Abstract
Previous studies have illustrated that bone marrow-derived mesenchymal stem cell (BMMSC) transplantation has therapeutic effects on diabetes and can prevent mice from renal damage and diabetic nephropathy (DN). Moreover, adipose-derived MSCs possess similar characteristics to BMMSCs. We investigated the effect of ADMSC transplantation on streptozotocin (STZ)-induced renal injury. Diabetes was induced in rats by STZ injection. After ADMSC treatment, renal histological changes and cell apoptosis were evaluated as were the expression of apoptosis-related proteins, Wnt/β-catenin pathway members, and klotho levels. We found that ADMSCs improved renal histological changes. Next, NRK-52E cells were exposed to normal glucose (NG; 5.5 mM glucose plus 24.5 mM mannitol)/high glucose (HG) or ADMSCs, and then measured for changes in the aforementioned proteins. Similarly, changes in these proteins were also determined following transient transfection of klotho siRNA. We found that both ADMSC transplantation and co-incubation reduced the rate of cellular apoptosis, decreased Bax and Wnt/β-catenin levels, and elevated Bcl-2 and klotho levels. Interestingly, klotho knockdown reversed the effects of ADMSCs on the expression of apoptosis-related proteins and Wnt/β-catenin pathway members. Taken together, ADMSCs transplantation might attenuate renal injury in DN via activating klotho and inhibiting the Wnt/β-catenin pathway. This study may provide evidence for the treatment of DN using ADMSCs.
Keywords: adipose-derived mesenchymal stem cells, diabetic nephropathy, klotho, siRNA, Wnt/β-catenin pathway
Introduction
Diabetic nephropathy (DN) is one of the most common complications of diabetes and the leading cause of end-stage renal disease (ESRD). It is characterized by tubulointerstitial fibrosis, microalbuminuria, thickened glomerular and tubular basement membranes, and renal inflammation (Abe et al. 2011; Dronavalli et al. 2008; Hovind et al. 2001). DN affects approximately one third of people who suffer with type 1 or type 2 diabetes (Gross et al. 2005). Numerous studies have shown that oxidative stress, inflammatory responses, renal hemodynamic changes and metabolic abnormalities may contribute to the occurrence and development of DN (Brownlee 2001; Burke et al. 2014; Duran-Salgado and Rubio-Guerra 2014; Forbes et al. 2008; Kume et al. 2014). However, the exact pathogenesis of DN has not yet been completely elucidated.
Mesenchymal stem cells (MSCs) are a group of cells that have the differentiative and proliferative potentials (Amable et al. 2014). MSCs can be isolated from multiple sources, including adipose tissue, bone marrow and other tissues (Montespan et al. 2014). MSCs have been reported to alleviate hyperglycemia, suppress oxidative stress and improve renal histopathological changes in diabetic rats with DN (Lv et al. 2014). Previous studies have illustrated that transplantation of BMMSCs has therapeutic effects on diabetes and can prevent mice from renal damage and DN (Ezquer et al. 2008). Adipose-derived MSCs (ADMSCs) are multipotent stem cells (Efimenko et al. 2011) and possess similar characteristics to BMMSCs (Schaffler and Buchler 2007). Accumulated reports have shown therapeutic efficacy of ADMSCs in the treatment of diabetic retinopathy (Yang et al. 2010). Moreover, we previously demonstrated that autologous transplantation of ADMSCs improves diabetes-induced metabolic abnormalities and renal injury, inhibits oxidative stress, and reduces pro-inflammatory cytokine release in a rat model of streptozotocin (STZ)-induced DN (Fang et al. 2012). Additionally, renal tubular injury (Xiao et al. 2014) and apoptosis of tubular epithelial cells (Sato et al. 2010) can be observed in diabetic rats.
α-Klotho is a transmembrane protein reported to be highly expressed in normal kidney (Lee et al. 2014). A report has found that the expression of α-klotho in kidney is significantly lowered in patients with DN, and α-klotho exerts protective effects on the function and structure of kidneys. Klotho deficiency, remarkably, aggravates the progression of DN (Lin et al. 2013). Other evidence has shown that BMMSC transplantation upregulates klotho expression in numerous organs of adult immunocompromised mice (Yamaza et al. 2009). However, the effect of ADMSCs on klotho remains unknown.
Based on the above findings, we hypothesized that ADMSC transplantation might ameliorate STZ-induced DN, and we therefore further explored the associated underlying molecular mechanisms.
Materials & Methods
Animals
Male Sprague-Dawley (SD) rats, weighting 200–250 g at 8 weeks of age, were purchased from Charles River (Beijing, China). The rats were kept in cages under 12 hr light/dark cycles and had access to standard diet and water ad libitum throughout the whole experiment. All of the animal experiments were performed following the Guide for the Care and Use of Laboratory Animals of China Medical University. This study was approved by the Institutional Animal Care and Use Committee at China Medical University.
The rats were randomly divided into four groups. (i) The control group (n=8), where the rats were intraperitoneally administrated with a volume of sodium citrate buffer (0.01 M, pH 4.5) equal to volumes used in the other groups. (ii) The DN group (n=8), where rats were intraperitoneally injected with 40 mg/kg streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO) in sodium citrate buffer for five consecutive days. Two weeks after STZ injection, the rats with glucose concentrations greater than 300 mg/dl were recruited in this study. (iii) The ADMSCs group (n=8), where the rats were administrated with 1×107 ADMSCs via tail-vein injection 4 weeks after STZ injection. (iv) The vehicle group (n=8), where the STZ-induced DN rats received an equal volume of culture medium as those volumes used in the other groups. After 8 weeks of treatment, the animals were sacrificed, and blood samples and kidneys were obtained for further analyses.
