). Therefore, the profound and rapid mitotic arrest that precedes apoptosis in cancer cells in culture may play only a minor role in patients, with the major therapeutic benefit arising from non-mitotic actions.
In addition to their effects in mitosis, microtubules play essential roles in cellular functions throughout each phase of the cell cycle. Proteins intricately involved in oncogenesis, including p53, c-Myc, BRCA1, androgen receptor, APC and Src, are known to associate with and/or traffic along microtubules (15, 21). Moreover, fully one-third of MAP kinase proteins are associated with microtubules (23). The abilities of MTAs to interrupt functions of interphase microtubules would be expected to attenuate the activity of these and other proteins implicated in cancer maintenance and progression. Notably, MTAs were recently shown to inhibit the translocation of DNA repair proteins to the nucleus explaining the synergistic actions and clinical efficacies of combining MTAs and DNA damaging agents (22). These findings strongly support the concept that the anti-mitotic effects of MTAs are not the sole mechanism leading to their anticancer activities.
To begin understanding the nature of the differences and similarities among MTAs, the effects of five clinically-approved microtubule stabilizers and de-stabilizers were evaluated in a panel of 8 molecularly diverse breast cancer cell lines. The results demonstrate that there are notable differences in the activities of these drug/cell line combinations that are not predicted from differences in doubling rate which indicates that the non-mitotic effects of these drugs may be important in their activities even in rapidly dividing cell cultures, that can be used to facilitate the understanding of the interphase mechanisms of action of these agents.
Materials and Methods
Cell Lines
BT-549 cells were obtained from the Lombardi Cancer Center, George Washington University (Washington, DC, USA) and authenticated by ATCC. All other cell lines were purchased from ATCC (Manassas, VA) and grown under standard tissue culturing conditions.
Drugs
Eribulin was provided by Eisai Inc. (Andover, MA, USA), paclitaxel and docetaxel were purchased from Sigma Aldrich (St. Louis, MO, USA) and ixabepilone and vinorelbine obtained from LC Laboratories (Woburn, MA, USA). All drugs were solubilized in DMSO and stored at −20°C.
Sulforhodamine B assay
The anti-proliferative and cytotoxic potencies of MTAs were evaluated using the sulforhodamine B (SRB) assay (25, 26). A time-0 plate was developed at the time of drug addition to measure initial cell density and evaluate cytotoxicity (3). The concentrations that caused a 50% reduction in cell growth (GI50), total growth inhibition (TGI) and 50% cytotoxicity (LC50) were determined by non-linear regression analysis using Graphpad Prism software (La Jolla, CA). The doubling time for each cell line was calculated from vehicle-treated controls.
Results
Differential sensitivity of breast cancer cell lines to MTAs
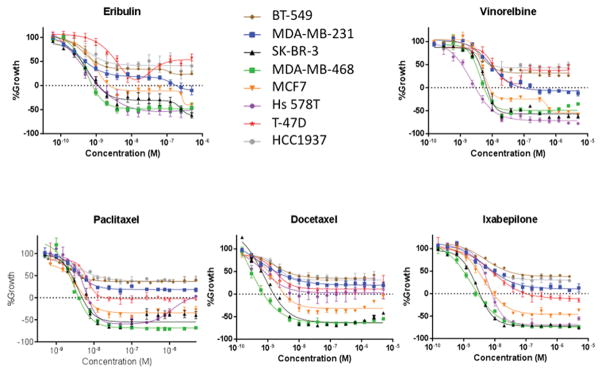
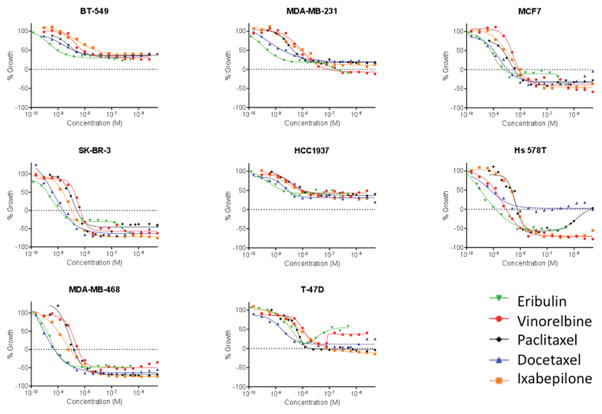
Concentration-response curves were generated for eribulin, vinorelbine, paclitaxel, docetaxel, and ixabepilone in a panel of 5 molecularly diverse triple-negative breast cancer cell lines (MDA-MB-468, HCC1937, BT-549, Hs 578T, and MDA-MB-231),(17) two ER-positive cell lines (MCF7 and T-47D) and the HER2-overexpressing SK-BR-3 line. The results for each drug are presented in Figure 1 to highlight the differential sensitivity of these cell lines to each MTA. Figure 2 shows the same data, grouped by each cell line so differential sensitivity of a cell line to individual drugs can be easily discerned.