Isolation and Identification of ADMSCs
Adipose tissues were obtained and washed with sterile Hank’s balanced salt solution. The tissues were cut into pieces (0.5 mm3) using scissors and digested with type I collagenase (2 mg/ml; Biosharp, Hefei, China) at 37°C for 40 min. After digestion, the cell suspension was centrifuged for 5 min at 1000 rpm. Then, the cell pellet was harvested, re-suspended in culture medium containing serum, and filtered through a 100-μm nylon mesh filter to remove undigested sections. After centrifugation, the cell pellet was re-suspended in 2 ml DMEM containing 10% FBS. Isolated ADMSCs were counted and then seeded into 6-well plates at a density of 1×105 cells/ml for culture. The cells were digested and incubated for 30 min on ice with anti-CD13 (BioLegend; San Diego, CA), anti-CD45 (BD Biosciences; San Jose, CA) and anti-CD90 antibodies (Invitrogen, Life Technologies; Carlsbad, CA), and then incubated with FITC-conjugated secondary antibodies. Finally, ADMSCs were analyzed by flow cytometry (C6; BD Biosciences). The experiment was repeated at least three times.
Histological Examination
The kidneys were removed, fixed in 4% paraformaldehyde, embedded in paraffin and then cut into 5-μm sections. Then, the sections were deparaffinized, hydrated, and stained with hematoxylin and eosin (HE) or periodic acid-Schiff (PAS). Renal injury and morphological changes were observed under a microscope (DP73; Olympus, Tokyo, Japan). The experiment was repeated five times. Semi-quantitative scoring of renal injury was performed according to the extent of glomerular changes and tubular dilation as follows: 0, absent; 1, mild; 2, moderate; and 3, severe (Ortiz-Munoz et al. 2010).
TUNEL Assay
A TUNEL assay was carried out using the In Situ Cell Death Detection Kit (Roche Applied Sciences; Shanghai, China) according to the manufacturer’s instructions. Briefly, sections were cut from paraffin-embedded renal tissues, treated with 0.1% Triton X-100 (Beyotime Institute of Biotechnology; Haimen, China) for 8 min at room temperature, blocked with 3% H2O2, and then placed in working solution (10% enzyme solution and 90% label solution) for 1 hr at 37°C. Diaminobenzidine (DAB; Solarbio, Beijing, China) substrate was added, and the apoptotic cells were observed under a microscope (DP73; Olympus).
Immunohistochemistry (IHC)
Renal tissue sections were blocked with 3% H2O2 and normal goat serum (Solarbio) and incubated with klotho antibody (1:100; Bioss, Beijing, China) at 4°C overnight, followed by incubation with biotinylated goat anti-rabbit secondary antibody (1:200; Beyotime Institute of Biotechnology) and HRP-conjugated avidin (1:200; Beyotime Institute of Biotechnology). After three washes with PBS, the sections were treated with diaminobenzidine (DAB) solution. The slides were then counterstained with hematoxylin (Solarbio) and photographed (DP73; Olympus).
ELISA
Klotho levels in serum were detected using an ELISA kit (Wanleibio; Shenyang, China) according to the manufacturer’s instructions. The absorbance was measured using a model ELX-800 microplate reader (Bio-Tek; Winooski, VT) at 450 nm.
Cell Culture and Treatment
The rat renal tubular epithelial cell line NRK-52E was purchased from Shanghai Institute of Biological Sciences, Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM (Gibco, Invitrogen) containing 10% FBS (HyClone, Thermo Scientific; Wilmington, DE) at 37°C in a 5% CO2 atmosphere.
NRK-52E cells were plated in 24-well plates. After reaching 90% confluence, the cells were stimulated with normal glucose (NG; 5.5 mM glucose plus 24.5 mM mannitol) or high glucose (HG; 30 mM) for 12 hr. Meanwhile, ADMSCs were seeded into the upper chamber of a Transwell chamber at a density of 1×105 cells/ml and cultured for 12 hr. The upper chamber was then placed on 6-well plate that contained NRK-52E cells. After co-culturing for 24 hr, NRK-52E cells were harvested for further analyses.
Annexin V-FITC/Propidium Iodide (PI) Assay
After 24 hr of co-culture, the apoptosis rates of NRK-52E cells in each group were measured using Annexin V-FITC/PI apoptosis detection kit (KeyGEN Biotech; Nanjing, China) according to the manufacturer’s instructions. In brief, NRK-52E cells in each group were harvested and washed twice with PBS. The cells were then re-suspended in 500 μl binding buffer. Equal parts of Annexin V-FITC (5 μl) and PI (5 μl) were added into the cell suspension. After incubation in the dark for 15 min at room temperature, the samples were detected by flow cytometry (C6; BD Biosciences).
Quantitative Real-Time PCR
Total RNA was isolated from renal tissue or from co-cultured NRK-52E cells using RNAsimple total RNA kit (Tiangen Biotech; Beijing, China) in accordance with the manufacturer’s instructions. Reverse-Transcription PCR (RT-PCR) was performed to synthesize cDNA using the Super M-MLV reverse transcriptase (Bio-Teke; Beijing, China). Real-time PCR was carried out on an ExicyclerTM 96 quantitative fluorescence analyzer (Bioneer; Daejeon, Korea). The cycling conditions were: 95°C for 10 min followed by 40 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 30 s. The primer sequences used were: Bax-F, 5’-GTGGTTGCCCTCTTCTACTTTGC-3’, Bax-R, 5’-CTCACGGAGGAAGTCCAGTGTCC-3’; Bcl-2-F, 5’-ATGCGACCTCTGTTTGATTTCTC-3’, Bcl-2-R, 5’-AACTTTGTTTCATGGTCCATCCT-3’; klotho-F, 5’-CCTCCTTTACCTGAGAACCA-3’, klotho-R, 5’-GCACATCCCACAGATAGACA-3’; β-actin-F, 5’-GGAGATTACTGCCCTGGCTCCTAGC-3’, β-actin-R, 5’- GGCCGGACTCATCGTACTCCTGCTT-3’. The relative expression levels of these genes were calculated using the 2-ΔΔCt formula (Livak and Schmittgen 2001) and normalized to β-actin levels.
siRNA Transfection
NRK-52E cells were seeded into the wells of 6-well plates. When reaching 70% to 80% confluence, the cells were transfected with klotho siRNA or control siRNA (75 pM) using Lipofectamine 2000 (Invitrogen). Target protein silencing was measured by western blot at 48 hr post transfection. β-actin served as an internal control.
After transfection, the cells were stimulated with HG for 12 hr and then co-cultured with ADMSCs for 24 hr. NRK-52E cells were harvested, and the expression levels of Bax, Bcl-2, Wnt1, Wnt3a, Snail and active-β-catenin were measured by western blot.
Western Blot
Renal tissues and co-cultured NRK-52E cells that had been transfected with/without siRNA were treated with RIPA lysis buffer for protein extraction (Beyotime Institute of Biotechnology). Protein concentrations were determined using the BCA assay (Beyotime Institute of Biotechnology). Proteins (40 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto PVDF membranes (Millipore, Bedford, MA). The membranes were blocked with 5% non-fat milk and incubated overnight with primary antibodies against Bax (1:1000; Boster, Wuhan, China), Bcl-2 (1:1000; Boster), Klotho (1:500; Bioss), Wnt1 (1:500; Bioss), Wnt3a (1:1000; Boster), Snail (1:1000; Bioss) and active-β-catenin (1:1000; Millipore) at 4°C. Subsequently, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5000; Beyotime Institute of Biotechnology) at 37°C for 45 min. Finally, the protein bands were visualized using enhanced chemiluminescence (ECL; Wanleibio) reagents and quantified using Gel-Pro-Analyzer software (Media Cybernetics, Bethesda, MD, USA). β-actin was used as an internal control.
Statistical Analysis
Data are expressed as the mean ± SD. The differences were analyzed by one-way ANOVA followed by Bonferroni post-hoc test using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA). P<0.05 was considered statistically significant.
Results
Surface Marker Expression of ADMSCs
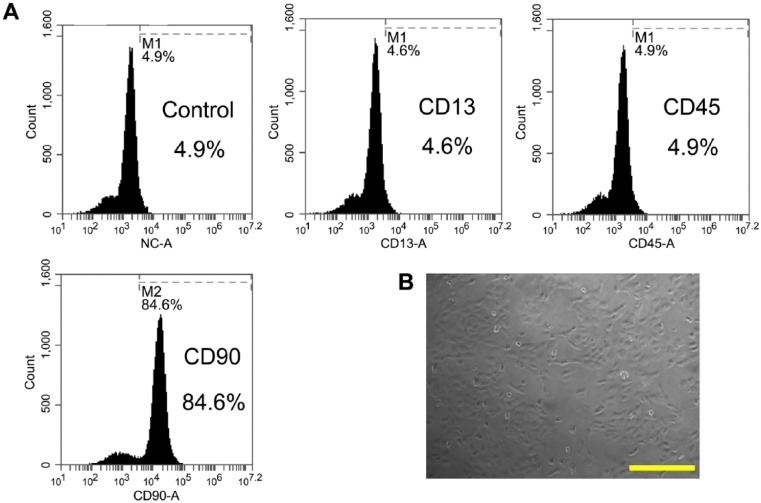
ADMSCs were analyzed for the expression of surface markers. Flow cytometry analysis showed that ADMSCs expressed high levels of CD90, but lacked CD13 and CD45 (Fig. 1A). The results showed that CD90+ cells contributed the highest population of stem cells, which is in line with a recent study (Jin et al. 2013). ADMSCs displayed spindle-shaped morphological features in the Petri dish (Fig. 1B).
Figure 1.
Flow cytometry and microscopic analysis of rat ADMSCs. (A) ADMSCs were obtained and analyzed by flow cytometry for the expression of CD13, CD45 and CD90. (B) Spindle-shaped morphology of ADMSCs was observed under a microscope. Scale, 200 μm.
Histological Examination of Kidneys
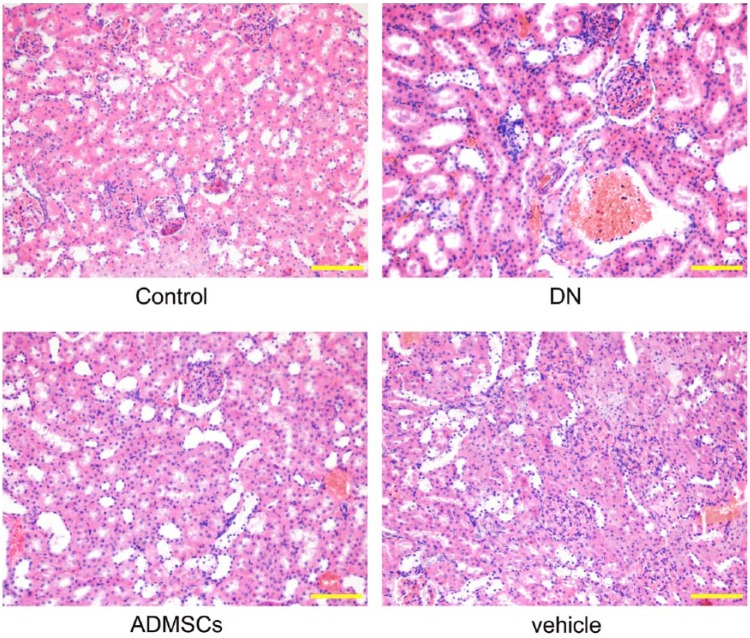
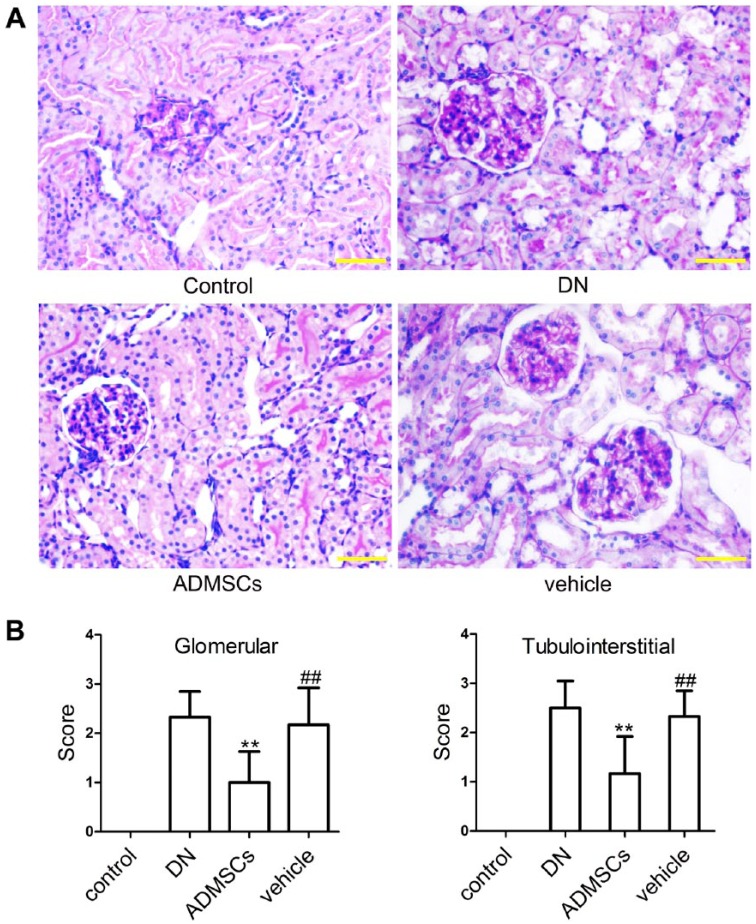