Figure 1.
Anti-proliferative and cytotoxic effects of diverse MTAs against breast cancer cell lines. The SRB assay was used to calculate the effect of increasing drug concentrations on the growth (positive values) and cytotoxicity (negative values) of breast cancer cell lines. The cell density at the time of drug addition is indicated by the dashed line at 0% growth.
Figure 2.
Sensitivity of molecularly diverse breast cancer cell lines to the anti-proliferative and cytotoxic effects of MTAs. The SRB assay was used to calculate the effect of increasing drug concentrations on the growth (positive values) and cytotoxicity (negative values) of breast cancer cell lines. The cell density at the time of drug addition is indicated by the dashed line at 0% growth.
The dashed line on the Y-axis in each graph indicates the cell density at the time of drug addition (time 0) to indicate a transition from antiproliferative to cytotoxic efficacy when the curve falls below the dashed line. For each cell line, the GI50, TGI and LC50 are listed in Table 1. This approach reveals significant differences in the drug sensitivity of these cell lines that are not captured by the GI50 alone.
Table I.
Average anti-proliferative and cytotoxic potencies of MTAs in breast cancer cell lines with different doubling times
| ER/HER2 status | Doubling Time (h) | Average GI50 (nM) | Average TGI (nM) | Average LC50 (nM) | |
|---|---|---|---|---|---|
| MDA-MB-468 | −/− | 62.8 | 1.4 | 2.8 | 11 |
| SK-BR-3 | −/+ | 44.6 | 1.8 | 3.5 | 62 |
| Hs 578T | −/− | 43.7 | 2.5 | 4.9* | 18* |
| MCF7 | +/− | 44.2 | 2.3 | 5.8 | 410*** |
| T-47D | +/− | 47.4 | 5.8 | 92** | > 5,000 |
| MDA-MB-231 | −/− | 31.1 | 4.9 | 132*** | > 5,000 |
| HCC1937 | −/− | 49.9 | 6.5 | > 5,000 | > 5,000 |
| BT-549 | −/− | 20.9 | 8.4 | > 5,000 | > 5,000 |
One of the drugs did not reach this criterion;
two of the drugs did not reach this criterion;
three of the drugs did not reach this criterion
From these data, global trends can be seen that allow a general ranking of drugs by potency or cell lines by sensitivity. The MDA-MB-468 and SK-BR-3 cells are generally the most sensitive to MTAs (Figure 1) whereas the BT-549 and HCC1937 cells are the most resistant. Additionally, although the differences in potency among drugs do not vary greatly, in Figure 2 it is evident that eribulin and docetaxel are, in general, the most potent MTAs against these cell lines while ixabepilone and vinorelbine are the least potent. However, there are also significant outliers to these trends, including the fact that Hs 578T cells are highly sensitive to all the MTAs except docetaxel. In this cell line, high concentrations of paclitaxel are also less effective than lower concentrations, indicated by the upward trend in the dose response curve such that 5 μM is no longer cytotoxic. Interestingly, this same pattern of a U-shaped dose response relationship is observed for both destabilizers but not the stabilizers in the T-47D cell line. These examples of marked differential sensitivity of specific cell line/MTA combinations highlight opportunities to identify the nature of biological differences among MTAs.