To assess the effects of ADMSCs on STZ-induced renal injury in DN rats, the kidneys were excised from the rats at the end of the experiments and histological examination was performed using HE and PAS staining. As shown in Figs. 2 and 3A, the control rats showed normal glomerular and tubular structures. Histological changes were observed in DN and vehicle rats, including tubulointerstitial lesions (inflammatory cell infiltration and tubular dilatation) and glomerular hypertrophy. However, ADMSC transplantation significantly improved each of these pathological abnormalities. Semi-quantitative scoring of renal tissues confirmed these observations (Fig. 3B and 3C, p<0.01).
Figure 2.
HE staining. Rats (8 rats in each group) were sacrificed after 8 weeks of ADMSC treatment and the kidneys were removed. The renal tissue was embedded, cut into 5-μm sections, stained with HE and examined under a microscope. Scale, 100 μm.
Figure 3.
PAS staining and semi-quantitative scoring of renal injury. (A) Representative photographs of renal tissues stained with PAS. (B) Semi-quantitative scoring of renal injury. Data shown are the mean ± SD, n=6. ##p<0.01, compared with the control group; **p<0.01, compared with the vehicle group. Scale, 50 μm.
ADMSC Transplantation Inhibits Renal Cell Apoptosis in Rats
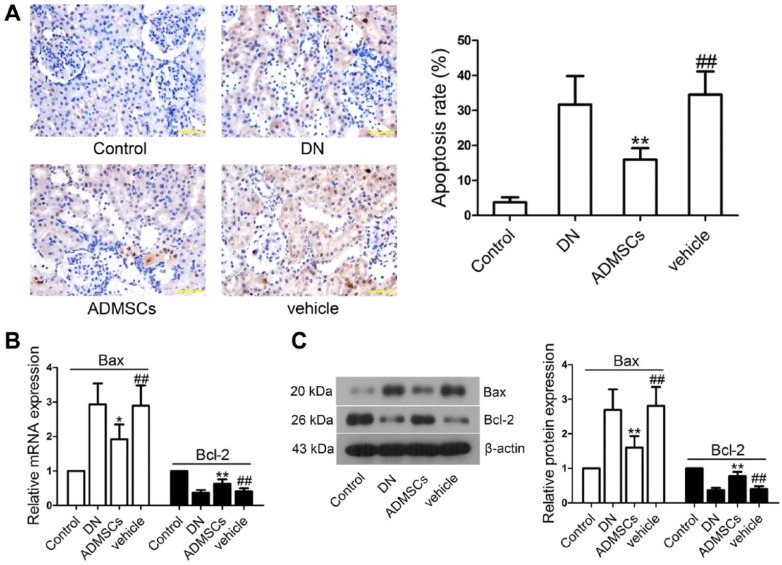
To study whether STZ-induced renal injury was correlated with renal cell apoptosis, paraffin-embedded renal tissues were cut into sections and TUNEL assay was performed. The apoptosis rate in the vehicle group was increased in comparison with the control group (p<0.01) and decreased after ADMSC transplantation (p<0.01; Fig. 4A). To further examine the effects of ADMSCs on apoptosis-related protein expression, we then measured Bax and Bcl-2 levels in renal tissues. Real-time PCR results showed that the mRNA level of Bax was upregulated and Bcl-2 downregulated in the vehicle group as compared with those levels in the control group (p<0.01; Fig. 4B). However, treatment with ADMSCs remarkably restored Bax (p<0.05) and Bcl-2 (p<0.01) expression to that of normal levels; these findings were further confirmed by western blot analysis (p<0.01; Fig. 4C).
Figure 4.
Effects of ADMSCs transplantation on the apoptosis of renal tissue cells in DN. (A) Cell apoptosis was detected by TUNEL staining and quantified as a percentage of apoptotic cells. (B) The mRNA levels of Bax and Bcl-2 were measured by real-time PCR. β-actin was used as an internal control. (C) Protein levels of Bax and Bcl-2 were measured by western blot. β-actin was used as an internal control. Data shown are the mean ± SD, n=5. ##p<0.01, compared with the control group; *p<0.05 and **p<0.01, compared with the vehicle group. Scale, 50 μm.
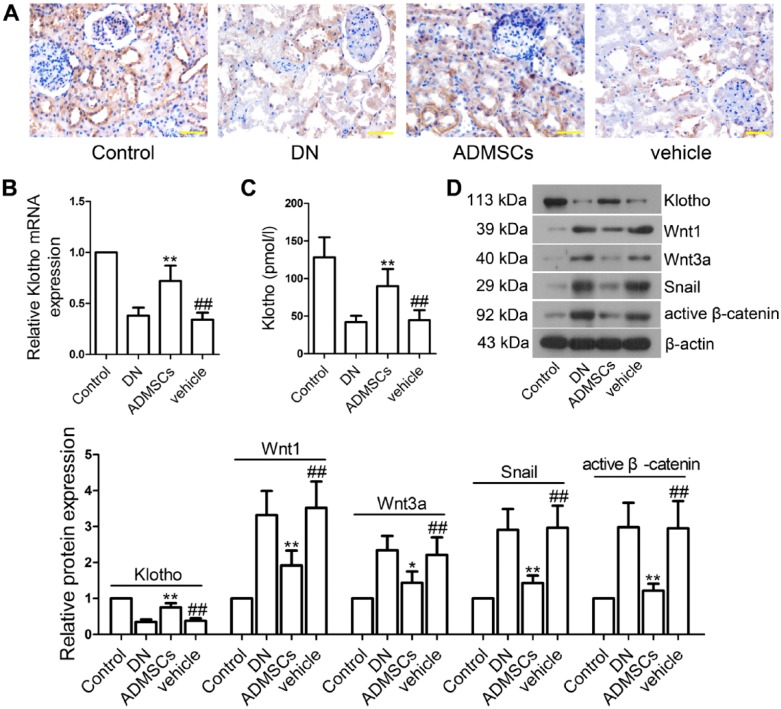
ADMSC Transplantation Promotes Renal Expression of Klotho and Inhibits Wnt/β-catenin Signaling Pathway in STZ-induced DN Rats
In order to investigate the effect of ADMSCs on klotho and the Wnt/β-catenin signaling pathway, we measured klotho, Wnt1, Wnt3a, Snail and active-β-catenin expression levels in vivo. Compared with the control group, klotho expression levels in serum and renal tissues were significantly lowered in the vehicle group (p<0.01; Fig. 5A–5D). Interestingly, the decreased klotho levels were elevated following ADMSC transplantation (p<0.01). As shown in Fig. 5D, Wnt1, Wnt3a, Snail and active-β-catenin levels were significantly elevated in renal tissues of the vehicle-treated rats as compared with the control rats (p<0.01). After ADMSCs treatment, these proteins levels were lowered (Wnt1, Snail and active-β-catenin, p<0.01; Wnt3a, p<0.05).
Figure 5.
Effects of ADMSC transplantation on klotho expression and Wnt/β-catenin signaling pathway in vivo. (A) Paraffin-embedded tissue sections of renal tissues were subjected to immunohistochemical (IHC) staining using klotho antibody. (B) The mRNA level of klotho was determined by real-time PCR. (C) Klotho expression in the serum was determined by ELISA. (D) Western blot analysis of klotho, Wnt1, Wnt3a, Snail and β-catenin expression in renal tissues. β-actin was used as an internal control. The intensities of the bands for these proteins were quantified. The relative expression of all controls was set to 1, and the values within the data set were compared to the respective controls. Data shown are the mean ± SD, n=5. ##p<0.01, compared with the control group; *p<0.05 and **p<0.01, compared with the vehicle group. Scale, 50 μm.
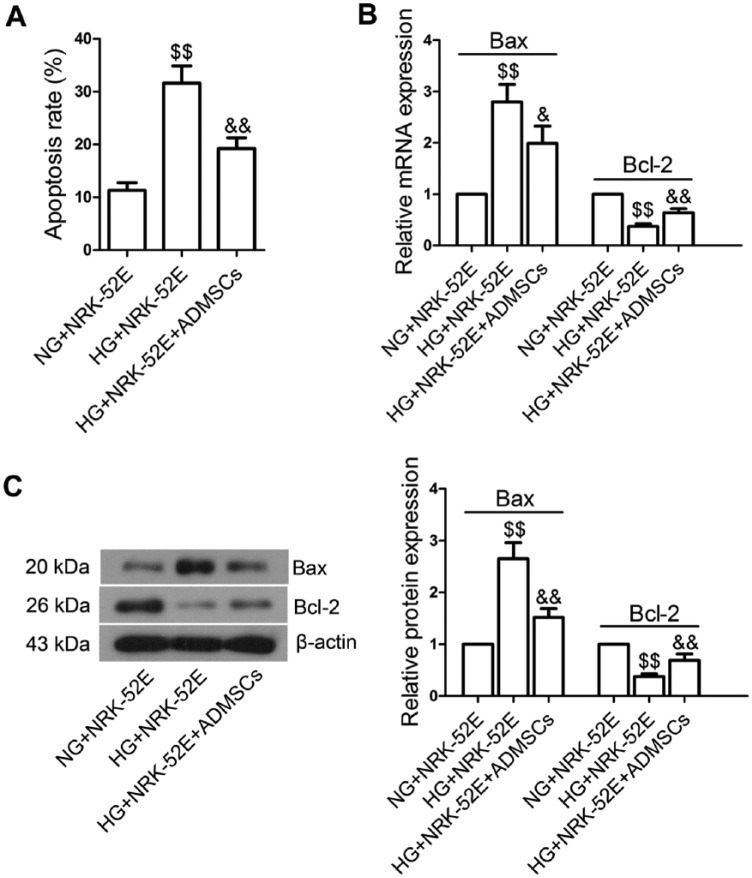
Co-cultured with ADMSCs Inhibited High Glucose (HG)-induced NRK-52E Cell Apoptosis
We further evaluated the impact of ADMSC transplantation on cell apoptosis in NRK-52E cells. We found that HG stimulation significantly increased the apoptosis rate of NRK-52E cells (Fig. 6A, p<0.01) and this effect was not induced by the hyperosmolarity of HG, as determined by Annexin V-FITC/PI assay. However, when co-cultured with ADMSCs, NRK-52E cells showed a markedly inhibited rate of apoptosis (p<0.01). Compared with the NG+NRK-52E group, HG caused significant increases in the mRNA (Fig. 6B; p<0.01) and protein (Fig. 6C; p<0.01) levels of Bax in the HG+NRK-52E group, along with decreases in Bcl-2 levels (p<0.01). However, ADMSCs significantly reduced Bax levels (mRNA, p<0.05; protein, p<0.01) and elevated Bcl-2 levels (mRNA and protein, P<0.01).