Differential sensitivity of breast cancer cell lines to MTAs does not correlate with doubling time
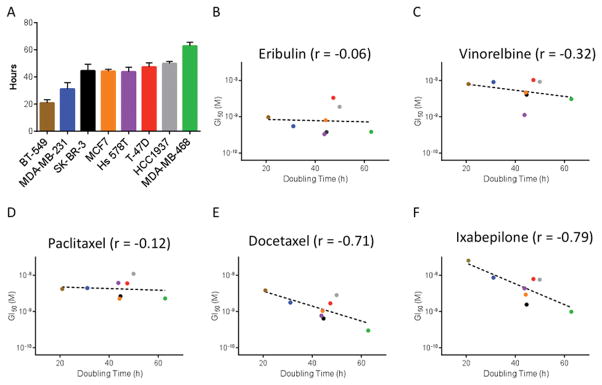
Due to the well-documented anti-mitotic effects of MTAs and the rapid rate of cell division observed in cell culture, we hypothesized that some differences in antiproliferative and/or cytotoxic potencies among the cell lines might be due simply to differences in proliferation rates such that more rapidly dividing cells might be more sensitive. To test this hypothesis, we compared the average doubling time of each cell line (Figure 3A, Table I) with the GI50 for each drug in that line. For eribulin (Figure 3B), vinorelbine (Figure 3C), and paclitaxel (Figure 3D), there was no correlation between the doubling time of the cell line and the GI50. Surprisingly, a slight inverse correlation was observed between doubling time and sensitivity to either docetaxel (Figure 3E) or ixabepilone (Figure 3F). Overall, the most rapidly dividing cell line, BT-549, was the least sensitive to these drugs while the slowest dividing cell line, MDA-MB-468, was the most sensitive
Figure 3.
Cell lines that proliferate faster are not more sensitive to MTAs. (A) The doubling time in hours of breast cancer cell lines used in this study. These doubling times were significantly different by one-way ANOVA (n=9–12, p<0.001). (B–F) Correlation between the proliferation rate of diverse breast cancer cell lines and their sensitivity to the antiproliferative effects of MTAs.
Discussion
The finding of no positive correlation between GI50 and doubling time indicates that not even the anti-proliferative activities of MTAs are dependent on cell doubling time. Moreover, a similar lack of correlation was observed between doubling time and cytotoxicity. These results strongly suggest that the antimitotic effects of MTAs are not the primary drivers of antiproliferative and/or cytotoxic effects even in rapidly dividing cells in culture. These results therefore indicate that cells in culture can serve as useful models to explore the non-mitotic effects of MTAs. Data by others also support this conclusion (6, 12, 19, 20, 22). Our results additionally indicate that non-mitotic effects of MTAs can be effectively studied at short time points after drug addition, thus precluding confounding anti-mitotic effects. Overall, the implications of our studies are that non-mitotic effects of MTAs play prominent roles in their anti-proliferative and cytotoxic effects, and that obtaining a deeper understanding of such processes through the use of molecularly-defined cell lines might provide valuable insights into the different mechanisms of action that contribute to their therapeutic effects. Such insights may facilitate clinical decision-making regarding the most effective use of these diverse agents in differing cancer types and patient populations.
Acknowledgments
We thank Dr. Bruce Littlefield for his thoughtful comments.
Funding
This research was funded by Eisai Inc. N.F. Dybdal-Hargreaves was supported in part by the COSTAR training grant (T32-DE014318) and a predoctoral fellowship training grant through the NIDCR (1F30DE025535-01).
Footnotes
Competing Interests
SLM has participated as a member of Eisai’s Eribulin Preclinical and Global Advisory Boards.
References
- 1.Andreu JM, Diaz JF, Gil R, de Pereda JM, Garcia de Lacoba M, Peyrot V, Briand C, Towns-Andrews E, Bordas J. Solution structure of Taxotere-induced microtubules to 3-nm resolution. The change in protofilament number is linked to the binding of the taxol side chain. J Biol Chem. 1994;269:31785–31792. [PubMed] [Google Scholar]
- 2.Binet S, Fellous A, Lataste H, Krikorian A, Couzinier JP, Meininger V. In situ analysis of the action of Navelbine on various types of microtubules using immunofluorescence. Semin Oncol. 1989;16:5–8. [PubMed] [Google Scholar]
- 3.Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer discovery screen. Drug Devel Res. 1995;34:91–109. [Google Scholar]
- 4.Chittajallu DR, Florian S, Kohler RH, Iwamoto Y, Orth JD, Weissleder R, Danuser G, Mitchison TJ. In vivo cell-cycle profiling in xenograft tumors by quantitative intravital microscopy. Nat Methods. 2015;12:577–585. doi: 10.1038/nmeth.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabydeen DA, Burnett JC, Bai R, Verdier-Pinard P, Hickford SJ, Pettit GR, Blunt JW, Munro MH, Gussio R, Hamel E. Comparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Mol Pharmacol. 2006;70:1866–1875. doi: 10.1124/mol.106.026641. [DOI] [PubMed] [Google Scholar]
- 6.Darshan MS, Loftus MS, Thadani-Mulero M, Levy BP, Escuin D, Zhou XK, Gjyrezi A, Chanel-Vos C, Shen R, Tagawa ST, Bander NH, Nanus DM, Giannakakou P. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–6029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dybdal-Hargreaves NF, Risinger AL, Mooberry SL. Eribulin Mesylate: Mechanism of Action of a Unique Microtubule-Targeting Agent. Clin Cancer Res. 2015;21:2445–2452. doi: 10.1158/1078-0432.CCR-14-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabre C, Czaplicki J, Wright M, Hill B, Barret JM, Fahy J, Milon A. Differential binding to the alpha/beta-tubulin dimer of vinorelbine and vinflunine revealed by nuclear magnetic resonance analyses. Biochem Pharmacol. 2002;64:733–740. doi: 10.1016/s0006-2952(02)01255-8. [DOI] [PubMed] [Google Scholar]
- 10.Fromes Y, Gounon P, Veitia R, Bissery MC, Fellous A. Influence of microtubule-associated proteins on the differential effects of paclitaxel and docetaxel. J Protein Chem. 1996;15:377–388. doi: 10.1007/BF01886864. [DOI] [PubMed] [Google Scholar]
- 11.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Janssen A, Beerling E, Medema R, van Rheenen J. Intravital FRET imaging of tumor cell viability and mitosis during chemotherapy. PLoS One. 2013;8:e64029. doi: 10.1371/journal.pone.0064029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 14.Khrapunovich-Baine M, Menon V, Yang CP, Northcote PT, Miller JH, Angeletti RH, Fiser A, Horwitz SB, Xiao H. Hallmarks of molecular action of microtubule stabilizing agents: effects of epothilone B, ixabepilone, peloruside A, and laulimalide on microtubule conformation. J Biol Chem. 2011;286:11765–11778. doi: 10.1074/jbc.M110.162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8:244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 16.Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res. 2012;18:51–63. doi: 10.1158/1078-0432.CCR-11-0999. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobert S, Vulevic B, Correia JJ. Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine. Biochemistry. 1996;35:6806–6814. doi: 10.1021/bi953037i. [DOI] [PubMed] [Google Scholar]
- 19.Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Mol Biol Cell. 2012;23:1–6. doi: 10.1091/mbc.E10-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orth JD, Kohler RH, Foijer F, Sorger PK, Weissleder R, Mitchison TJ. Analysis of mitosis and antimitotic drug responses in tumors by in vivo microscopy and single-cell pharmacodynamics. Cancer Res. 2011;71:4608–4616. doi: 10.1158/0008-5472.CAN-11-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker AL, Kavallaris M, McCarroll JA. Microtubules and their role in cellular stress in cancer. Front Oncol. 2014;4:153. doi: 10.3389/fonc.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poruchynsky MS, Komlodi-Pasztor E, Trostel S, Wilkerson J, Regairaz M, Pommier Y, Zhang X, Kumar Maity T, Robey R, Burotto M, Sackett D, Guha U, Fojo AT. Microtubule-targeting agents augment the toxicity of DNA-damaging agents by disrupting intracellular trafficking of DNA repair proteins. Proc Natl Acad Sci USA. 2015;112:1571–1576. doi: 10.1073/pnas.1416418112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reszka AA, Seger R, Diltz CD, Krebs EG, Fischer EH. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci USA. 1995;92:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohena CC, Mooberry SL. Recent progress with microtubule stabilizers: new compounds, binding modes and cellular activities. Nat Prod Rep. 2014;31:335–355. doi: 10.1039/c3np70092e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 26.Tinley TL, Leal RM, Randall-Hlubek DA, Cessac JW, Wilkens LR, Rao PN, Mooberry SL. Novel 2-methoxyestradiol analogues with antitumor activity. Cancer Res. 2003;63:1538–1549. [PubMed] [Google Scholar]
- 27.Verweij J, Clavel M, Chevalier B. Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol. 1994;5:495–505. doi: 10.1093/oxfordjournals.annonc.a058903. [DOI] [PubMed] [Google Scholar]
- 28.Von Hoff DD. The taxoids: same roots, different drugs. Semin Oncol. 1997;24:S13-13–S13-10. [PubMed] [Google Scholar]