Figure 6.
Effects of ADMSC co-incubation on the apoptosis of NRK-52E cells exposed to high glucose (HG). (A) After growing to 90% confluence, NRK-52E cells were stimulated with normal glucose (NG, 5.5 mM glucose plus 24.5 mM mannitol) or HG (30 mM) for 12 hr. Then, the cells were co-incubated with ADMSCs for 24 hr. The apoptosis rates of NRK-52E cells in each group were measured by Annexin V-FITC/PI assay. Representative images are shown. (B) Real-time PCR analysis of Bax and Bcl-2 expression. (C) Western blot analysis of Bax and Bcl-2 expression. Data shown are the mean ± SD; n=3. $$p<0.01, compared with the NG+NRK-52E group; &p<0.05 and &&p<0.01, compared with the HG+NRK-52E group.
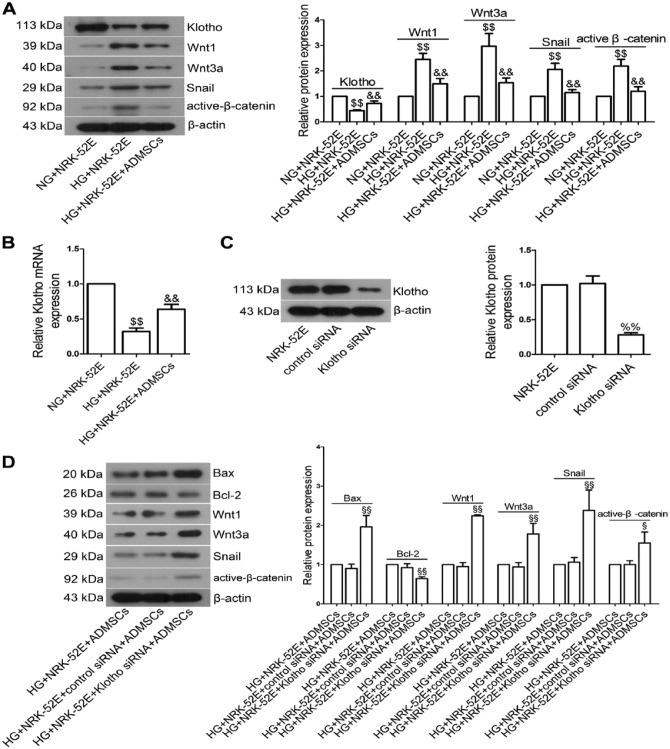
ADMSCs Promoted Klotho Expression and Inhibited Wnt/β-catenin Signaling Pathway in NRK-52E Cells
We next examined the effects of ADMSCs on klotho expression and Wnt/β-catenin signaling pathway in vitro. Compared with the NG+NRK-52E group, klotho protein expression in the HG+NRK-52E group was significantly lowered (Fig. 7A; p<0.01). However, klotho expression was significantly higher in the HG+NRK-52E+ADMSCs group than in the HG+NRK-52E group (p<0.01). Similar results were observed when we measured klotho mRNA levels by real-time PCR (Fig. 7B; p<0.01). HG exposure remarkably elevated the levels of Wnt1, Wnt3a, Snail and active-β-catenin in NRK-52E cells (p<0.01), which was not due to the hyperosmolarity of HG. As expected, ADMSC treatment significantly blocked HG-induced upregulation of these proteins (p<0.01).
Figure 7.
Effects of ADMSC co-incubation with NRK-52E cells on klotho expression and Wnt/β-catenin signaling pathway in vitro. (A) NRK-52E cells were incubated with NG (5.5 mM glucose plus 24.5 mM mannitol)/HG and ADMSCs, and then the expression of klotho, Wnt1, Wnt3a, Snail and β-catenin was measured by western blot. (B) Real-time PCR analysis of klotho mRNA expression. (C) NRK-52E cells were transfected with klotho siRNA (75 pM) for 12 hr. After further culturing for 48 hr, klotho expression was measured by western blot. β-actin was served as an internal control. (D) After transfection with klotho siRNA or control siRNA, NRK-52E cells were incubated with HG and ADMSCs. The expression of klotho, Wnt1, Wnt3a, Snail and β-catenin expression were measured by western blot. Data shown are the mean ± SD; n=3. $$p<0.01, compared with the NG+NRK-52E group; &&p<0.01, compared with the HG+NRK-52E group; %%p<0.01, compared with the control siRNA group. §p<0.05 and §§p<0.01, compared with the HG+NRK-52E+control siRNA+ADMSCs group.
Klotho Knockdown Could Activate the Wnt/β-catenin Pathway and Induce Apoptosis in NRK-52E Cells
To determine the roles of klotho and the Wnt/β-catenin pathway in the apoptosis of NRK-52E cells, we knocked down klotho expression using klotho siRNA. As shown in Fig. 7C, western blot analysis confirmed that klotho was dramatically reduced after siRNA transfection (p<0.01). Klotho knockdown upregulated the protein expression of Bax when compared with the HG+NRK-52E+control siRNA+ADMSCs group (Fig. 7D, p<0.01), whereas downregulated Bcl-2 expression (p<0.01). Meanwhile, the levels of Wnt1, Wnt3a, Snail and active-β-catenin (all p<0.01) were greatly increased after klotho siRNA transfection.
Discussion
MSCs were first discovered and isolated from bone marrow by A. J. Friedenstein in the 1960s (Wong 2011). In actuality, MSCs can be found in various tissues and organs throughout the body, including adipose tissue, kidney, bone marrow, lung, spleen, brain and liver (Lai et al. 2015). ADMSCs and BMMSCs share similar characteristics regarding morphology and immune phenotype (Kern et al. 2006). ADMSCs are able to differentiate into several cell lineages of mesodermal origin, including adipocytes, chondrocytes and osteoblasts. Due to their properties of self-renewal and differentiation into different cell types, MSCs have been widely applied in clinical research for the treatment of numerous diseases (de Girolamo et al. 2013). Our previous study demonstrated that ADMSCs autologous transplantation protects the rats against DN by suppressing oxidative stress, the release of pro-inflammatory cytokines, and the p38 MAPK pathway (Fang et al. 2012). However, the therapeutic effects and the mechanisms of ADMSC implantation in STZ-induced DN needed further investigation.
In the present study, we investigated the protective effects of ADMSC transplantation in STZ-induced renal injury and renal tissue cell apoptosis in DN rats and we further discussed its possible mechanisms. We found that ADMSC treatment improved histological changes in kidney, decreased the apoptosis rate and the expression of Wnt/β-catenin, and upregulated klotho expression. Interestingly, knockdown of klotho using siRNA reversed the inhibitory effects of ADMSCs on Bax and Wnt/β-catenin pathway members.
Apoptosis plays a vital role in the development and homeostasis of tissues (Harada et al. 2004). It is regulated by two major pathways, namely the death receptor pathway and the mitochondrial pathway (Jin and El-Deiry 2005). Bcl-2 family proteins are key regulators of cell apoptosis in the mitochondrial pathway (Hardwick and Soane 2013). Bcl-2 is an anti-apoptotic protein and it can prevent the release of cytochrome c from the mitochondria into the cytosol (Makpol et al. 2012). In contrast, Bax, which also belongs to Bcl-2 family, is identified as an inhibitory binding protein of Bcl-2 and exerts pro-apoptotic effects (Oltvai et al. 1993). A previous study has reported upregulation of the pro-apoptotic protein Bax and the downregulation of anti-apoptotic protein Bcl-2 in STZ-induced diabetic rats (Pal et al. 2014).
Klotho is a gene correlated with aging and mainly expressed in kidneys (Lee et al. 2014; Nabeshima et al. 2014). Klotho can be used as an indicator of the progression of renal disease and extra renal complications (Hu et al. 2012). It has been found that klotho expression level decreases in DN in humans and mice (Asai et al. 2012). Moreover, klotho deficiency can promote the development and exacerbation of DN in mice. However, upregulated klotho expression exerts renoprotective effects against renal disease and complications in chronic kidney disease (Hu et al. 2012). In the present study, we found an increase in the rate of cell apoptosis and a decrease in klotho expression in renal tissues of DN rats and HG-stimulated NRK-52E cells; we also saw an upregulation in Bax expression and a downregulation in Bcl-2 expression. Our results are thus consistent with previous findings (Asai et al. 2012; Pal et al. 2014). As expected, ADMSC treatment markedly inhibited apoptosis of renal tissue cells and NRK-52E cells, and ADMSC transplantation upregulated klotho expression both in renal tissues of DN rats and in NRK-52E cells exposed to HG. However, Bax and Bcl-2 expressions were reversed by klotho siRNA transfection. These results suggest that ADMSCs may suppress cell apoptosis through elevating klotho expression in DN.
Earlier reports have demonstrated that klotho physically binds to multiple Wnt ligands, including Wnt1, Wnt3a, Wnt4 and Wnt7a, and serves as an endogenous antagonist of Wnt/β-catenin signaling. Furthermore, klotho is negatively correlated with the expression of Wnt/β-catenin signaling (Liu et al. 2007; Zhou et al. 2013). Evidence also shows that activated Wnt/β-catenin signaling protects the cells against cellular damage, whereas aberrant activation of the Wnt/β-catenin pathway may lead to adverse effects and the development of DN, suggesting that Wnt/β-catenin plays a dual role in cell proliferation and apoptosis under DN conditions (Xiao et al. 2013). HG can upregulate the expression of Wnt proteins and activates Wnt/β-catenin signaling in tubular epithelial cells (Zhou et al. 2012), and thus induces the expression of β-catenin target genes, including Snail and Twist (Liu 2010). Our present studies have demonstrated that Wnt/β-catenin is activated in renal tissues of DN rats and in HG-induced NRK-52E cells. However, ADMSC transplantation ameliorates the activation of the Wnt/β-catenin signaling pathway. Knockdown of klotho via siRNA greatly reverses Wnt/β-catenin expression, which is consistent with an earlier study (Zhou et al. 2013). These results suggest that ADMSCs may suppress Wnt/β-catenin signaling through the upregulation of klotho expression in DN.
In summary, we have demonstrated that ADMSCs may ameliorate renal injury by promoting klotho expression and inhibiting the Wnt/β-catenin signaling pathway. Therefore, transplantation of ADMSCs may be a rational strategy for the treatment of DN.
Footnotes
Author Contributions: WS, RZ, XL contributed to data acquisition. JL, XL contributed to statistical analysis. WN, YF, LX performed the experiments and drafted the manuscript. YF reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Competing Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (No.: 81300601), the Natural Science Foundation of Liaoning Province (No.: 2013022025), the Excellent talent Foundation of Liaoning Province (No.: LJQ2013092) and the President grand of LNMU (No.: XZJJ20130243).
References
- Abe H, Matsubara T, Arai H, Doi T. (2011). Role of Smad1 in diabetic nephropathy: Molecular mechanisms and implications as a diagnostic marker. Histol Histopathol 26:531-541. [DOI] [PubMed] [Google Scholar]
- Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. (2014). Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther 5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y. (2012). Decreased renal alpha-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 81:539-547. [DOI] [PubMed] [Google Scholar]
- Brownlee M. (2001). Biochemistry and molecular cell biology of diabetic complications. Nature 414:813-820. [DOI] [PubMed] [Google Scholar]
- Burke M, Pabbidi MR, Farley J, Roman RJ. (2014). Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12:845-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Girolamo L, Lucarelli E, Alessandri G, Avanzini MA, Bernardo ME, Biagi E, Brini AT, D’Amico G, Fagioli F, Ferrero I, Locatelli F, Maccario R, Marazzi M, Parolini O, Pessina A, Torre ML, Italian Mesenchymal Stem Cell G. (2013). Mesenchymal stem/stromal cells: a new “cells as drugs” paradigm. Efficacy and critical aspects in cell therapy. Curr Pharm Des 19:2459-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronavalli S, Duka I, Bakris GL. (2008). The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 4:444-452. [DOI] [PubMed] [Google Scholar]
- Duran-Salgado MB, Rubio-Guerra AF. (2014). Diabetic nephropathy and inflammation. World J Diabetes 5:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimenko A, Starostina E, Kalinina N, Stolzing A. (2011). Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med 9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. (2008). Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 14:631-640. [DOI] [PubMed] [Google Scholar]
- Fang Y, Tian X, Bai S, Fan J, Hou W, Tong H, Li D. (2012). Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med 30:85-92. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Coughlan MT, Cooper ME. (2008). Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57:1446-1454. [DOI] [PubMed] [Google Scholar]
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. (2005). Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28:164-176. [DOI] [PubMed] [Google Scholar]
- Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, Paschopoulos M, Paraskevaidis E, Terakawa N. (2004). Apoptosis in human endometrium and endometriosis. Hum Reprod Update 10:29-38. [DOI] [PubMed] [Google Scholar]
- Hardwick JM, Soane L. (2013). Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. (2001). Progression of diabetic nephropathy. Kidney Int 59:702-709. [DOI] [PubMed] [Google Scholar]
- Hu MC, Kuro-o M, Moe OW. (2012). The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant 27:2650-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin ES, Min J, Jeon SR, Choi KH, Jeong JH. (2013). Analysis of molecular expression in adipose tissue-derived mesenchymal stem cells : prospects for use in the treatment of intervertebral disc degeneration. J Korean Neurosurg Soc 53:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. (2005). Overview of cell death signaling pathways. Cancer Biol Ther 4:139-163. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294-1301. [DOI] [PubMed] [Google Scholar]
- Kume S, Koya D, Uzu T, Maegawa H. (2014). Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy. Biomed Res Int 2014:315494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RC, Yeo RW, Lim SK. (2015). Mesenchymal stem cell exosomes. Semin Cell Dev Biol [DOI] [PubMed] [Google Scholar]
- Lee EY, Kim SS, Lee JS, Kim IJ, Song SH, Cha SK, Park KS, Kang JS, Chung CH. (2014). Soluble alpha-klotho as a novel biomarker in the early stage of nephropathy in patients with type 2 diabetes. PLoS One 9:e102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Kuro-o M, Sun Z. (2013). Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology 154:3855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. (2007). Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317:803-806. [DOI] [PubMed] [Google Scholar]
- Liu Y. (2010). New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21:212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- Lv S, Cheng J, Sun A, Li J, Wang W, Guan G, Liu G, Su M. (2014). Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting oxidative stress. Diabetes Res Clin Pract 104:143-154. [DOI] [PubMed] [Google Scholar]
- Makpol S, Abdul Rahim N, Hui CK, Ngah WZ. (2012). Inhibition of mitochondrial cytochrome c release and suppression of caspases by gamma-tocotrienol prevent apoptosis and delay aging in stress-induced premature senescence of skin fibroblasts. Oxid Med Cell Longev 2012:785743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montespan F, Deschaseaux F, Sensebe L, Carosella ED, Rouas-Freiss N. (2014). Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: implications in bone repair therapy. J Immunol Res 2014:230346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y, Washida M, Tamura M, Maeno A, Ohnishi M, Shiroishi T, Imura A, Razzaque MS. (2014). Calpain 1 inhibitor BDA-410 ameliorates alpha-klotho-deficiency phenotypes resembling human aging-related syndromes. Sci Rep 4:5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. (1993). Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609-619. [DOI] [PubMed] [Google Scholar]
- Ortiz-Munoz G, Lopez-Parra V, Lopez-Franco O, Fernandez-Vizarra P, Mallavia B, Flores C, Sanz A, Blanco J, Mezzano S, Ortiz A, Egido J, Gomez-Guerrero C. (2010). Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol 21:763-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal PB, Sinha K, Sil PC. (2014). Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFalpha related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS One 9:e107220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Feng GG, Huang L, Fan JH, Li C, An J, Tsunekawa K, Kurokawa S, Fujiwara Y, Komatsu T, Kondo F, Ishikawa N. (2010). Enhanced expression of naofen in kidney of streptozotocin-induced diabetic rats: possible correlation to apoptosis of tubular epithelial cells. Clin Exp Nephrol 14:205-212. [DOI] [PubMed] [Google Scholar]
- Schaffler A, Buchler C. (2007). Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells 25:818-827. [DOI] [PubMed] [Google Scholar]
- Wong RS. (2011). Mesenchymal stem cells: angels or demons? J Biomed Biotechnol 2011:459510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Wang M, Yang S, Liu F, Sun L. (2013). A glimpse of the pathogenetic mechanisms of Wnt/beta-catenin signaling in diabetic nephropathy. Biomed Res Int 2013:987064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Zhu X, Yang S, Liu F, Zhou Z, Zhan M, Xie P, Zhang D, Li J, Song P, Kanwar YS, Sun L. (2014). Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes 63:1366-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza T, Miura Y, Akiyama K, Bi Y, Sonoyama W, Gronthos S, Chen W, Le A, Shi S. (2009). Mesenchymal stem cell-mediated ectopic hematopoiesis alleviates aging-related phenotype in immunocompromised mice. Blood 113:2595-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Li K, Yan X, Dong F, Zhao C. (2010). Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefes Arch Clin Exp Ophthalmol 248:1415-1422. [DOI] [PubMed] [Google Scholar]
- Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. (2013). Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol 24:771-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, He X, Cheng R, Zhang B, Zhang RR, Chen Y, Takahashi Y, Murray AR, Lee K, Gao G, Ma JX. (2012). Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia 55:255-266. [DOI] [PubMed] [Google Scholar]